Abstract
Background
The aim of this study is to evaluate the clinical significance of cystatin C(CysC) in the newborns who show normal serum creatinine (Cr) and who are in an intensive care unit.
Methods
From July 2009 to May 2010, a total of 106 patients (53 male and 53 female newborns) in a neonatal intensive care unit at Kyung Hee Medical Center were enrolled in this study. When clinicians ordered CysC, it was tested using HiSens Cystatin‐C LTIA(HBi, An‐yang, Korea) on a Toshiba chemical analyzer (Toshiba, Nasushiobara, Japan).
Results
The range of serum Cr and CysCwas from 0.1 to 0.8 mg/dL and from 1.0 to 2.3 mg/L, respectively. CysCpresented the wider amplitude of the changes in acute renal failure.
Conclusion
In this study, CysCwithout an increased Cr showed only a mild increase. However, CysCreflected more delicate changes in newborns than the serum Cr. This characteristic of CysCcould make it very appropriate for a pediatric population, especially for critically ill newborns. J. Clin. Lab. Anal. 26:267‐271, 2012. © 2012 Wiley Periodicals, Inc.
Keywords: cystatin C, creatinine, newborn, acute renal failure
INTRODUCTION
Estimating the glomerular filtration rate (GFR) is important to assess the renal function. Precise and accurate methods for assessing the GFR such as insulin clearance or radioactive markers are laborious for routine clinical use and they are inappropriate for children, especially for newborn infants (1, 2, 3). Therefore, serum creatinine (Cr) is the most widely used indicator for routine GFR estimation in children. However, it is well known that the blood Cr level is influenced by the body muscle mass and it is insensitive to slightly reduced renal function 4, 5. For these reasons, there are practical needs to search for a more sensitive and accurate marker than the Cr level. The blood cystatin C (CysC) level has recently been proposed as a novel marker for the GFR 3.
CysC is an inhibitor of lysosomal cystein proteinases, and it is a 13‐kD basic protein produced by all nucleated cells 6, 7. CysC is produced at a constant rate, it is freely filtered by the glomerular basement membranes, and it is almost completely reabsorbed 2. Unlike Cr, CysC is not secreted by tubules, even in cases with a reduced GFR, and it is eliminated from circulation almost exclusively by glomerular filtration 2, 5. The level of CysC does not appear to be affected by muscle mass and gender 8. Also unlike Cr, CysC does not cross the placental barrier and the CysC level after birth probably reflects the degree of maturation of the glomerular filtration capacity 9.
However, there are only a few studies on CysC that have focused on newborns or premature infants 10. With this background, we conducted this study to evaluate the clinical significance of CysC as an early and sensitive marker in newborns in the neonatal intensive care unit (NICU), especially in newborns with normal Cr levels, and traced serial follow‐up results of these two markers in newborns with acute renal failure (ARF).
MATERIALS AND METHODS
From July 2009 to May 2010, a total of 106 patients (53 male and 53 female infants) in the NICU were enrolled in this study at Kyung Hee Medical Center. When clinicians ordered CysC, it was tested using HiSens Cystatin‐C LTIA (HBi, An‐yang, Korea) on a Toshiba chemical analyzer (Toshiba, Nasushiobara, Japan). Serum Cr (Kanto Chemical Co., Tokyo, Japan) and C‐reactive protein (CRP) (Sekisui, Tokyo, Japan) were measured on the same chemical analyzer. The clinical information was retrospectively obtained by extensive medical chart reviews. Diagnoses of ARF were made by neonatologist according to the risk, injury, failure, loss of kidney function, and end‐stage kidney disease (RIFLE) criteria 11.
The independent t‐test was used to compare the mean values determined in this study. Statistical analyses were performed with Medcalc 12.0 (Medcalc Software, Mariakerke, Belgium) and Excel 2003 (Microsoft, Redmond, WA). Statistical significance was set at P values < 0.05.
