Abstract
Background
Cytokeratin 19 fragment antigen (CYFRA 21–1) is used to diagnose and monitor neoplasms. However, the main disadvantages of the currently available CYFRA 21–1 assays include heterogenous technology, being time‐consuming, and having low through‐put with low insensitivity. This study investigated the use of amplified luminescent proximity homogeneous immunoassay (AlphaLISA) for the quantization of CYFRA 21–1 in human serum.
Methods
The AlphaLISA kit was developed based on AlphaScreen detection technology with two different anti‐CYFRA 21–1 monoclonal antibodies. One was coated on AlphaLISA acceptor beads and the other was biotinylated. Donor beads were coated with streptavidin. The test conditions were optimized and analytical performance was studied.
Results
The measurement range of AlphaLISA CYFRA 21–1 kit was 0.08–500 ng/ml. Assay detection limit was 0.08 ng/ml. The intra‐ and interassay coefficients of variation were 3.00–9.00% and 4.00–10.00%, respectively. There was no cross‐reaction to alpha‐fetoprotein (AFP), carcinoembryonic antigen (CEA), neuron‐specific enolase (NSE), cancer antigen 19–9 (CA19–9), cytokeratins 8 (CK8), and cytokeratins 18 (CK18). The correlation coefficient of blood samples involved was 0.974 between CYFRA 21–1‐AlphaLISA assay and a commercial electrochemiluminescence immunoassay (ECLIA) CYFRA 21–1 kit (Roche).
Conclusions
The AlphaLISA CYFRA 21–1 kit developed in this study had favorable performance characteristics for clinical application with acceptable analytical sensitivity, specificity, and accuracy.
Keywords: cytokeratin fragments, CYFRA 21–1, AlphaLISA, homogeneous immunoassays, singlet oxygen
Abbreviations
- A beads
unconjugated Eu‐acceptor beads
- AFP
alpha‐fetoprotein
- CA19–9
cancer antigen 19–9
- CEA
carcinoembryonic antigen
- CK18
cytokeratins 18
- CK8
cytokeratins 8
- CMO
carboxy‐methoxy lamine
- CV
coefficients of variation
- CYFRA 21–1
cytokeratin 19 fragment antigen
- DTPA
diethylene triamine pentacetic acid
- ECLIA
electrochemiluminescence immunoassay
- ELISA
enzyme‐linked immunosorbent assay
- LOCI
luminescent oxygen channeling immunoassay
- NaBH3CN
sodium cyanoborohydride
- NSCLC
nonsmall cell lung cancer
- NSE
neuron‐specific enolase
- RIA
radioimmunoassay
- SA‐D beads
streptavidin‐coated donor beads
- SD
standard deviation
INTRODUCTION
Cytokeratin 19 fragment antigen (CYFRA 21–1) is an epitope of a polypeptide that is released following cell death 1, 2, 3. Cytokeratin 19 has an isoelectric pH of 5.2 and a molecular weight of 40 kDa and is present in intermediate filaments of the cytoskeletal structure of normal epithelium and in malignant epithelium 4, 5. In malignant epidermal tumors, activated proteinases accelerate cell degeneration and cause the release of soluble CYFRA 21–1 to tissues and body fluids. CYFRA 21–1 has been reported to be the most sensitive tumor marker for nonsmall cell lung cancer (NSCLC) and appears to correlate with the development of disease 6, 7, 8. The levels of CYFRA 21–1 increase specifically in squamous cell carcinoma and lung adenocarcinoma 9, 10, 11. Approximately 70–85% of various types of NSCLC are CYFRA 21–1 positive. Thus, measurement of CYFRA 21–1 is useful for lung cancer differential diagnosis and therapeutic monitoring 12, 13. Furthermore, CYFRA 21–1 is also a clinical tumor marker of gastrointestinal tumors 14, 15, liver cancer 16, 17, esophageal cancer 4, 18, and gynecologic oncology 19, 20. Therefore, CYFRA 21–1 is beneficial for the diagnosis and monitoring of some neoplasms.
A number of methods have been developed to measure CYFRA 21–1 concentrations in human serum, including radioimmunoassay (RIA) 21, 22, enzyme‐linked immunosorbent assay (ELISA; 23, 24), and electrochemiluminescence immunoassay (ECLIA) 25, 26. These methods have a number of disadvantages including radiation hazards, short half‐life of iodinated labels for RIA 27, low sensitivity and instability for ELISA, and expense and difficulty to set up an open system for ECLIA. Because of these intrinsic limitations, alternative innovative assays that are robust, cost‐effective, and easy to automate with high‐throughput are urgently required for clinical applications. The amplified luminescent proximity homogeneous immunoassay (AlphaLISA) developed from luminescent oxygen channeling immunoassay (LOCI) technology has all of the desired features. AlphaLISA is a homogenous technology, where the proximity of donor beads and acceptor beads causes emission of light through chemiluminescence 28, 29. This technology has been commercialized by PerkinElmer (Waltham, MA).
