Abstract
Background
Breast cancer (BC), a prevalent and heterogeneous disease of glandular breast tissue, is the most common cancer in women. The interaction between Kaempferol and IQ motif containing GTPase-activating protein 3 (IQGAP3) in BC and its underlying mechanism are poorly defined.
Material/Methods
After natural phytochemicals treatment, the expression of IQGAP3 in BC cells (ZR-75-30 and BT474) was detected by real-time PCR. Then, the proliferation and apoptosis in BC cells with different gradient concentrations (10, 25, 50, and 100 μmol/l) of Kaempferol treatment were detected. After treatment with Kaempferol or epidermal growth factor (EGF), we assessed apoptosis and expression of related genes.
Results
We found that natural phytochemicals, especially Kaempferol, decreased IQGAP3 expression in BC cells. Kaempferol significantly induced proliferation inhibition and apoptosis in BC cells, concurrent with decreased IQGAP3 expression. Upregulation of IQGAP3 inhibited apoptosis in BC cells, along with increased expression of phosphorylated extracellular signal-regulated kinases 1/2 (p-ERK1/2) and B cell lymphoma 2 (Bcl2) and decreased Bcl-2-associated X protein (Bax) expression, which was counteracted by Kaempferol treatment. EGF markedly inhibited Kaempferol-induced apoptosis in BC cells, and ERK1/2 inhibitor PD98059 had an effect similar to that of Kaempferol.
Conclusions
IQGAP3 may be a potential target gene for Kaempferol in the treatment of BC, and upregulation of IQGAP3 inhibits Kaempferol-induced apoptosis in BC cells by ERK1/2 signaling activation. Targeting IQGAP3 may contribute to the study of natural phytochemicals as anti-tumor drugs in BC.
MeSH Keywords: Apoptosis, Cell Proliferation, Inflammatory Breast Neoplasms, Kaempferols
Background
Breast cancer (BC) affects glandular breast tissue and is the most common cancer in women, with high prevalence and heterogeneity [1,2]. Since the late 1970s, the incidence of BC worldwide has been on the rise. Most patients diagnosed with BC at the early stage can be treated with surgery, but this does not guarantee prevention of metastasis [3,4]. Chemotherapy is also used to treat BC [5]. Flavonoids, a class of natural polyphenolic compounds, have a variety of biological properties, including anti-carcinogenic effects [6,7]. For example, Kaempferol (3,5,7-trihydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one), a flavonoid found in plants, is reported to inhibit proliferation and induce apoptosis in many cancers, including BC [8–10]. Despite the advances in BC therapies, the mortality rates remain high due to the failure to prevent recurrence.
The IQ motif containing GTPase-activating protein 3 (IQGAP3), along with IQGAP1 and IQGAP2, are 3 members of the IQGAP family, which is highly conserved in organisms [11,12]. Studies have revealed that IQGAP1 is overexpressed in human cancers [13,14] and is involved in enhanced tumor proliferation and invasion in various cancers, including BC [15]. IQGAP2, when coupled with Wnt/β-catenin pathway activation, appears to be a tumor suppressor [16]. IQGAP3 is only found in proliferating cells [17], and IQGAP3 is a recently discovered effector of Rac1 and Cdc42, which are members of the Rho family of GTPases [18]. Rac1 and Cdc42 are reported to control various cellular processes like cell migration through their effectors [18]. Silencing of IQGAP3 in pancreatic cancer cells significantly induces cell apoptosis [19]. In addition, downregulation of IQGAP3 can suppress cell proliferation and invasion in BC cells [20]. However, its role in Kaempferol-induced apoptosis of BC cells and its underlying mechanisms are unclear.
In the present study, we found that natural phytochemicals, especially Kaempferol, decreased IQGAP3 expression in BC cells (ZR-75-30 and BT474). BC cell proliferation was inhibited by Kaempferol (10, 25, 50, and 100 μmol/l), whereas apoptosis was promoted. Upregulation of IQGAP3 suppressed apoptosis in BC cells, which was counteracted by Kaempferol, and epidermal growth factor (EGF) inhibited the induction of Kaempferol. In addition, extracellular signal-regulated kinases 1/2 (ERK1/2) inhibitor PD98059 had an effect similar to that of Kaempferol. Our results suggest that IQGAP3 is a potential target gene for Kaempferol in the treatment of BC, which may involve ERK1/2 signaling.
Material and Methods
Cell culture
Two human BC cell lines, ZR-75-30 and BT474, were purchased from the Cell Bank of the Chinese Academy of Science (Shanghai, China). The cells were placed in a 37°C, 5% CO2 incubator (Thermo, Thermo Forma 3111, USA) and cultured with RPMI-1640 medium (HyClone, SH30809.01B, USA) supplement with 10% fetal bovine serum (GIBCO, USA) and 1% antibiotic (penicillin and streptomycin, Solarbio, P1400-100, Beijing, China). The medium was refreshed every 2 days during incubation.
