Abstract
Background
Serum cystatin C (Cys‐C), an inhibitor of cysteine proteases, has been suggested as an ideal biomarker of glomerular filtration rate (GFR).
Objectives
The objective of this study was to describe the reference intervals of serum Cys‐C and identify factors associated with serum Cys‐C or its variability, including age, gender, creatinine (Crea), blood urea nitrogen (BUN), and uric acid (UA).
Design and Methods
Serum Cys‐C, Crea, BUN, and UA were measured in 4,517 healthy participants aged 8–89 years attending our hospital. Serum Cys‐C was analyzed using a latex‐enhanced immunoturbidimetric method. Crea were tested by picric acid jaffe method, BUN, and UA by kinetic UV assays.
Results
The predominant characteristic of Cys‐C distribution was that Cys‐C concentration in age ≥60 years group was the highest (P < 0.05). The differences of Cys‐C concentration between males and females existed for subjects aged from 30 to 59 years (P < 0.05). In a multiple model adjusted only for gender and age, gender (β = 0.007) has stronger effect on Cys‐C levels, compared with age (β = 0.003). The clinical variables, comprised of age, gender, Crea, BUN, and UA, involved in the fully adjusted equation accounted for 37.6% of variation of Cys‐C.
Conclusions
Ninety‐five percent reference intervals for healthy population were partitioned into three categories only by age, 0.59–1.07 mg/L for subjects aged 19–59 years; 0.74–1.14 mg/L for the older aged ≥60 years; and 0.63–1.11 mg/L for children aged ≤18 years. Serum Cys‐C is significantly related to gender, age, UA, Crea, and BUN. Besides, there are still other factors contributing to variation of Cys‐C levels.
Keywords: cystatin C, gender identity, age factors, reference values, China
INTRODUCTION
Chronic kidney disease (CKD) is a worldwide public health problem. Glomerular filtration rate (GFR) is considered to monitor kidney function and diagnose renal disease. In clinical practice, serum creatinine (Crea) is measured as a routine biomarker of GFR, on the assumption that the Crea is completely filtered across the glomerulus and that Crea biogenesis and excretion are constant. However, the biogenesis of Crea is affected by dietary intake and muscle mass, which itself varies by age, height, gender, and ethnicity. Besides, the level of Crea does not elevate until advanced stages of CKD.
At present, serum cystatin C (Cys‐C), an inhibitor of cysteine proteases, has been deemed as an ideal biomarker of GFR. Serum Cys‐C is more sensitive than serum Crea to detect early and moderate deterioration of GFR 1, 2, 3, 4. It is cleared exclusively through the kidney. Therefore, its concentration depends solely on GFR. Narvaez‐Sanchez et al. suggested that Cys‐C could be a replacement to serum Crea for diagnosing and monitoring kidney function in children 5.
Little is known about the reference intervals for Cys‐C in a large healthy population in our area. In addition, there are still some factors that should be taken into consideration. It is uncertain whether variables such as age and gender affect Cys‐C levels in healthy populations. Some previous studies suggested that Cys‐C levels are independent of gender and age 2, others showed different opinions 6. To fully exploit the value of serum Cys‐C as a GFR marker, reliable age and sex‐correlated reference intervals are required. The objective of this study is to establish the reference intervals in a sufficiently large healthy population and to verify whether serum levels are indeed independent of age and gender, and to explore the association between Cys‐C and other markers of kidney function such as Crea, blood urea nitrogen (BUN), and uric acid (UA).
MATERIALS AND METHODS
Study Population
A total of 4,517 healthy subjects (range from 8 to 89 years) attending West China Hospital of Sichuan University of China for routine health checks were enrolled, comprised of 2,273 males (42.4 ± 13.1 years) and 2,244 females (42.3 ± 11.9 years). Duplicate samples from same subjects were eliminated. Individuals were not selected if they were either infected with HBV, HCV, HIV, or suffered from renal disease, cardiovascular diseases, or diabetes. The criteria to exclude subjects with renal disease were listed as follows: (1) no history and signs/symptoms of any renal disease, (2) no medications, (3) normal dipstick urine test, (4) participants with Crea concentrations outside the reference interval were excluded from the project. This study received ethical approval from the Ethical Committee of West China Hospital of Sichuan University.