The aim of this study was to establish and validate the characteristics of a double‐antibody‐sandwiched immunoassay (CYFRA 21–1‐AlphaLISA, Fig. 1). The results showed that CYFRA 21–1‐AlphaLISA has favorable performance characteristics for clinical applications including no radioactive waste, relatively simple operation, higher sensitivity and specificity than other methods, and a wide linear range. Hence, CYFRA 21–1‐AlphaLISA demonstrates excellent performance characteristics for the quantitative measurement of CYFRA 21–1 in human serum.
Figure 1.
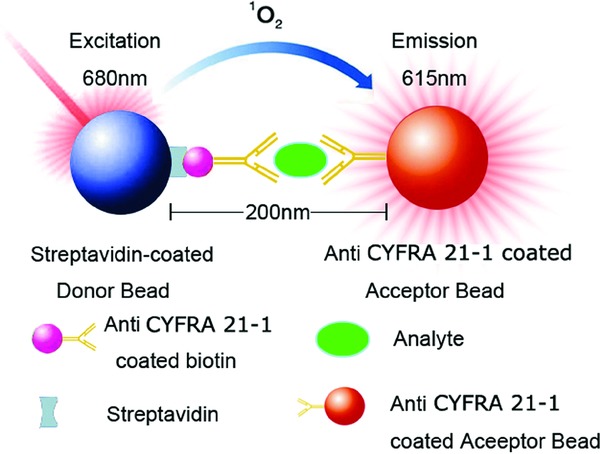
Principle of AlphaLISA for the detection of CYFRA21–1.
MATERIALS AND METHODS
Material and Chemicals
Anti‐CYFRA 21–1 monoclonal antibodies were obtained from Exbio Praha (Vestec, Czech Republic) and Progen (Heidelberg, Germany). CYFRA 21–1 antigen was purchased from BioDesign (Memphis, TN). Unconjugated Eu‐acceptor beads (A beads) and streptavidin‐coated donor beads (SA‐D beads) were purchased from PerkinElmer. The quality control serum was purchased from Bio‐Rad (Hercules, CA). Biotin‐NHS was from Sigma‐Aldrich (St. Louis, MO). EnVision Multilabel Reader and 96‐well Optiplates were from PerkinElmer.
Samples
The protocol was approved by the Southern Medical University Institutional Review Board. Clinical serum samples (n = 175) were collected from NanFang Hospital, Guangzhou, China, and analyzed by the CYFRA 21–1‐ECLIA (Roche Diagnostica, Switzerland) with a cutoff limit of 4.0 ng/ml. The clinical serum samples included 58 patients with lung cancer (19 lung squamous cell carcinoma, 25 lung adenocarcinoma, 6 bronchioloalveolar carcinoma, and 8 small cell lung cancer), 40 patients with other benign lung diseases (23 pulmonary infection, 7 pulmonary fibrosis, 6 emphysema, and 4 bronchitis), 49 patients with other cancers (13 liver cancer, 9 colon cancer, 5 breast cancer, 12 gastric cancer, and 10 pancreatic cancer), and 28 patients with other benign diseases (9 diabetes, 5 intestinal obstruction, 5 liver cirrhosis, 4 pleurisy, and 5 angina pectoris). The mean age was 57.5 years (SD, 14.0; range, 31–92 years); 58.6% were male and 41.4% were female. Venous blood samples were collected in sterile vacationers without anticoagulant. Samples were stored at −20°C for 2 months before analysis. The Ethical Committee of Science and Technology Department of Southern Medical University approved the collection of these samples.
Biotinylated Antibody
One milligram of anti‐CYFRA 21–1 antibody was dissolved in 0.05 ml of 0.1 mol/l carbonate buffer (pH 9.5) containing 0.1% NaN3 and NHS‐D‐Biotin (Catalog Number: H1759) in DMSO immediately prior to use in the dark at 22 mg/ml. A volume equal to 10% of the total volume of the antibody solution was added into the NHS‐D‐Biotin solution with gentle stirring and incubated at room temperature for 4 hours. The reaction solution was dialyzed against several changes of PBS buffer (0.01 M sodium phosphate, 0.15 M sodium chloride, pH 7.4) at 2–8°C. The dialyzed biotinylated antibody was stored (0.5 mg/ml) at −20°C until use.