Construction of the lentivirus
According to the experimental needs, pLVX-Puro construct, the overexpression of lentivirus, was selected. The coding DNA sequence (CDS) region of IQGAP3 (AY253300.1), with a full length of 4896 bp, containing restriction sites of EcoR I and BamH I, was synthesized by Genewiz Company (Shanghai, China) and then inserted into EcoR I/BamH I restriction sites of a pLVX-Puro plasmid (Clontech). Primer sequences were as follows (underlined for restriction sites): IQGAP3-Forward: 5′-CGGAATTCATGGAGAGGAGAGCAGC-3′ (EcoR I), IQGAP3-Reverse: 5′-CGGGATCCTCACTTCCGCAAAAACTTC-3′ (BamH I). After DNA sequencing (Majorbio, Shanghai, China), the sequencing results were compared with the IQGAP3 sequence in NCBI, and the plasmid retention liquid with a ratio of 100% was retained. After the extraction of pLVX-Puro-IQGAP3, pLVX-Puro, psPAX2, and pMD2G (Addgen, USA), and cell resuscitation and subculture of 293T, pLVX-Puro-IQGAP3 (1000 ng) or pLVX-Puro (1000 ng) was co-transfected into 293T cells with viral packaging plasmids psPAX2 (100 ng) and pMD2G (900 ng) using Lipofectamine 2000 (Invitrogen, USA) according to the manufacturer’s instructions. The virus particles in the cultured medium were collected after 48-h transfection.
Experimental grouping
ZR-75-30 and BT474 cells were respectively divided into groups infected with the lentiviruses of IQGAP3 overexpression (IQGAP3) and negative control (Vector), respectively, with RPMI-1640 medium-treated cells as control. After 48 h of transfection, the expression of IQGAP3 in ZR-75-30 and BT474 cells was detected by real-time PCR and Western blot analysis.
To further investigate the interaction between Kaempferol and IQGAP3, as well as the potential underlying mechanism, ZR-75-30 and BT474 cells were divided into groups treated with Vector+DMSO, IQGAP3+DMSO, 20 μM PD98059 (ERK inhibitor, Selleck, S1177; solvent: DMSO), Vector+25 μmol/l Kaempferol (Source Leaf Biotechnology Co., B21126, Shanghai, China; solvent: DMSO), IQGAP3+25 μmol/l Kaempferol, Vector+25 μmol/l Kaempferol+100 ng/ml EGF (R&D, 236-EG, USA; solvent: PBS), or IQGAP3+25 μmol/l Kaempferol+100 ng/ml EGF. Cell apoptosis and Western blot assays were performed after 48 h of treatment.
Real-time polymerase chain reaction (RT-PCR) assay
BC cells were treated with natural phytochemicals (solvents: DMSO or PBS) or IQGAP3 lentiviruses, and then the total RNA from these cells was extracted using Trizol reagent (Invitrogen, 1596-026, USA). After quantification using a reverse transcription kit (Fermentas, #K1622, USA), an aliquot of 1 μg RNA was reverse-transcribed into cDNA at 37°C for 30 min, 85˚C for 5 min, and 4°C for 5 min. Subsequently, cDNA was used as a template for RT-PCR reaction on a real-time PCR system (Applied Biosystems, ABI-7300, USA) using the SYBR Green PCR kit (Thermo Fisher Scientific, Inc., #K0223). The expression of IQGAP3 mRNA relative to GAPDH was analyzed by 2−ΔΔCq method [21]. The primers used were:
IQGAP3, Forward: 5′-TATGGGATGCGATATGTG-3′,
Reverse: 5′-GGTTCAGGAAGCGGTAG-3′;
GAPDH: Forward: 5′-CACCCACTCCTCCACCTTTG-3′,
Reverse: 5′-CCACCACCCTGTTGCTGTAG-3′.
The RT-PCR procedures were: 95°C for 10 min (95°C for 15 s; 60°C for 45 s)×40; 95°C for 15 s; 60°C for 1 min; 95°C for 15 s; and 60°C for 15 s [22].
Cell proliferation assay
The cell proliferation of BC cells was evaluated using the Cell Counting kit-8 (CCK-8). ZR-75-30 and BT474 cells in logarithmic growth phase were digested with 0.25% trypsin (Solarbio, T1300-100, Beijing, China) and then counted under a microscope (Shanghai Caikang Optical Instrument Co., XDS-500C) to prepare 3×104 cells/ml of cell suspension. Each 100 μl/well of cell suspension was inoculated into 96-well culture plates and cultured overnight at 37°C in a 5% CO2 incubator. The next day, the cells were treated with 100 μl of gradient concentrations of Kaempferol (10, 25, 50, and 100 μmol/l). After 0, 24, 48, and 72 h, 100 μl of CCK-8 solution (CCK-8: serum-free RPMI-1640 medium=1: 10, SAB Biotherapeutics, Inc., CP002) was added to incubate for 1 h. The optical density of the absorbance at 450 nm was evaluated at 0, 24, 48, and 72 h using a microplate reader (Perlong, DNM-9602, Beijing, China).
Cell apoptosis assay
Flow cytometry analysis (FCM) was applied to evaluate apoptosis in BC cells. Treated ZR-75-30 and BT474 cells were collected and Annexin V-fluorescein isothiocyanate (FITC)/propidium iodide (PI) double staining (Beyotime, C1063) was performed. According to the manufacturer’s instructions, 5×105–1×106 cells were resuspended in 195 μl Annexin V-FITC binding buffer, followed by incubation in 5 μl Annexin V-FITC for 15 min at 4°C in the dark. Subsequently, the cells were incubated in 5 μl PI for 5 min at 4°C in the dark. A tube without incubation of both Annexin V-FITC and PI was used as a control. The percentage of apoptotic ZR-75-30 and BT474 cells was evaluated on a flow cytometer using BD AccuriTM C6 software (Version 1.0.264.21, BD Biosciences, USA).