Biochemical Tests and Data Collection
Laboratory diagnostic data on enrolled subjects including age, gender, Crea, Cys‐C, BUN, and UA, were retrospectively recorded.
Serum Cys‐C samples were analyzed using a latex‐enhanced immunoturbidimetric method (Sichuan Maker Biotech Co., Ltd. Chengdu, China). Crea were tested by picric acid jaffe method (cobas), BUN, and UA by kinetic UV assay (cobas). All analyses work on Roche automated clinical chemistry analyzers modular. According to the Cys‐C manufacture's instruction, the within‐run precision is less than 3.9% and the inaccuracy is less than 15%. And moreover, all internal quality control plans were carried out to assure the precision of tests. The controls of Cys‐C was supplied by Sichuan Maker Biotech Co., Ltd., while the controls of BUN, Crea, and UA were purchased from Bio‐Rad (Bio‐Rad Laboratories, Cal., USA).
Statistical Analysis
Age, Cys‐C, Crea, BUN, and UA were expressed as a range with means ± 1 SD (standard deviation). One‐way ANOVA and S–N–K tests (or Games–Howell test where appropriate) were used to multiple compare the different Cys‐C, Crea, BUN, and UA levels in six age groups. Student's t‐test was used to compare the different distribution of Cys‐C levels between female and male individuals in different three age groups. Ninety‐five percent confidence intervals of Cys‐C in different gender in different clusters were deemed as reference ranges for Cys‐C. According to the partitioning criterion of Sinton et al. 11, the difference between subgroup means should exceed 25% of the reference range calculated for the combined distribution if the subgroups are to be considered separately. Multiple linear regression was used to study the contributions of different clinical variables to Cys‐C concentrations. All statistical processes were finished in SPSS software (Version 16.0, SPSS Inc., Chicago, IL). The differences were considered significant if P < 0.05.
RESULTS
Age‐Varying Characteristics of Cys‐C and Crea
As Figure 1 shows, the means levels of Cys‐C and Crea were age‐varying. The curve of the Cys‐C means was relatively flatter than that of Crea. Meanwhile Cys‐C changes more with 20–70 years of age than Crea in the young (age < 20 years) and old subjects (age > 70 years). Cys‐C concentrations in persons aged ≥60 years were higher than those in the other age groups (P < 0.05). As suggested in Table 1, Crea concentration in individuals aged <19 years was significantly lower than that in individuals aged >18 (P < 0.05).
Figure 1.
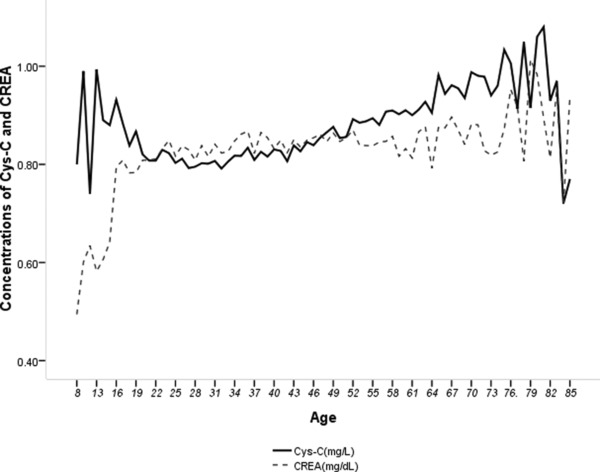
Age‐varying curves of Cys‐C and Crea.
Table 1.