Coupling of Antibody to Beads
Anti‐CYFRA 21–1 antibody, 0.2 mg was dissolved in 0.1 ml sodium phosphate buffer (0.13 mol/l, pH 8.0). The solution was added to 1 mg acceptor beads with 10 μl of 25 mg/ml NaBH3CN and 1.25 μl of 10% Tween‐20 and then incubated at 37°C for 48 hours. The total volume of the reaction solution was 200 μl. To block nonconjugated sites, a fresh carboxy‐methoxy lamine (CMO) solution (65 mg/ml) was prepared in 0.8 M NaOH. Ten microliters of CMO solution was added to the reaction and incubated for an hour at 37°C. The reaction solution was centrifuged (14,000 rpm, 25 min) and the supernatant was removed. Then the bead pellet was resuspended in 200 μl of 0.1 M Tris‐HCl, pH 8.0, centrifuged, and washed. After the last centrifugation, the beads were resuspended in storage buffer (200 μl of PBS + 0.05% Proclin‐300 as a preservative) at a concentration of 5 mg/ml.
CYFRA 21–1 Calibrations
For calibration purposes, a calibrator series was developed by diluting CYFRA 21–1 antigen in a buffer containing 50 mM Tris‐HCl, 0.9% NaCl, 0.05% sodium azide, 1.5% bovine serum albumin, and 0.01% Tween‐20, pH 7.8. This step generated the desired standard concentrations of 0, 2, 5, 20, 100, and 500 ng/ml, designated as A, B C, D, E, and F, correspondingly. The standards were stored at 4°C.
AlphaLISA Assay Protocol
The assay buffer contained 25 mM HEPES, 50 mM NaCl, 5 mM DTPA (tripotassium salt), 2 mg/ml Dextran T500 (Sigma‐Aldrich, St. Louis, MO), 0.5% bovine serum albumin (PAA Laboratories, Bethridge Road Etobicoke, Ontario), 0.05 mg/ml bovine gamma globulin (BioDesign, Wilfong Rd., Memphis, TN, 38134 USA), 0.01 mg/ml mouse immunoglobulin (BioDesign), 0.1% Tween‐20 (w/v) (Sigma‐Aldrich), 0.01% Proclin 300 (Sigma‐Aldrich), and 0.01% gentamycin sulfate and was adjusted to pH 7.4.
Two‐step assay procedures were used. Briefly, the test samples and standards (25 μl) were added to the 96‐well plates. A 50 μl mix of antibody‐A beads (1:400) and biotinylated antibody (1:400) in the assay buffer was added to the wells. The plates were covered with a lid and incubated at 37°C for 15 min. Subsequently, 175 μl of SA‐D beads (0.012 mg/ml) in assay buffer were added. The plates were covered with a lid and incubated at 37°C for 15 min in the dark. The AlphaLISA signal was measured on a 2300 EnVision Multilabel Reader.
Interference
Interference testing was performed as previously described 30. Briefly, interference was assessed by the addition of interfering substances to serum samples at the stated final concentrations as follows: hemoglobin from washed hemolyzed erythrocytes, bilirubin (unconjugated) prepared in sodium carbonate/dimethyl sulfoxide, and triglyceride in the form of intralipid 20% fat emulsion, and sodium ascorbate.
Comparison Method
CYFRA 21–1‐ECLIA was purchased from Roche Diagnostica Inc. (Switzerland). Twenty microliters of sample, a biotinylated monoclonal CYFRA 21–1‐specific antibody, and a monoclonal CYFRA 21–1‐specific antibody labeled with a ruthenium complex were reacted and incubated to form a sandwich complex. After the addition of streptavidin‐coated microparticles, the complex became bound to the solid phase via the interaction of biotin and streptavidin. The reaction mixture was aspirated into a measuring cell where the microparticles were magnetically captured onto the surface of an electrode. Unbound substances were then removed with ProCell. Application of voltage to the electrode induced chemiluminescent emission, which was measured by a photomultiplier. Results were determined via a calibration curve that was generated by two‐point calibration and a master curve was provided via the reagent bar code.
Statistical analysis
The data were expressed as mean values from duplicate measurements. The data were analyzed with Origin software (version 7.5, OriginLab, MA), and other statistical analyses were performed using SPSS software (version 13.0, SPSS Inc., Chicago, IL). A P‐value of <0.05 was considered statistically significant.
RESULTS
Calibration Curve and Detection Limit
Figure 2 shows a representative calibration curve. A CYFRA 21–1 A linearized standard curve (Y = 1.095X + 2.7, R 2 = 0.999) was obtained on a log‐to‐log plot with six concentrations including the zero calibrator. The standard curve possessed an intensity range between 1,000 and 430,000 counts per second (cps) from 0 to 500 ng/ml. The assay detection limit was 0.08 ng/ml based on 10 samples and calculated from the mean of the zero calibrator + 2 SD.
Figure 2.