Western blot analysis
The expression of apoptosis-related proteins was evaluated by Western blot analysis. Using RIPA buffer containing protease and phosphatase inhibitors (Solarbio, R0010, Beijing, China), the total proteins were extracted from treated ZR-75-30 and BT474 BC cells. After quantification with the BCA Kit (Thermo, PICPI23223), 25 μg of proteins were separated using 10% SDS-polyacrylamide gels and then transferred to polyvinylidene fluoride (PVDF) membranes (Millipore, HATF00010, USA). Subsequently, the membranes were blocked with 5% skim milk (BD Biosciences, BYL40422) for 1 h, followed by incubation in primary antibodies against IQGAP3 (1: 1000, Abcam, Ab88353), ERK1/2 (1: 1000, Cell Signaling Technology [CST], #9102), p-ERK1/2 (1: 1000, CST, #9101), Bax (1: 1000, Abcam, Ab32503), Bcl2 (1: 1000, Abcam, Ab196495), and GAPDH (1: 2000, CST, #5174) overnight at 4°C. The next day, at room temperature, the second antibodies labeled with HRP (1: 1000, Beyotime, Shanghai) goat anti-rabbit (A0208) and goat anti-mouse (A0216) antibodies were used to incubate the membranes for 2 h. The blots were developed by 5-min incubation with chemiluminescent reagent (Millipore, WBKLS0100), and then visualized on an ECL imaging system (Tanon, Tanon-5200, Shanghai). The protein expression, normalized to GAPDH, was analyzed and calculated by version 1.47v Image J software (Bethesda, MD, USA).
Statistical analysis
Statistical significance in the present study was analyzed using GraphPad prism 7.0 software (GraphPad Software, Inc., La Jolla, CA, USA). One-way analysis of variance (ANOVA) with a post-test of Tukey’s multiple comparison was used to determine the significance among multiple comparisons. Experimental values are presented as mean ±SD with 3 independent experiments, and P value less than 0.05 indicated a statistically significant difference.
Results
Natural phytochemicals decreased the expression levels of IQGAP3 in BC cells
It is reported that natural phytochemicals such as Tanshinone IIA, Amygdalin, and Kaempferol can regulate cellular processes in BC, such as cell growth and apoptosis [8,23,24]. In the present study we treated ZR-75-30 cells with Ligustrazine (1 and 2 mg/ml; injection: 4 mg/ml), Tanshinone IIA (10 and 20 μmol/l; solvent: DMSO), Amygdalin (50 and 100 μmol/l; solvent: DMSO), Succinic acid (25 and 50 μmol/l; solvent: PBS), Curcumin (25 and 50 μmol/l; solvent: DMSO), or Kaempferol (25 and 50 μmol/l; solvent: DMSO), and then the expression of IQGAP3 in ZR-75-30 cells was detected after 48-h treatment. As shown in Figure 1, we found that the expression of IQGAP3 in ZR-75-30 cells was decreased by these natural phytochemicals and Kaempferol had a best effect. Therefore, due to its superior regulation effect, Kaempferol was selected for further study.
Figure 1.
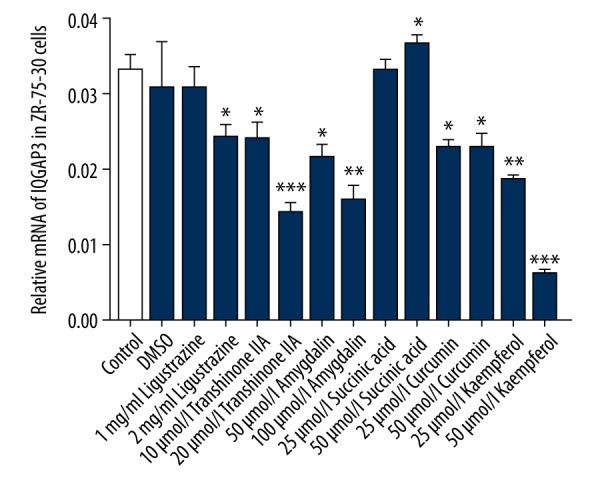
Natural phytochemicals decreased the expression levels of IQGAP3 in breast cancer cells. ZR-75-30 cells were divided into groups treated with Ligustrazine (1 and 2 mg/ml), Tanshinone IIA (10 and 20 μmol/l), Amygdalin (50 and 100 μmol/l), Succinic acid (25 and 50 μmol/l), Curcumin (25 and 50 μmol/l), or Kaempferol (25 and 50 μmol/l). After 48-h treatment, the expression of IQGAP3 in ZR-75-30 cells was detected. Concentrations of DMSO in each experimental group were the same as in the DMSO group. Data are shown as mean ±SD (n=3), * P<0.05, ** P<0.01 and *** P<0.001 compared to DMSO.