RESULTS
The mean body weight was 2,545 g and the mean height was 46.84 cm. The mean body mass index (BMI) was 10.7 (Table 1). The main causes of admission to the NICU were hyperbilirubinemia of the newborn and respiratory problem such as apnea, respiratory distress syndrome, and transient tachypnea of the newborn. There were 61 preterm infants among the total newborns. The range of the serum Cr and CysC levels was from 0.1 to 0.8 mg/dL and from 1.0 to 2.3 mg/L, respectively. Eleven newborns (10.4%) had clinical manifestations associated with their kidney function. One‐hundred‐three newborns (the 97.5 percentile) showed a CysC level under the 2.1 mg/L (Fig. 1. There were no significant differences for the CysC levels between the male and female groups, between the preterm and full‐term infants, and between lasix‐used infants and not. The CysC levels showed no significant correlation with BMI. However, among preterm infants, there was the significant increase in small for gestational age group (N = 26) than appropriate for gestational age group (N = 34, P = 0.0003).
Table 1.
The patients’ demographics and renal parameters
| Total patient number | 106 |
|---|---|
| Male/Female | 53/53 |
| Mean BMI (kg/m2) | 10.7 |
| Mean Weight (g) | 2,545 |
| Mean height (cm) | 46.84 |
| Range of serum Cr (mg/dL) | 0.1–0.8 |
| Mean of serum CysC (mg/L) | 1.0–2.3 |
| Main diagnosis of the disease entities | |
| Hyperbilirubinemia of newborns | 22 |
| Apnea | 8 |
| Respiratory distress syndrome | 7 |
| Transient tachypnea of newborn | 6 |
| Low birth weight infant | 5 |
| Meconium plug syndrome | 4 |
| Acute gastroenteritis | 4 |
| Fetal disatress | 3 |
| Convulsion disorder | 3 |
| Germinal matrix hemorrhage | 3 |
| Hypocalcemia | 3 |
| Hypoglycemia | 3 |
| Asymmetric intrauterine growth retardation | 3 |
| Othersa | 32 |
Megacolon, congenital ALL, hematochezia, intraventricular hemorrhage, hyponatremia, premature rupture of membrane, hyperammonemia, neutropenia, anemia, neonatal graves’ disease, amniotic fluid aspiration syndrome, hypotonia, hydronephrosis, pneumonia, ventricular septal defect, ileal atresia, etc.
BMI, body mass index; Cr, creatinine; CysC, cystatin C.
Figure 1.
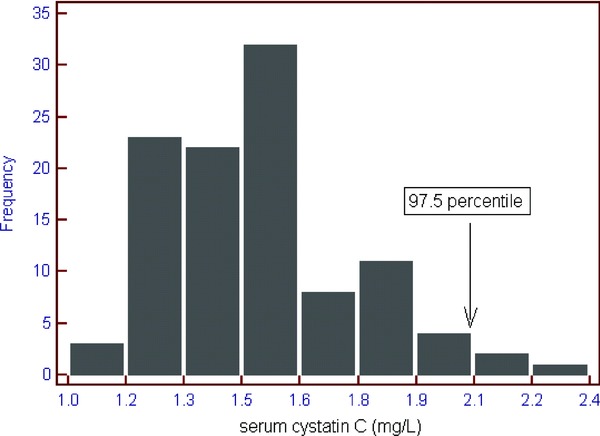
The distribution plot of the serum Cystatin C (CysC) in the critically ill newborns with normal serum Creatinine (Cr). One hundred three newborns (97.5 percentile, the vertical arrow) showed a CysC level under 2.1 mg/L.
Through the medical chart reviews, we found that the five patients showed ARF and another six patients presented with oliguria among the total 106 newborns (Table 2). On the retrospective follow‐up data of the five ARF patients, the Cr and CysC levels showed accompanying changes with similar patterns. The CysC levels showed wider amplitudes of fluctuation than Cr (Fig. 2).
Table 2.