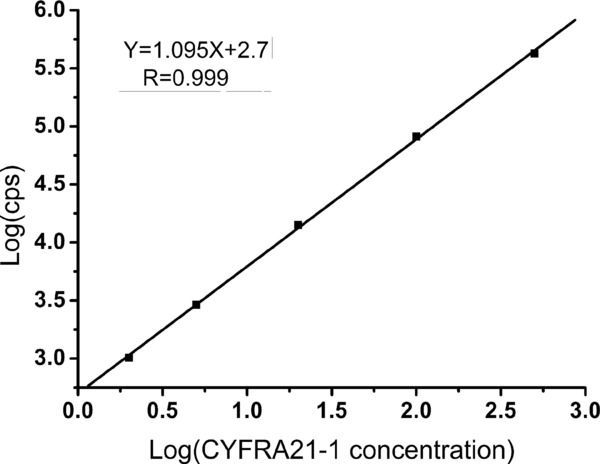
Representative standard curve of CYFRA21–1 AlphaLISA.
Analytical Recovery
Two serum samples containing 56.2 ng/ml and 358.4 ng/ml CYFRA 21–1 were diluted with the CYFRA 21–1‐depleted serum pool, respectively. Evaluations were performed by calculating the ratio between the calculated and the expected values. The results from the serum samples are shown in Table 1. The recovery rate was 90.98% and 108.82% relative to the expected values exhibiting a complete parallelism. The data indicated that the calibrator matrix performed similarly to serum samples and there was a linear relationship between the assay response and the sample volume.
Table 1.
Linearity of Dilution of Human EDTA Plasma Samples With Calibrator Matrixa
| Measured | Expected | |||
|---|---|---|---|---|
| CYFRA21–1 | CYFRA21–1 | |||
| Sample | Dilution | (ng/ml) | (ng/ml) | Recovery (%) |
| 16 | Neat | 23.38 | 23.38 | 100.00 |
| 1:2 | 12.10 | 24.20 | 103.51 | |
| 1:4 | 6.00 | 24.00 | 102.65 | |
| 1:8 | 3.13 | 25.04 | 107.10 | |
| 17 | Neat | 56.24 | 56.24 | 100.00 |
| 1:2 | 28.93 | 57.86 | 102.89 | |
| 1:4 | 14.87 | 59.48 | 105.76 | |
| 1:8 | 7.65 | 61.20 | 108.82 | |
| 36 | Neat | 358.48 | 358.48 | 100.00 |
| 1:2 | 175.59 | 351.18 | 97.96 | |
| 1:4 | 85.14 | 340.56 | 95.00 | |
| 1:8 | 40.77 | 326.16 | 90.98 | |
| 113 | Neat | 77.51 | 77.51 | 100.00 |
| 1:2 | 37.8 | 75.60 | 97.53 | |
| 1:4 | 19.7 | 78.80 | 101.66 | |
| 1:8 | 9.96 | 79.68 | 102.80 |
Sample 16 was from a patient of pleural endotheliomas, male, 33 years old. Samples 17 was from a patient of widely metastatic cancer, male, 65 years old. Sample 36 was from a patient of poor differentiation squamous cell lung cancer, male, 83 years old. Sample 113 was from a patient of hydrothorax, female, 40 years old.
Spiking recovery was further assessed by adding final exogenous CYFRA 21–1 concentrations of 2, 20, and 100 ng/ml to three serum samples from different patients. The concentrations of CYFRA 21–1 in these three patients were 4.37, 87.84, and 326.42 ng/ml, respectively. Ten microliters of each of the exogenous CYFRA 21–1 protein was spiked into 190 μl of serum samples at a ratio of 1:19, leaving the serum matrix of the spiked sample relatively intact. To calculate the expected values, 95% of the unspiked value was added to 5% of the spiking solution concentration.
Evaluations were performed by calculating the ratio between measured and expected values (Table 2). The percentage recovery of CYFRA 21–1 was between 94.35% and 105.42%, suggesting that recovery of the different serum samples was quantitative and that CYFRA 21–1 was accurately measured in the real samples.
Table 2.
Analytical Recovery of CYFRA21–1 Added to Plasma Samples
| Measured | Expected | ||
|---|---|---|---|
| Added | CYFRA21–1 | CYFRA21–1 | Recovery |
| (ng/ml) | (ng/ml) | (ng/ml) | (%) |
| 2 | 4.01 | 4.25 | 94.35 |
| 80.23 | 83.55 | 96.03 | |
| 307.48 | 310.20 | 99.12 | |
| 20 | 4.89 | 5.15 | 94.95 |
| 79.32 | 83.45 | 95.05 | |
| 314.47 | 311.10 | 101.08 | |
| 100 | 8.78 | 9.15 | 95.96 |
| 91.37 | 88.45 | 103.30 | |
| 332.17 | 315.10 | 105.42 |
Precision
Precision was assessed by performing a number of duplicate tests of the control serum with a range of 0.08–500 ng/ml (Table 3). For the intraassay (within‐assay, within‐plate) study, eight duplicates of each sample were placed randomly on the same plate and analyzed in the same test. The intraassay variation was calculated from the variation of the eight determinations of CYFRA 21–1 concentrations. The intraassay CV% was low, from 3.00% to 9.00%. For the interassay study (between plates, between runs), we assessed the variation by analyzing samples in the same manner in five independent tests, one duplicate per day, and each sample in a different position on the plate in each test. The interassay CV% was slightly higher, from 4.00% to 10.00%.