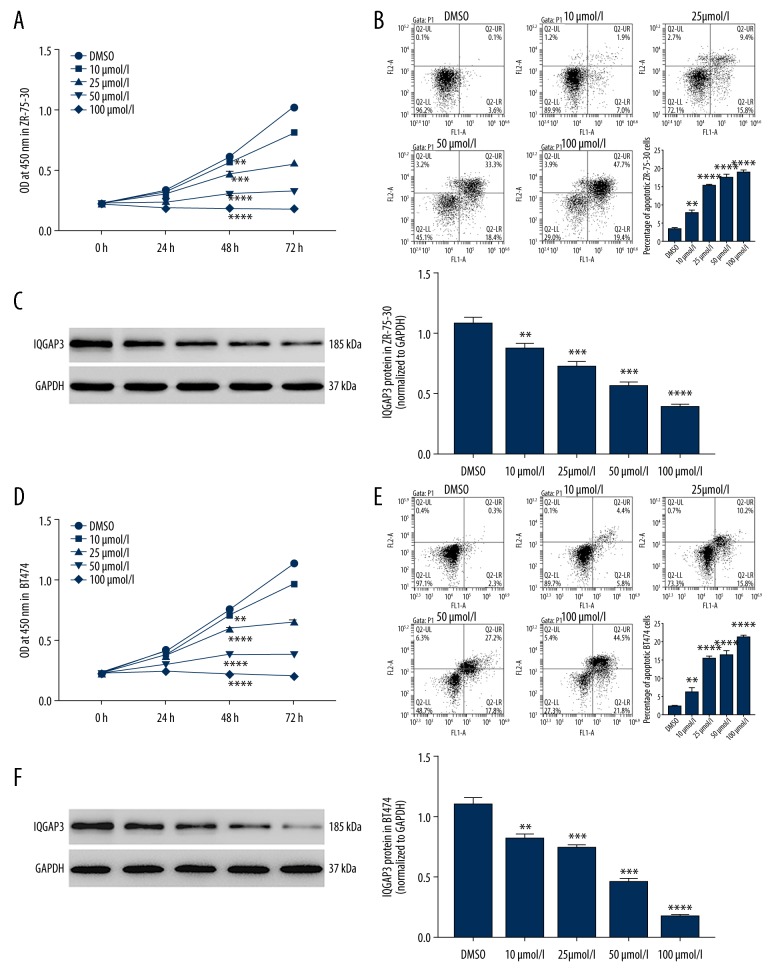
Kaempferol regulated cell proliferation and apoptosis in BC cells
In vitro, cultured ZR-75-30 and BT474 cells were treated with gradient concentrations of Kaempferol (10, 25, 50, 100 μmol/l), and the cell proliferation and apoptosis were evaluated. The results in Figure 2 show that Kaempferol significantly inhibited cell proliferation (Figure 2A, 2D) and promoted cell apoptosis (Figure 2B, 2E) in BC cells, concurrent with a significant decrease of IQGAP3 expression (Figure 2C, 2F). Importantly, as the concentration increased, the regulation effect of Kaempferol was increased, which is consistent with a previous observation that Kaempferol can induce apoptosis of BC cells [8]. These results indicated that Kaempferol could treat BC by promoting cell apoptosis by inhibiting IQGAP3 expression.
Figure 2.
Kaempferol regulates the cell proliferation and apoptosis in breast cancer cells. In vitro, cultured ZR-75-30 and BT474 cells were divided into groups treated with gradient concentrations of Kaempferol (10, 25, 50, and 100 μmol/l). (A, D) The cell proliferation of ZR-75-30 (A) and BT474 (D) cells were evaluated at 0, 24, 48, and 72 h by CCK-8. (B, E) The percentage of apoptotic ZR-75-30 (B) and BT474 (E) cells was evaluated after 48-h treatment. (C, F) The protein expression of IQGAP3 in ZR-75-30 (C) and BT474 (F) cells was detected by Western blot. Concentrations of DMSO in each experimental group were similar to that in the DMSO group. All data are expressed as mean ±SD (n=3), ** P<0.01, *** P<0.001 and **** P<0.0001 compared to DMSO.
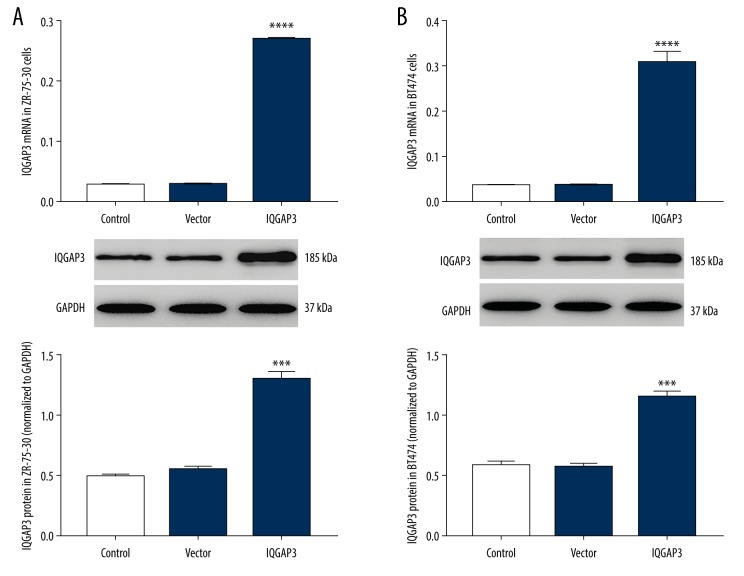
Upregulation of IQGAP3 expression in BC cells
Our previous study found that the expression of IQGAP3 in tumor tissues of BC is much higher than in adjacent normal tissues [19]. In the present study, to further investigate the role of IQGAP3 in BC, ZR-75-30 and BT474 cells were infected with IQGAP3 lentivirus. The results are shown in Figure 3. Both in ZR-75-30 (Figure 3A) and BT474 cells (Figure 3B), the levels of IQGAP3 mRNA (upper) and protein (lower) were markedly upregulated by IQGAP3 lentiviruses. Thus, the IQGAP3 lentivirus was used for follow-up experiments.