The initial tests and nearest follow up laboratory findings for the 12 patients with increased serum CysC levels accompanied with a normal serum SCr level
| Serial no. | The present or past diagnosis associated with renal impairment | Main diagnosis | Sex | (mg/dL) | (g) | (mg/dL) | (mg/L) |
|---|---|---|---|---|---|---|---|
| 1a | ARF | PDA, neonatal convulsion, DIC | F | NA | 2,510 | 0.9 | 1.3 |
| 2a | ARF | RDS, apnea | M | <0.5 | 1,158 | 0.3 | 1.4 |
| 3a | ARF | ELBW, RDS PDA, DIC | M | <0.5 | 762 | 0.5 | 1.4 |
| 4a | ARF | RDS, apnea | F | <0.5 | 1,036 | 0.7 | 1.5 |
| 5a | ARF | RDS, IVH | F | NA | 626 | 0.7 | 1.8c |
| 6b | oliguria | Birth aspixia, DIC | F | <0.5 | 1,614 | 0.5 | 1.2 |
| 7b | oliguria | VLBW, RDS, PDA, ASD, | M | <0.5 | 13 | 0.8 | 1.3 |
| 8b | oliguria | VLBW, Pn | M | 2.44 | 1,234 | 1.1 | 1.4 |
| 9b | oliguria | ELBW, multiple organ failure | F | NA | 782 | 1.1 | 1.4 |
| 10b | oliguria | VLBWI, RDS | M | <0.5 | 106 | 0.6 | 1.5 |
| 11b | oliguria | NEC, Apnea, DIC | F | <0.5 | 1,518 | 0.5 | 1.5 |
The serial follow‐up data of the serum SCr and CysC levels of these five patients with ARF is presented in Figure 2.
Lasix treatment.
Among newborns with having the history of ARF, only one patient shows increased SCysC above 1.7 mg/l [14].
CRP, C‐reactive protein; SCr, serum SCr; SCysC, serum cystatin C; ARF, acute renal failure; PDA, patent ductus arteriosus; DIC, intravascular coagulation; RDS, respiratory distress syndrome; ELBW, extremely low birth weight; IVH, intraventricular hemorrhage; VLBW, very low birth weight; ASD, atrial septal defect; Pn, pneumonia; NEC, necrotizing enterocolitis; UTI, urinary tract infection; AGE, acute gastroenteritis; M, male: F, female; NA, not available.
Figure 2.
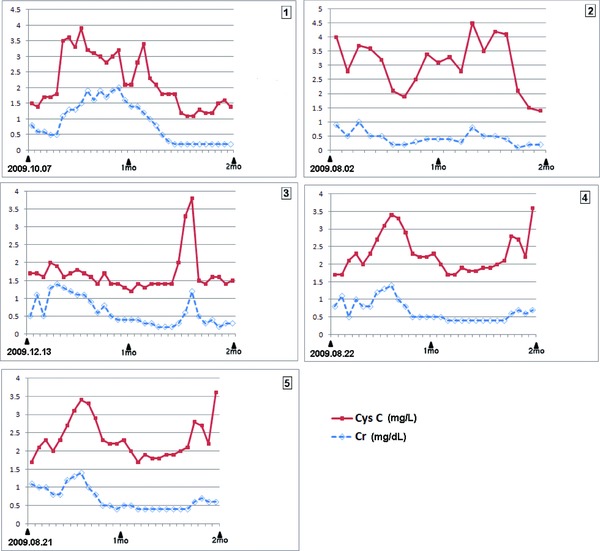
Serial follow‐up data of the serum creatinine (Cr) and cystatin C (CysC) in the five newborns having the medical history of acute renal failure in Table 2. During the 2 months of follow‐up, these two markers moved with the similar pattern. However, CysC showed the wider amplitude of changes. Patients number 3 and 5 showed increased CRPlevels (patient 3; 1.0–1.5 mg/dL, patient 5; 0.7–5.0 mg/dL), while patients number 1, 2, and 4 had CRPvalue within the reference range.
DISCUSSION
Despite being the most commonly used marker of kidney function, serum Cr is insensitive to small changes in renal function and it is also proportional to muscle mass and body weight, which increase with growth. Besides the bilirubin interface in the Jaffe method, Cr has significant shortcomings to assess the neonatal renal function as follows. First, Cr in the first few days of life reflects the mother's renal function and not the infant's renal function 12. Second, the distribution of the normal serum Cr values is dependent on the level of prematurity and age 13. Finally, once an infant receives dialysis, Cr can no longer be used to assess kidney function since Cr is easily dialyzed 12.
Many other renal markers such as neutrophil gelatinase‐associated lipocalin (NGAL) and CysC have been developed, and they are undergoing clinical verification in pediatric patients. The CysC concentrations may more closely reflect the GFR in premature infants as they are unaffected by these physiological variables 10. We previously investigated CysC in pediatric group 7, 14. In this study, we planned to evaluate the clinical meaning of CysC in newborns with normal Cr levels.