Table 3.
Intraassay and Interassay of Precisiona
| Intraassay | Interassay | |||
|---|---|---|---|---|
| Lot. number | Concentration | CV | Concentration | CV |
| of sample | (ng/ml) | (%) | (ng/ml) | (%) |
| 54408 | 0.08 ± 0.007 | 9.00 | 0.08 ± 0.008 | 10.00 |
| 54501 | 6.16 ± 0.19 | 3.08 | 6.16 ± 0.35 | 5.68 |
| 54503 | 28.2 ± 1.1 | 3.90 | 28.2 ± 1.5 | 5.32 |
| 54406 | 100 ± 3 | 3.00 | 100 ± 4 | 4.00 |
| 54509 | 300 ± 11 | 3.67 | 300 ± 15 | 5.00 |
| 54208 | 500 ± 17 | 3.40 | 500 ± 23 | 4.60 |
The samples were Lyphochek Tumor Marker Plus Control from Bio‐Rad Co., which were levels 1, 2, and 3, respectively.
Interference
The effect of hemolysis, lipemia, and bilirubinemia was assessed by adding hemolysate, bilirubin (unconjugated), triglyceride, and sodium ascorbate to the serum samples of four patient volunteers. The recoveries of CYFRA 21–1 in these samples were calculated from CYFRA 21–1 concentrations determined before and after addition of interfering substances. The recoveries were between 89.42% and 105.16% (Table 4).
Table 4.
Interference From Addition of Hemolysate, Bilirubin, Triglyceride, and Ascorbic Acid to Patients Serum Samples
| Interfering substance | CYFRA21–1 (ng/ml) | Recovery (%) |
|---|---|---|
| Hemoglobin(ng/ml) | ||
| 0 | 67.31 | 100.00 |
| 200 | 63.48 | 94.31 |
| Bilirubin(ng/ml) | ||
| 0 | 104.64 | 100.00 |
| 50 | 97.13 | 92.82 |
| Triglyceride (ng/ml) | ||
| 0 | 158.06 | 100.00 |
| 1000 | 166.22 | 105.16 |
| Ascorbic acid (ng/ml) | ||
| 0 | 182.57 | 100.00 |
| 200 | 163.25 | 89.42 |
Specificity
To investigate the specificity of the developed CYFRA 21–1 assay, the effect of adding other commonly measured proteins alpha‐fetoprotein (AFP), carcinoembryonic antigen (CEA), neuron‐specific enolase (NSE), cancer antigen 19–9 (CA19–9), cytokeratins 8 (CK8), and cytokeratins 18 (CK18) to normal human serum samples was assessed. The cross‐reactivity was expressed as the percent ratio between measured and expected values. Results in Table 5 show that no major cross‐reactivity was identified among the analogous compounds.
Table 5.
Specificity of CYFRA21–1 AlphaLISA®
| CYFRA21–1 | |||
|---|---|---|---|
| Cross‐reactivity | |||
| Antigens | Concentration | Value (ng/ml) | Cross‐reactivity rate (%) |
| AFP | 1000 U/ml | 0.10 | 0.01 |
| CEA | 600 ng/ml | 0.12 | 0.02 |
| NSE | 1000 ng/ml | 0.05 | 0.005 |
| CA 19–9 | 400 U/ml | 0.06 | 0.015 |
| CK 8 | 500 ng/ml | 0.20 | 0.04 |
| CK 18 | 500 ng/ml | 0.21 | 0.04 |
Comparison of CYFRA 21–1 and ECLIA
To further validate the assay, 175 samples were tested concurrently with the new CYFRA 21–1 assay (x‐axis) and Roche ECLIA assay (y‐axis). Regression analysis revealed a positive relationship between CYFRA 21–1 determined in the two assays (Fig. 3). A significant association (R 2 = 0.948, P < 0.001) between the two assays was observed. This comparison confirmed the accuracy of the AlphaLISA system.
Figure 3.
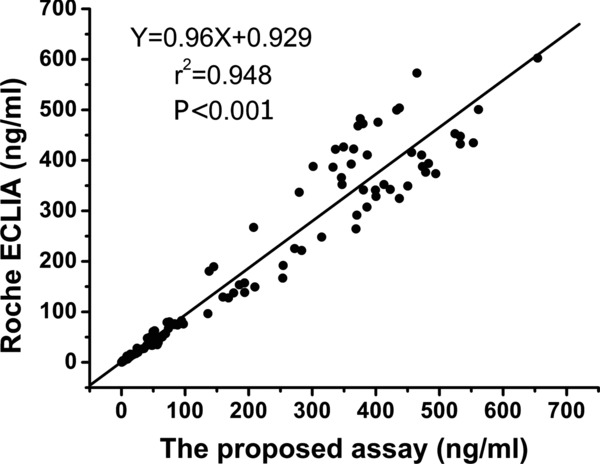
Correlation between CYFRA21–1 levels in serum samples measured by CYFRA21–1‐AlphaLISA and ECLIA.