Figure 3.
Upregulation of IQGAP3 expression in breast cancer cells. In vitro, cultured ZR-75-30 and BT474 cells were infected with lentiviruses of IQGAP3/Vector for 48 h, with medium-treated cells as control. (A) The levels of IQGAP3 mRNA (upper) and protein (lower) in ZR-75-30 cells were detected by RT-PCR and Western blot, respectively. (B) The expression of IQGAP3 in BT474 cells was also detected. All data are shown as mean ±SD (n=3), *** P<0.001 and **** P<0.0001 compared to Vector.
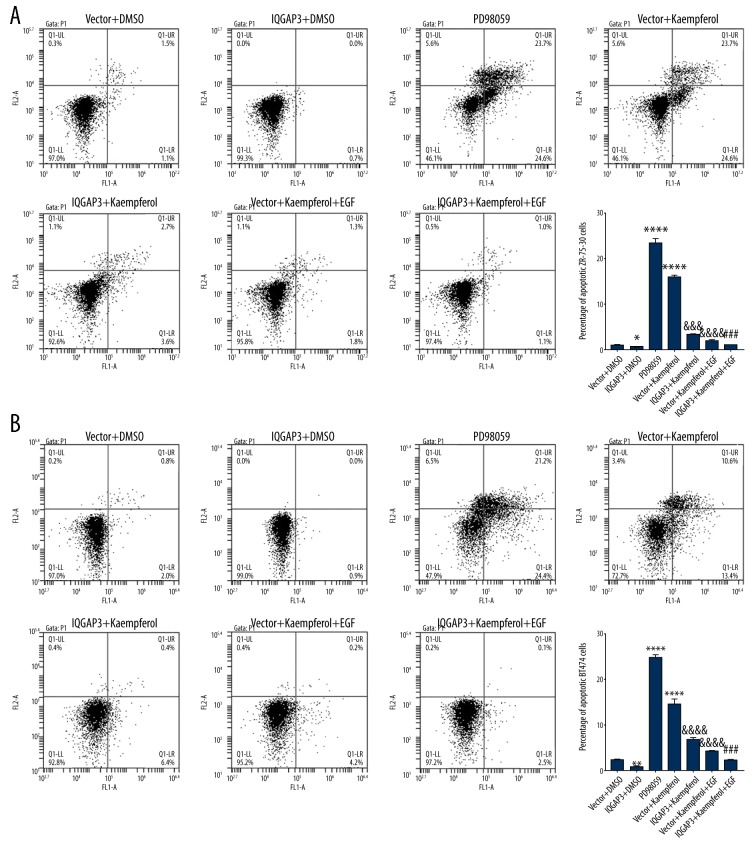
Kaempferol-induced apoptosis in BC cells was significantly suppressed by IQGAP3 upregulation
To explore the interaction between IQGAP3 and Kaempferol in BC cells, after treatment, the percentage of apoptotic ZR-75-30 and BT474 cells was evaluated. Data in Figure 4 show that upregulation of IQGAP3 inhibited apoptosis in BC cells and significantly suppressed Kaempferol-induced apoptosis. Furthermore, similar to IQGAP3 upregulation, treatment with EGF also markedly inhibited Kaempferol-induced apoptosis in BC cells, and PD98059, an inhibitor of ERK, had a similar effect to that of Kaempferol. EGF is a growth factor that can promote the proliferation and differentiation of cells, thereby replacing aging and dead cells with new cells. These results further show that Kaempferol-induced apoptosis in BC cells may occur by inhibiting the expression of IQGAP3, which may further involve the ERK signaling pathway.
Figure 4.
Kaempferol-induced apoptosis in breast cancer cells was significantly suppressed by IQGAP3 upregulation ZR-75-30 and BT474 cells were divided into groups treated with Vector+DMSO, IQGAP3+DMSO, 20 μM PD98059 (ERK inhibitor), Vector+25 μmol/l Kaempferol, IQGAP3+25 μmol/l Kaempferol, Vector+25 μmol/l Kaempferol+100 ng/ml EGF, or IQGAP3+25 μmol/l Kaempferol+100 ng/ml EGF. (A, B) The percentage of apoptotic ZR-75-30 (A) and BT474 (B) cells was evaluated 48 after treatment. Concentrations of DMSO experimental groups were the same as in the DMSO group. Data are presented as mean ±SD (n=3), * P<0.05, ** P<0.01 and **** P<0.0001 compared to Vector & DMSO, &&& P<0.001 and &&&& P<0.0001 compared to Vector+Kaempferol, ### P<0.001 compared to IQGAP3+Kaempferol.