In our study, 103 newborns (97.5 percentile) showed CysC levels under the 2.1 mg/L, and CysC levels without increased Cr showed only mild increases. There were 11 patients (10.4%) who had clinical manifestations associated with their impaired renal function. However, Novo et al. suggested the reference range of CysC for the neonatal period. In their study, one patient showed the CysC level above the upper reference limit, >1.7 mg/L 15. Figure 2 shows the serial follow‐up data of serum Cr and CysC in the five newborns with ARF (see Table 2. In these graphs, although two markers move with the similar pattern, CysC shows wider amplitude of the fluctuation. Therefore, clinical progress such as the improvement or aggravation of disease could be more easily recognized through CysC than Cr. This characteristic of CysC, that it is more sensitive to small changes, could be an advantage for a pediatric population, and especially to newborns in whom the monitoring of minute changes should not be overlooked.
The Cr levels fall to a nadir at the age of 4 months and then they gradually rise to adult levels by about 15–17 years of age as the muscle mass develops 16, 17, 18. Although higher values of Cr were reported in newborns during the first months of life, the age cut off remains under discussion 8. In CysC, the currently used normal reference intervals are the same for adults and children, although it has been reported that higher values are found in newborns regardless of gender, weight, or the child's height 19. Kaneko suggested that the decrease in the CysC levels reflects renal maturation because the GFR develops rapidly to maturity in the first 2 to 3 years of life, and children younger than 3 years are characterized by a “physiologically impaired GFR” 16, 20. Another previous study of CysC in healthy controls showed that reference serum CysC levels gradually decreased, from about 1.5 to 0.8 mg/L, during the year after birth, and then remained about 0.7 mg/L 21. Therefore, caution is required for very young children and premature infants in whom increased CysC may reflect a low GFR as part of the renal maturation process 22, 23. Our study had some limitations. Because almost newborns had comorbidities shown in Table 1 and the majority was under 1 week in chronologic age after birth, comparative studies among subgroups according to the comorbiditiy or chronological age were not performed in this study. Therefore, further study must be followed in these subjects. We also hope that studies could be performed in the near future to determine the relationship between CysC and the levels of other parameters such as birth weight, gestational age, CysC/Cr ratio, and CRP in newborns 23.
ACKNOWLEDGMENTS
We would like to thank Dr. Tae Sung Park, an assistant professor, Dr. Min Jin Kim, and Dr. Gayoung Lim, residents in the Department of Laboratory Medicine (School of Medicine, Kyung Hee University), for their invaluable support for this study.
REFERENCES
- 1. Harmoinen A, Ylinen E, Ala‐Houhala M, Janas M, Kaila M, Kouri T. Reference intervals for CysC in pre‐ and full‐term infants and children. Pediatr Nephrol 2000;15:105–108. [DOI] [PubMed] [Google Scholar]
- 2. Treiber M, Pecovnik‐Balon B, Gorenjak M. CysC versus SCr as a marker of glomerular filtration rate in the newborn. Wien Klin Wochenschr 2006;118:66–70. [DOI] [PubMed] [Google Scholar]
- 3. Franco MC, Nishida SK, Sesso R. GFRestimated from CysC versus SCr in children born small for gestational age. Am J Kidney Dis 2008;51:925–932. [DOI] [PubMed] [Google Scholar]
- 4. Westhuyzen J. CysC: A promising marker and predictor of impaired renal function. Ann Clin Lab Sci 2006;36:387–394. [PubMed] [Google Scholar]
- 5. Filler G, Witt I, Priem F, Ehrich JH, Jung K. Are CysC and beta 2‐microglobulin better markers than serum SCr for prediction of a normal glomerular filtration rate in pediatric subjects? Clin Chem 1997;43:1077–1078. [PubMed] [Google Scholar]