Basic Characteristics of Cys‐C, Crea, BUN, and Uric Acid
| Age groups (years) | n | Age (years) Mean ± 1SD | Cys‐C (mg/L) Mean ± 1SD (Range) | Crea (μmol/L) Mean ± 1SD (Range) | BUN (mmol/L) Mean ± 1SD (Range) | Uric Mean ± 1SD (Range) |
|---|---|---|---|---|---|---|
| ≤18 | 44 | 16.1 ± 2.4 | 0.874 ± 0.121 | 65.4 ± 14.5 | 5.1 ± 1.26 | 317.7 ± 71.3 |
| (0.62,1.09) | (39.3,99.70) | (2.44,7.04) | (184.0,487.0) | |||
| 19–29 | 697 | 25.4 ± 2.6 | 0.808 ± 0.119 | 72.8 ± 14.9 | 5.00 ± 1.1 | 310.5 ± 73.1 |
| (0.52,1.09) | (40.6,121.90) | (2.74,7.92) | (166.0,524.0) | |||
| 30–39 | 1,258 | 35.0 ± 2.8 | 0.814 ± 0.116 | 74.6 ± 14.5 | 5.12 ± 1.10 | 313 ± 76.7 |
| (0.51,1.09) | (41,118.6) | (2.80,10.58) | (158.0,609.0) | |||
| 40–49 | 1,297 | 44.0 ± 2.7 | 0.837 ± 0.121 | 74.7 ± 14.4 | 5.26 ± 1.15 | 311.5 ± 75.7 |
| (0.51,1.18) | (38.0,130.1) | (2.86,9.53) | (151.0,587.0) | |||
| 50–59 | 769 | 54.3 ± 2.5 | 0.887 ± 0.108 | 74.8 ± 14.1 | 5.5 ± 1.17 | 312.9 ± 72.3 |
| (0.54,1.17) | (38.4,127.1) | (3.15,8.56) | (160.0,551.0) | |||
| ≥60 | 452 | 66 ± 5.2 | 0.939 ± 0.101 | 75.30 ± 13.6 | 5.6 ± 1.21 | 308.0 ± 65.7 |
| (0.63,1.19) | (46.3,112.5) | (3.16,11.30) | (132.0,485.0)) | |||
| Total | 4,517 | 42 ± 12.5 | 0.845 ± 0.122 | 74.4 ± 14.4 | 5.2 ± 1.15 | 311.7 ± 74.0 |
| (0.51,1.19) | (39.3,130.1) | (2.44,11.30) | (132.0,609.0) |
Cys‐C Levels of Subjects of Different Gender and Age
The mean Cys‐C level of males was 0.84 ± 0.12 mg/L, lower than that of females (0.85 ± 0.12 mg/L; P < 0.05). The differences of Cys‐C concentration between males and females existed when subjects were aged from 30 to 60 years (P < 0.05). But the mean differences between those groups were smaller than the SD of Cys‐C in all individual enrolled. No significant differences of Cys‐C levels between males and females younger than 29 years or older than 60 years were found (P > 0.05; Table 2).
Table 2.
Cys‐C Levels of Subjects of Different Gender and Age
| Cys‐C(mg/L) | Crea (μmol/L) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Male | Female | Male | Female | |||||||
| Age groups (years) | n | Mean ± SD | n | Mean ± SD | P | n | Mean ± SD | n | Mean ± SD | P |
| ≤18 | 34 | 0.88 ± 0.11 | 10 | 0.86 ± 0.17 | 0.732 | 34 | 66.5 ± 15.3 | 10 | 61.7 ± 11.3 | 0.369 |
| 19–29 | 363 | 0.81 ± 0.12 | 334 | 0.80 ± 0.12 | 0.309 | 363 | 73.7 ± 14.4 | 334 | 72.0 ± 15.1 | 0.129 |
| 30–39 | 609 | 0.81 ± 0.12 | 649 | 0.82 ± 0.12 | 0.020 | 609 | 75.1 ± 15.0 | 649 | 74.1 ± 14.1 | 0.199 |
| 40–49 | 627 | 0.83 ± 0.12 | 670 | 0.84 ± 0.12 | 0.021 | 627 | 73.4 ± 14.5 | 670 | 75.4 ± 14.3 | 0.075 |
| 50–59 | 395 | 0.87 ± 0.11 | 374 | 0.89 ± 0.10 | 0.002 | 395 | 73.6 ± 14.4 | 374 | 76.0 ± 13.8 | 0.019 |
| ≥60 | 245 | 0.94 ± 0.10 | 207 | 0.94 ± 0.10 | 0.462 | 245 | 74.7 ± 13.1 | 207 | 76.0 ± 14.1 | 0.297 |
| Total | 2,273 | 0.84 ± 0.12 | 2,244 | 0.85 ± 0.12 | 0.009 | 2,273 | 74.2 ± 14.5 | 2,244 | 74.6 ± 14.3 | 0.275 |
Association with Cys‐C and Covariates
As Figure 2 shows, there is positive correlation between Cys‐C and Crea (r = 0.516) or BUN (r = 0.225). In a multiple model adjusted only for gender and age, both gender and age contributed to variants of Cys‐C levels. Compared with age (β = 0.003), gender (β = 0.007) has stronger effect on Cys‐C levels. All covariates involved in the fully adjusted equation accounted for 37.6% of variation of Cys‐C, while factors, comprising of age and gender accounted for 10.6% of variation of Cys‐C. The fully adjusted equation suggests that there are more factors contributing to variation in serum levels of Cys‐C, with all factors in this study only accounted for 37.6% of variation of Cys‐C levels (Table 3).