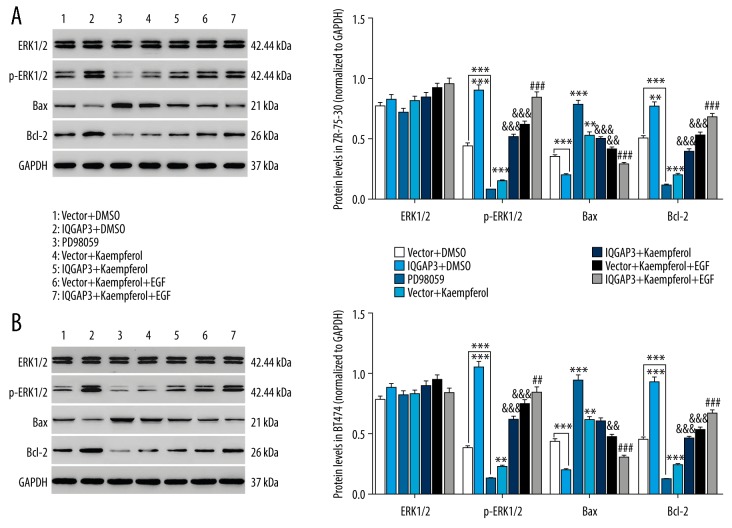
IQGAP3 inhibited Kaempferol-induced apoptosis in BC cells by activation of ERK signaling pathway
The mechanism underlying the anti-apoptotic effect of IQGAP3 on BC cells was further investigated in this study. Studies have reported that B cell lymphoma 2 (Bcl2) is an antiapoptotic protein in cancers, whereas Bcl-2-associated X protein (Bax) has a proapoptotic effect, and the ratio of Bax to Bcl-2 is used to determine whether the cells are committed to apoptosis or not [25,26]. As shown in Figure 5, the expression of p-ERK1/2 and Bcl2 in BC cells was significantly increased by IQGAP3 upregulation, whereas Bax was decreased, and Kaempferol had an opposite effect to that of IQGAP3. Furthermore, the effect of Kaempferol on the expression of p-ERK1/2, Bax, and Bcl2 was counteracted by IQGAP3. Similar to IQGAP3 upregulation, EGF treatment also increased p-ERK1/2 and Bcl2 and decreased Bax, which was consistent with previous findings that ERK1 and 2 are preferentially activated in response to growth factors [27,28]. In addition, the ERK inhibitor PD98059 had a similar effect to that of Kaempferol. Our results suggest that upregulation of IQGAP3 inhibits Kaempferol-induced apoptosis in BC cells, probably through activation of the ERK signaling pathway.
Figure 5.
IQGAP3 inhibited Kaempferol-induced apoptosis in breast cancer cells via activation of ERK signaling pathway. In vitro, cultured ZR-75-30 and BT474 cells were divided into groups treated with Vector, IQGAP3, 20 μM PD98059 (ERK inhibitor), Vector+25 μmol/l Kaempferol, IQGAP3+25 μmol/l Kaempferol, Vector+25 μmol/l Kaempferol+100 ng/ml EGF, or IQGAP3+25 μmol/l Kaempferol+100 ng/ml EGF. (A, B) The expression of ERK1/2, p-ERK1/2, Bax, and Bcl-2 proteins in ZR-75-30 (A) and BT474 (B) cells was detected by Western blot at 48 h after treatment. Concentrations of DMSO in each experimental group were the same as in the DMSO group. Data are expressed as mean ±SD (n=3), ** P<0.01 and *** P<0.001 compared to Vector, && P<0.01 and &&& P<0.001 compared to Vector+Kaempferol, ## P<0.01 and ### P<0.001 compared to IQGAP3+Kaempferol.
Discussion
As more and more natural phytochemicals are found to have anti-cancer effects, the application of natural phytochemicals in anti-tumor drugs development has become an important research field in recent years. For example, paclitaxel has already been used as an anti-cancer drug in clinical practice [29]. Flavonoids are reported to inhibit cell growth in multiple cancers, including breast and prostate cancer [30]. Our previous study showed that, compared to other BC cell lines, IQGAP3 has a higher expression level in ZR-75-30 and BT474 cells [31]. Thus, ZR-75-30 and BT474, 2 specific BC cell lines, were chosen as a model system for use in the present study. This study demonstrated that IQGAP3 can inhibit Kaempferol-induced apoptosis in BC cells (ZR-75-30 and BT474) by activating the ERK1/2 signaling pathway.