- 6. Randers E, Krue S, Erlandsen EJ, Danielsen H, Hansen LG. Reference interval for serum cystatin C in children. Clin Chem 1999;45:1856–1858. [PubMed] [Google Scholar]
- 7. Cho SY, Lee HJ, Suh JT, et al. The significance of serum cystatin C accompanied with a normal serum creatinine level in pediatric patients with chronic kidney disease. Labmed 2011;2:5–8. [Google Scholar]
- 8. Fischbach M, Graff V, Terzic J, Bergère V, Oudet M, Hamel G. Impact of age on reference values for serum concentration of CysC in children. Pediatr Nephrol 2002;17:104–106. [DOI] [PubMed] [Google Scholar]
- 9. Bökenkamp A, Dieterich C, Dressler F, Gembruch U, Bald R, Kirschstein M. Fetal serum concentrations of CysC and a2‐microglobulin as predictors of postnatal kidney function. Am J Obstet Gynecol 2001;185:468–475. [DOI] [PubMed] [Google Scholar]
- 10. Donadio C, Lucchesi A, Ardini M, Giordani R. CysC, a2‐microglobulin and retinol‐binding protein as indicators of glomerular filtration rate: Comparison with plasma SCr. J Pharm Biomed Anal 2001;24:835–842. [DOI] [PubMed] [Google Scholar]
- 11. Bagshaw SM, George C, Bellomo R; ANZICS Database Management Committee . A comparison of the RIFLEand AKINcriteria for acute kidney injury in critically ill patients. Nephrol Dial Transplant 2008;23:1569–1574. [DOI] [PubMed] [Google Scholar]
- 12. Bökenkamp A, Domanetzki M, Zink R, Schumann G, Byrd D, Brodehl J. CysC – A new marker of glomerular filtration rate in children independent of age and height. Pediatrics 1998;101:875–881. [DOI] [PubMed] [Google Scholar]
- 13. Ylinen EA, Houhala MA, Harmoinen APT, Knip M. CysC as a marker for glomerular filtration rate in pediatric patients. Pediatr Nephrol 1999;13:506–509. [DOI] [PubMed] [Google Scholar]
- 14. Cho SY, Lee HJ, Suh JT, Cho BS, Suh JS. Cystatin C/creatinine ratio in pediatric kidney disease. Clin Exp Nephrol 2011;15:976–977. [DOI] [PubMed] [Google Scholar]
- 15. Novo AC, Sadeck Ldos S, Okay TS, Leone CR. Longitudinal study of Cystatin C in healthy term newborns. Clinics (Sao Paulo). 2011;66:217–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Armangil D, Yurdakök M, Canpolat FE, Korkmaz A, Yiğit S, Tekinalp G. Determination of reference values for plasma CysC and comparison with SCr in premature infants. Pediatr Nephrol 2008;23:2081–2083. [DOI] [PubMed] [Google Scholar]
- 17. Askenazi DJ, Ambalavanan N, Goldstein SL. Acute kidney injury in critically ill newborns: What do we know? What do we need to learn? Pediatr Nephrol 2009;24:265–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gallini F, Maggio L, Romagnoli C, Marrocco G, Tortorolo G. Progression of renal function in preterm newborns with gestational age < or = 32 weeks. Pediatr Nephrol 2000;15:119–124. [DOI] [PubMed] [Google Scholar]
- 19. Séronie‐Vivien S, Delanaye P, Piéroni L, Mariat C, Froissart M, Cristol JP; SFBC“Biology of renal function and renal failure” working group . CysC: Current position and future prospects. Clin Chem Lab Med 2008;46:1664–1686. [DOI] [PubMed] [Google Scholar]
- 20. Kaneko K. Serum CysC as a possible marker to detect renal maturation. Pediatr Nephrol 2010;25:561–562. [DOI] [PubMed] [Google Scholar]
- 21. Andersen TB, Erlandsen EJ, Frøkiaer J, Eskild‐Jensen A, Brøchner‐Mortensen J. Comparison of within‐ and between‐subject variation of serum cystatin Cand serum creatinine in children aged 2–13 years. Scand J Clin Lab Invest 2010;70:54–59. [DOI] [PubMed] [Google Scholar]
- 22. Finney H, Newman DJ, Thakkar H, Fell JM, Price CP. Reference ranges for plasma CysC and SCr measurements in premature infants, newborns, and older children. Arch Dis Child 2000;82:71–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bahar A, Yilmaz Y, Unver S, Gocmen I, Karademir F. Reference values of umbilical cord and third‐day CysC levels for determining glomerular filtration rates in newborns. J Int Med Res 2003;31:231–235. [DOI] [PubMed] [Google Scholar]