Figure 2.
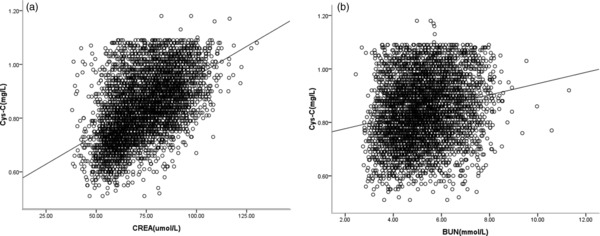
(a) Correlation between Cys‐C and Crea. (b) Correlation between Cys‐C and BUN. There was positive correlation between Cys‐C and Crea (r = 0.516) or BUN (r = 0.225). Lines in two figures is regression curve.
Table 3.
Linear Analysis of Factors Contributing to Variants of Cys‐C
| Unadjusted association | Adjusted associationa | Fully adjusted modela | |||||
|---|---|---|---|---|---|---|---|
| Dependent | Independent | β | P | β | P | β | P |
| Cys‐C (mg/L) | Gender | / | / | 0.007 | 0.030 | 0.008 | 0.007 |
| (0.001,0.014) | (0.002,0.014) | ||||||
| Age | 0.003 | 0.000 | 0.003 | 0.000 | 0.003 | 0.000 | |
| (0.003,0.003) | (0.003,0.004) | (0.003,0.003) | |||||
| Crea (mg/dL)b | 0.387 | 0.000 | / | / | 0.313 | 0.000 | |
| (0.369,0.406) | (0.290,0.336) | ||||||
| BUN (mmol/L) | 0.021 | 0.000 | / | / | 0.005 | 0.000 | |
| (0.018,0.024) | (0.003,0.008) | ||||||
| Uric A (mg/dL)b | 0.039 | 0.000 | / | / | 0.013 | 0.000 | |
| (0.036,0.041) | (0.010, 0.016) | ||||||
| R 2 | N/A | 0.106 | 0.376 | ||||
Adjusted means that covariates were put into multiple linear regression equation.
Conversion factors: Crea mg/dL × 88.4 = μmol/L; uric acid mg/dL × 59.48 = mmol/L.
β: The higher the β, the more association with Cys‐C.
DISCUSSION
Previous studies of Cys‐C in the healthy group have been conducted in relatively small and selected study populations 7, 8, 9, 10, 11. Thus, one of the largest advantages of our study was the generalizability of the results from a large population. This study was to establish the reference intervals of Cys‐C and evaluate the association between Cys‐C and its covariates, including Crea, BUN, UA, age, and gender.