IQGAP3 is a newly discovered IQGAP and has been found to be overexpressed in BC tissues in our previous studies [20,32]. In the present study, we found that the high expression of IQGAP3 in BC cells was significantly reduced by natural phytochemicals, especially Kaempferol. Kaempferol is a flavonoid and functions in many cancers by regulating cell proliferation and apoptosis [9,10], which is consistent with our results showing that Kaempferol significantly inhibits cell proliferation and promotes apoptosis in BC cells, accompanied with markedly decreased IQGAP3 expression. Our results suggest that IQGAP3 is a target gene of Kaempferol in BC. In our study, upregulation of IQGAP3 inhibited apoptosis of BC cells, accompanied with increased p-ERK1/2 and Bcl2 expression and decreased Bax expression, and Kaempferol counteracted the effect of IQGAP3 upregulation. ERK1/2, one of the most extensively studied groups of MAPKs, is a major determinant in diverse cellular processes, including apoptosis, proliferation, and survival [33,34], and has been observed to be associated with BC [35]. It has been reported that Kaempferol-induced apoptosis of BC cells involves sustained activation of ERK signaling [8], and IQGAP3 is reported to regulate cell proliferation by mediating Ras-dependent ERK signaling [17]. Thereby, we hypothesized that upregulation of IQGAP3 inhibits Kaempferol-induced apoptosis in BC cells, probably through activating the ERK1/2 signaling pathway. This hypothesis is further supported by the observations that PD98059, an inhibitor of ERK1/2, also markedly induces apoptosis in BC cells, similar to the effect of Kaempferol. In addition, EGF treatment had an inhibitory effect on Kaempferol-induced apoptosis, suggesting that Kaempferol-induced BC cell apoptosis may be correlated to its cell proliferation.
Conclusions
In this study, we demonstrated that IQGAP3 is a potential target gene for Kaempferol in the treatment of BC, and IQGAP3 may inhibit Kaempferol-induced apoptosis in BC cells through activating the ERK1/2 signaling pathway. Targeting IQGAP3 is a potential novel therapeutic method for BC, and may contribute to the study of natural phytochemicals as anti-tumor drugs in BC.
Footnotes
Source of support: Departmental sources
References
- 1.Feuer EJ, Wun LM, Boring CC, et al. The lifetime risk of developing breast cancer. J Natl Cancer Inst. 1993;85:892–97. doi: 10.1093/jnci/85.11.892. [DOI] [PubMed] [Google Scholar]
- 2.Wolff MS, Collman GW, Barrett JC, Huff J. Breast cancer and environmental risk factors: Epidemiological and experimental findings. Annu Rev Pharmacol Toxicol. 1996;36:573–96. doi: 10.1146/annurev.pa.36.040196.003041. [DOI] [PubMed] [Google Scholar]
- 3.Demicheli R, Valagussa P, Bonadonna G. Does surgery modify growth kinetics of breast cancer micrometastases? Br J Cancer. 2001;85:490–92. doi: 10.1054/bjoc.2001.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hansen E, Wolff N, Knuechel R, et al. Tumor cells in blood shed from the surgical field. Arch Surg. 1995;130:387–93. doi: 10.1001/archsurg.1995.01430040049007. [DOI] [PubMed] [Google Scholar]
- 5.Osta WA, Chen Y, Mikhitarian K, et al. EpCAM is overexpressed in breast cancer and is a potential target for breast cancer gene therapy. Cancer Res. 2004;64:5818–24. doi: 10.1158/0008-5472.CAN-04-0754. [DOI] [PubMed] [Google Scholar]
- 6.Kanno S, Tomizawa A, Hiura T, et al. Inhibitory effects of naringenin on tumor growth in human cancer cell lines and sarcoma S-180-implanted mice. Biol Pharm Bull. 2005;28:527–30. doi: 10.1248/bpb.28.527. [DOI] [PubMed] [Google Scholar]
- 7.Harborne JB, Williams CA. Advances in flavonoid research since 1992. Phytochemistry. 2000;55:481–504. doi: 10.1016/s0031-9422(00)00235-1. [DOI] [PubMed] [Google Scholar]
- 8.Kim BW, Lee ER, Min HM, et al. Sustained ERK activation is involved in the kaempferol-induced apoptosis of breast cancer cells and is more evident under 3-D culture condition. Cancer Biol Ther. 2008;7:1080–89. doi: 10.4161/cbt.7.7.6164. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Y, Chen AY, Li M, et al. Ginkgo biloba extract kaempferol inhibits cell proliferation and induces apoptosis in pancreatic cancer cells. J Surg Res. 2008;148:17–23. doi: 10.1016/j.jss.2008.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nguyen TTT, Tran E, Ong CK, et al. Kaempferol-induced growth inhibition and apoptosis in A549 lung cancer cells is mediated by activation of MEK-MAPK. J Cell Physiol. 2003;197:110–21. doi: 10.1002/jcp.10340. [DOI] [PubMed] [Google Scholar]
- 11.Weissbach L, Settleman J, Kalady MF, et al. Identification of a human rasGAP-related protein containing calmodulin-binding motifs. J Biol Chem. 1994;269:20517–21. [PubMed] [Google Scholar]
- 12.Briggs MW, Sacks DB. IQGAP1 as signal integrator: Ca2+, calmodulin, Cdc42 and the cytoskeleton. FEBS Lett. 2003;542:7–11. doi: 10.1016/s0014-5793(03)00333-8. [DOI] [PubMed] [Google Scholar]