DISCUSSION
In this study, we established a 96‐well plate‐based homogeneous chemiluminescent sandwich immunoassay for the quantization of CYFRA 21–1 in human serum. As shown in Figure 1, one anti‐CYFRA 21–1 monoclonal antibody was coated on AlphaLISA acceptor beads and another anti‐CYFRA 21–1 antibody was biotinylated. The kit also contains donor beads coated with streptavidin. The resulting complex was formed between acceptor beads and monoclonal antibody–analyte–antibody–biotin–streptavidin–donor beads, and was quantified in the well by excitation of the donor beads with laser irradiation at 680 nm. The specific delayed sharp chemiluminescent emission peak was monitored at about 615 nm 31.
AlphaLISA is simple and quick to develop, and is a small and automatable method 31, 32, 33, 34. The total assay time is short because of fast mix‐and‐measure protocols 31. The assay can be handled manually or as an automatic setup, which facilitates assay development and makes the technology more versatile.
The CYFRA 21–1‐AlphaLISA assay is very sensitive with a detection limit of 0.08 ng/ml, which allows the measurement of very small concentrations of CYFRA 21–1. The higher sensitivity was obtained because of the amplified signal resulting from the 60,000 singlet oxygen molecules generated by each donor bead 31. At these low signal levels, an assay may generate false results because of interference. However, the assay was found to be robust to serum interference and was not affected by autofluorescence. Thus, the accuracy, even at very low CYFRA 21–1 concentrations, should be acceptable and robust.
Homogenous assay systems are potentially more sensitive to serum interference. The most prominent types of general interference are inner filter effects and singlet oxygen quenchers. By using Eu complex in the acceptor beads emitting light at 615 nm 31, the inner filter effects are minimal when testing serum. Ascorbic acid and heme iron are the main potential singlet oxygen quenchers in serum. However, our results indicated that the assay was unaffected by interfering substances including hemolysate, bilirubin, triglyceride, and ascorbic acid at concentrations that might be expected in a routine clinical laboratory setting. Notably, the system is free of problems with sample autofluorescence by using the time‐resolved mode and because of the difference between the emission and excitation wavelengths. In addition, cross‐reactivity data with major interfering tumor markers showed the assay was highly specific for CYFRA 21–1.
The validity of AlphaLISA for measurement of serum CYFRA 21–1 was confirmed by the good correlation of results obtained by AlphaLISA and ECLIA. Comparison of the CYFRA 21–1‐AlphaLISA assay with a homogeneous assay such as ECLIA demonstrated the performance standards and analysis capability were similar. Comparison of CYFRA 21–1‐AlphaLISA assay with a heterogeneous assay such as ELISA showed that AlphaLISA was a quick and easy mix‐and‐read assay, as it did not require multiple washing steps and could be performed on an automated platform. Overall, AlphaLISA may be superior to other assays when considering a range of important performance characteristics such as sensitivity, analytical range, sample volume, assay time, and ease of handling.
In conclusion, the CYFRA 21–1‐AlphaLISA assay presented here provides a rapid and sensitive method for the measurement of serum CYFRA 21–1. The CYFRA 21–1‐AlphaLISA assay demonstrated excellent performance characteristics for the quantitative measurement of CYFRA 21–1 in serum, indicating that this tumor marker can be precisely and accurately measured using this method. Therefore, compared with other CYFRA 21–1 assay methods, the CYFRA 21–1‐AlphaLISA assay may be considered as a model system to measure drugs and biomarkers in biological samples for biomedical studies and clinical examination.
CONFLICT OF INTEREST
The authors declare that no competing interests exist.
ACKNOWLEDGMENTS
The authors thank the Committee of National Science and Technology, the Ministry of Education of China, the Education Committee of Guangdong Province, the Guangdong Committee of Science and Technology, and the Education Department of Southern Medical University. They thank the staff of the department for their support and suggestions. They also thank Nan Fang Hospital, Guangzhou, China, for providing reagents at a reduced cost.
Grant sponsor: National Science and Technology Major Project of China (2009ZX10607); Grant sponsor: The National Natural Science Foundation of China (30901382 and 81271931); Grant sponsor: The Science Foundation of Guangdong Province (S2012010009547); Grant sponsor: The New Teacher for Doctoral Fund of Ministry of Education of China (20094433120008); Grant sponsor: Special funds for Colleges and Universities Talents by Guangdong Province (2009); Grant sponsor: Special funds for Colleges and Universities Talents by Southern Medical University (2009).