- 13.Johnson M, Sharma M, Henderson BR. IQGAP1 regulation and roles in cancer. Cell Signal. 2009;21:1471–78. doi: 10.1016/j.cellsig.2009.02.023. [DOI] [PubMed] [Google Scholar]
- 14.White CD, Brown MD, Sacks DB. IQGAPs in cancer: A family of scaffold proteins underlying tumorigenesis. FEBS Lett. 2009;583:1817–24. doi: 10.1016/j.febslet.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jadeski L, Mataraza JM, Jeong HW, et al. IQGAP1 stimulates proliferation and enhances tumorigenesis of human breast epithelial cells. J Biol Chem. 2008;283:1008–17. doi: 10.1074/jbc.M708466200. [DOI] [PubMed] [Google Scholar]
- 16.Schmidt VA, Chiariello CS, Capilla E, et al. Development of hepatocellular carcinoma in Iqgap2-deficient mice is IQGAP1 dependent. Mol Cell Biol. 2008;28:1489–502. doi: 10.1128/MCB.01090-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nojima H, Adachi M, Matsui T, et al. IQGAP3 regulates cell proliferation through the Ras/ERK signalling cascade. Nat Cell Biol. 2008;10:971–78. doi: 10.1038/ncb1757. [DOI] [PubMed] [Google Scholar]
- 18.Wang S, Watanabe T, Noritake J, et al. IQGAP3, a novel effector of Rac1 and Cdc42, regulates neurite outgrowth. J Cell Sci. 2007;120:567–77. doi: 10.1242/jcs.03356. [DOI] [PubMed] [Google Scholar]
- 19.Xu W, Xu B, Yao Y, et al. Overexpression and biological function of IQGAP3 in human pancreatic cancer. Am J Transl Res. 2016;8:5421–32. [PMC free article] [PubMed] [Google Scholar]
- 20.Hu G, Xu Y, Chen W, et al. RNA interference of IQ motif containing GTPase-activating protein 3 (IQGAP3) inhibits cell proliferation and invasion in breast carcinoma cells. Oncol Res. 2016;24:455–61. doi: 10.3727/096504016X14685034103635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 22.Hong JY, Kang B, Kim A, et al. Development of a highly sensitive real-time one step RT-PCR combined complementary locked primer technology and conjugated minor groove binder probe. Virol J. 2011;8:330. doi: 10.1186/1743-422X-8-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin C, Wang L, Wang H, et al. Tanshinone IIA inhibits breast cancer stem cells growth in vitro and in vivo through attenuation of IL-6/STAT3/NF-κB signaling pathways. J Cell Biochem. 2013;114:2061–70. doi: 10.1002/jcb.24553. [DOI] [PubMed] [Google Scholar]
- 24.Min LH, Aree M. Amygdalin regulates apoptosis and adhesion in Hs578T triple-negative breast cancer cells. Biomol Ther (Seoul) 2016;24:62–66. doi: 10.4062/biomolther.2015.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gao G, Dou QP. G(1) phase-dependent expression of bcl-2 mRNA and protein correlates with chemoresistance of human cancer cells. Mol Pharmacol. 2000;58:1001–10. doi: 10.1124/mol.58.5.1001. [DOI] [PubMed] [Google Scholar]
- 26.Green DR, Reed JC. Mitochondria and apoptosis. Science. 1998;281:1309–12. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- 27.Pearson G, Robinson F, Beers TG, et al. Mitogen-activated protein (MAP) kinase pathways: Regulation and physiological functions. Endocr Rev. 2001;22:153–83. doi: 10.1210/edrv.22.2.0428. [DOI] [PubMed] [Google Scholar]
- 28.Peyssonnaux C, Eychène A. The Raf/MEK/ERK pathway: New concepts of activation. Biol Cell. 2001;93:53–62. doi: 10.1016/s0248-4900(01)01125-x. [DOI] [PubMed] [Google Scholar]
- 29.Sheppard BC, Rutten MJ, Meichsner CL, et al. Effects of paclitaxel on the growth of normal, polyposis, and cancerous human colonic epithelial cells. Cancer. 1999;85:1454–64. [PubMed] [Google Scholar]
- 30.Sarkar FH, Adsule S, Padhye S, et al. The role of genistein and synthetic derivatives of isoflavone in cancer prevention and therapy. Mini Rev Med Chem. 2006;6:401–7. doi: 10.2174/138955706776361439. [DOI] [PubMed] [Google Scholar]
- 31.Hu G, Xu Y, Chen W, et al. RNA interference of IQ motif containing GTPase-activating protein 3 (IQGAP3) inhibits cell proliferation and invasion in breast carcinoma cells. Oncol Res. 2016;24:455–61. doi: 10.3727/096504016X14685034103635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hua X, Long Z, Zhang W, et al. IQGAP3 overexpression correlates with poor prognosis and radiation therapy resistance in breast cancer. 2018 doi: 10.3389/fphar.2020.584450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roux PP, Blenis J. ERK and p38 MAPK-activated protein kinases: A family of protein kinases with diverse biological functions. Microbiol Mol Biol Rev. 2004;68:320–44. doi: 10.1128/MMBR.68.2.320-344.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kohno M, Pouyssegur J. Targeting the ERK signaling pathway in cancer therapy. Ann Med. 2006;38:200–11. doi: 10.1080/07853890600551037. [DOI] [PubMed] [Google Scholar]
- 35.Nunesxavier CE, Elson A, Pulido R. Epidermal growth factor receptor (EGFR)-mediated positive feedback of protein-tyrosine phosphatase ɛ (PTPɛ) on ERK1/2 and AKT protein pathways is required for survival of human breast cancer cells. J Biol Chem. 2012;287:3433–44. doi: 10.1074/jbc.M111.293928. [DOI] [PMC free article] [PubMed] [Google Scholar]