REFERENCES
- 1. Stieber P, Dienemann H, Hasholzner U, et al. Comparison of cytokeratin fragment 19 (CYFRA 21–1), tissue polypeptide antigen (TPA) and tissue polypeptide specific antigen (TPS) as tumour markers in lung cancer. Eur J Clin Chem Clin Biochem 1993;31(10):689–694. [DOI] [PubMed] [Google Scholar]
- 2. Bodenmuller H, Donie F, Kaufmann M, Banauch D. The tumor markers TPA, TPS, TPACYK and CYFRA 21–1 react differently with the keratins 8, 18 and 19. Int J Biol Markers 1994;9(2):70–74. [DOI] [PubMed] [Google Scholar]
- 3. Kramer G, Erdal H, Mertens HJ, et al. Differentiation between cell death modes using measurements of different soluble forms of extracellular cytokeratin 18. Cancer Res 2004;64(5):1751–1756. [DOI] [PubMed] [Google Scholar]
- 4. Kawaguchi H, Ohno S, Miyazaki M, et al. CYFRA 21–1 determination in patients with esophageal squamous cell carcinoma: Clinical utility for detection of recurrences. Cancer 2000;89(7):1413–1417. [DOI] [PubMed] [Google Scholar]
- 5. Chyczewski L, Niklinski J, Chyczewska E, Laudanski J, Furman M. Immunohistochemical analysis of tissue localization of cytokeratin 19 in lung cancer. Rocz Akad Med Bialymst 1997;42 Suppl 1:162–172. [PubMed] [Google Scholar]
- 6. Tomita M, Shimizu T, Ayabe T, Yonei A, Onitsuka T. Prognostic significance of tumour marker index based on preoperative CEA and CYFRA 21–1 in non‐small cell lung cancer. Anticancer Res 2010;30(7):3099–3102. [PubMed] [Google Scholar]
- 7. Edelman MJ, Hodgson L, Rosenblatt PY, et al. CYFRA 21–1 as a prognostic and predictive marker in advanced non‐small‐cell lung cancer in a prospective trial: CALGB 150304. J Thorac Oncol 2012;7(4):649–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pavicevic R, Bubanovic G, Franjevic A, Stancic‐Rokotov D, Samarzija M. CYFRA 21–1 in non‐small cell lung cancer: Standardisation and application during diagnosis. Coll Antropol 2008;32(2):485–498. [PubMed] [Google Scholar]
- 9. Yeh JJ, Liu FY, Hsu WH, Wang JJ, Ho ST, Kao A. Monitoring cytokeratin fragment 19 (CYFRA 21–1) serum levels for early prediction of recurrence of adenocarcinoma and squamous cell carcinoma in the lung after surgical resection. Lung 2002;180(5):273–279. [DOI] [PubMed] [Google Scholar]
- 10. Song WA, Liu X, Tian XD, et al. Utility of squamous cell carcinoma antigen, carcinoembryonic antigen, Cyfra 21–1 and neuron specific enolase in lung cancer diagnosis: A prospective study from China. Chin Med J (Engl) 2011;124(20):3244–3248. [PubMed] [Google Scholar]
- 11. Huang WW, Tsao SM, Lai CL, Su CC, Tseng CE. Diagnostic value of Her‐2/neu, Cyfra 21–1, and carcinoembryonic antigen levels in malignant pleural effusions of lung adenocarcinoma. Pathology 2010;42(3):224–228. [DOI] [PubMed] [Google Scholar]
- 12. Lee JH, Chang JH. Diagnostic utility of serum and pleural fluid carcinoembryonic antigen, neuron‐specific enolase, and cytokeratin 19 fragments in patients with effusions from primary lung cancer. Chest 2005;128(4):2298–2303. [DOI] [PubMed] [Google Scholar]
- 13. Ferrer J, Villarino MA, Encabo G, et al. Diagnostic utility of CYFRA 21–1, carcinoembryonic antigen, CA 125, neuron specific enolase, and squamous cell antigen level determinations in the serum and pleural fluid of patients with pleural effusions. Cancer 1999;86(8):1488–1495. [DOI] [PubMed] [Google Scholar]
- 14. Hara A, Watanabe H. [A case of advanced gastric cancer showing high serum CYFRA21–1 level responding to chemotherapy with S‐1]. Gan To Kagaku Ryoho 2008;35(13):2405–2407. [PubMed] [Google Scholar]
- 15. Miyashita T, Nishimura G, Michiwa Y, et al. [Clinical usefulness of serum CYFRA21–1 in colorectal cancer]. Gan To Kagaku Ryoho 1996;23(12):1693–1696. [PubMed] [Google Scholar]
- 16. Wu F, Fujita J, Murota M, et al. CYFRA 21–1 is released in TNF‐alpha‐induced apoptosis in the hepatocellular carcinoma cell line HuH‐7. Int J Oncol 2002;21(2):441–445. [PubMed] [Google Scholar]
- 17. Uenishi T, Yamazaki O, Yamamoto T, et al. Clinical significance of serum cytokeratin‐19 fragment (CYFRA 21–1) in hepatocellular carcinoma. J Hepatobiliary Pancreat Surg 2006;13(3):239–244. [DOI] [PubMed] [Google Scholar]
- 18. Moll R, Franke WW, Schiller DL, Geiger B, Krepler R. The catalog of human cytokeratins: Patterns of expression in normal epithelia, tumors and cultured cells. Cell 1982;31(1):11–24. [DOI] [PubMed] [Google Scholar]
- 19. Marrakchi R, Ouerhani S, Benammar S, et al. Detection of cytokeratin 19 mRNA and CYFRA 21–1 (cytokeratin 19 fragments) in blood of Tunisian women with breast cancer. Int J Biol Markers 2008;23(4):238–243. [DOI] [PubMed] [Google Scholar]
- 20. Xiong Y, Peng XP, Liang LZ, Zheng M, Li JD. Clinical significance of combined examination of pretreatment serum CYFRA21–1 and SCCAg in cervical cancer patients. Ai Zheng 2009;28(1):64–67. [PubMed] [Google Scholar]
- 21. Pujol JL, Grenier J, Daures JP, Daver A, Pujol H, Michel FB. Serum fragment of cytokeratin subunit 19 measured by CYFRA 21–1 immunoradiometric assay as a marker of lung cancer. Cancer Res 1993;53(1):61–66. [PubMed] [Google Scholar]
- 22. Holdenrieder S, Von Pawel J, Duell T, et al. Clinical relevance of thymidine kinase for the diagnosis, therapy monitoring and prognosis of non‐operable lung cancer. Anticancer Res 2010;30(5):1855–1862. [PubMed] [Google Scholar]
- 23. Takada M, Masuda N, Matsuura E, et al. Measurement of cytokeratin 19 fragments as a marker of lung cancer by CYFRA 21–1 enzyme immunoassay. Br J Cancer 1995;71(1):160–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chapman MH, Sandanayake NS, Andreola F, et al. Circulating CYFRA 21–1 is a specific diagnostic and prognostic biomarker in biliary tract cancer. J Clin Exp Hepatol 2011;1(1):6–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kuropkat C, Werner JA. Analytical and clinical evaluation of CYFRA 21–1 by electrochemiluminescent immunoassay in head and neck squamous cell carcinoma. J Laryngol Otol 2003;117(12):1007–1008; author reply 1008–1009. [DOI] [PubMed] [Google Scholar]
- 26. Alkotyfan K, Wiegand S, Muller HH, Windfuhr JP, Werner JA, Sesterhenn AM. Cyfra 21–1 as a tumor marker for follow‐up of patients with squamous cell carcinoma of the oropharynx. Anticancer Res 2010;30(6):2291–2296. [PubMed] [Google Scholar]
- 27. Shan G, Huang W, Gee SJ, Buchholz BA, Vogel JS, Hammock BD. Isotope‐labeled immunoassays without radiation waste. Proc Natl Acad Sci USA 2000;97(6):2445–2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ullman EF, Kirakossian H, Singh S, et al. Luminescent oxygen channeling immunoassay: Measurement of particle binding kinetics by chemiluminescence. Proc Natl Acad Sci U S A 1994;91(12):5426–5430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ullman EF, Kirakossian H, Switchenko AC, et al. Luminescent oxygen channeling assay (LOCI): Sensitive, broadly applicable homogeneous immunoassay method. Clin Chem 1996;42(9):1518–1526. [PubMed] [Google Scholar]
- 30. Glick MR, Ryder KW, Jackson SA. Graphical comparisons of interferences in clinical chemistry instrumentation. Clin Chem 1986;32(3):470–475. [PubMed] [Google Scholar]
- 31. Bielefeld‐Sevigny M. AlphaLISA immunoassay platform‐ the “no‐wash” high‐throughput alternative to ELISA. Assay Drug Dev Technol 2009;7(1):90–92. [DOI] [PubMed] [Google Scholar]
- 32. Poulsen F, Jensen KB. A luminescent oxygen channeling immunoassay for the determination of insulin in human plasma. J Biomol Screen 2007;12(2):240–247. [DOI] [PubMed] [Google Scholar]
- 33. McGiven JA, Sawyer J, Perrett LL, et al. A new homogeneous assay for high throughput serological diagnosis of brucellosis in ruminants. J Immunol Methods 2008;337(1):7–15. [DOI] [PubMed] [Google Scholar]
- 34. Szekeres PG, Leong K, Day TA, Kingston AE, Karran EH. Development of homogeneous 384‐well high‐throughput screening assays for Abeta1–40 and Abeta1–42 using AlphaScreen technology. J Biomol Screen 2008;13(2):101–111. [DOI] [PubMed] [Google Scholar]