In this study, contrary to previous reports of Cys‐C levels, being independent of age and gender 12, gender and age were statistically associated with Cys‐C levels 13. Overall, the mean Cys‐C level was 0.845 mg/L and was higher in females (0.85 mg/L) than in males (0.84 mg/L). Some studies 8, 14 showed Cys‐C in females was significantly higher than that in males, whereas Groesbeck et al. 15 observed an opposite Cys‐C distribution in gender in US adolescents aged from 12 to 19 years, with an explanation that young male adults have had GFR reported as 127 ml/min per 1.73 m2 compared with 118 mL/min per 1.73 m2 in female adults. However, there were significant differences of Cys‐C level between males and females when the age ranges from 30 to 60 years (Table 4). It is unclear why female individuals have higher Cys‐C levels than male individuals. Knight et al. 16 observed factors influencing serum Cys‐C levels other than renal function and the impact on renal function measurement. Age, gender, weight, height, current cigarette smoking, and CRP levels were independently associated with Cys‐C levels 16. More attention should be paid on the old group (aged ≥60 years). The Cys‐C level was the highest (P < 0.05) in all age groups, and no differences of mean Cys‐C concentration were found in gender (P > 0.05) in age ≥60 group in this study. A study in elders aged >70 years suggested that significant increase with age was found with Cys‐C 17. Torner et al. 18 reported that renal function in community‐dwelling elders were frail. Crea levels in the young population aged from 8 to 18 years change dramatically (see Fig. 1), so the use of Crea as an index of GFR has long been considered problematic in youth. It is reported that Cys‐C is an alternative for assessing renal function in premature infants 19 and children 20, 21. Furthermore, there was no significant male–female difference in children aged from 1 to 15 years 22. Fischbach et al. indicated that mean Cys‐C is higher in infants than older children 22.
Table 4.
Ninety‐Five Percent Reference Intervals of Cys‐C
| Cys‐C (mg/L) | ||||
|---|---|---|---|---|
| Age group (years) | n | Mean | SD | 95% reference intervals (M − 2SD, M + 2SD) |
| ≥8 and ≤18 | 44 | 0.87 | 0.12 | (0.63, 1.11) |
| 19–59 | 4,021 | 0.83 | 0.12 | (0.59, 1.07) |
| ≤89 and ≥60 | 452 | 0.94 | 0.10 | (0.74, 1.14) |
| Total | 4,517 | 0.845 | 0.122 | (0.601, 1.089) |
Although there was difference in Cys‐C levels between male and female, it was without clinical importance. Because the difference was small (0.01 mg/L), and more considerations should taken into actual practice, including SD of Cys‐C (higher than 0.1), intraindividual variance, precision or accuracy of kits and so on. Besides, 95% confidence intervals were partitioned into three categories by age instead of gender according to the partitioning criterion of Sinton et al. (11; Table 4). Therefore, 95% reference intervals for healthy population were partitioned into three categories only by age, 0.59–1.07 mg/L for adults aged 19–59 years; 0.74–1.14 mg/L for the older aged ≥60 years; and 0.63–1.11 mg/L for all subjects aged from 8 to 18 years (Table 4). The reference intervals in children (aged ≤18) listed in Table 4, may be not reliable, for there were only 44 children included in this study.
Cys‐C and Crea levels differed significantly by age in this study. Crea levels in individuals aged from 8 to 18 years were lower than those in the other age groups. Figure 1 suggests that Cys‐C is more constant than Crea in juveniles, but is not a replacement for Crea because the intraindividual variance was greater for Cys‐C than for Crea in healthy subjects 23, 24. As expected by linear analyses, Crea compared with BUN was much more correlated with Cys‐C (β = 0.313). Another interesting finding was that UA contributed to variation of Cys‐C levels. The UA was higher in patients with elevated Cys‐C levels than that in patients with normal Cys‐C levels 25. Previous studies described association between high UA and reduced GFR in nonproteinuric patients with type I diabetes 26, 27. The clinical variables, comprising of gender, age, Crea, BUN, and UA, included in the multivariable equation only accounted for 37.6% of variation of Cys‐C. Other variables contributing to variation of Cys‐C need to be investigated.
In conclusion, this study provides gender‐/age‐specific reference values of Cys‐C for a large population in China. There was a clear gender difference in Cys‐C levels with female subjects having higher levels than male subjects, although the effect may be minimal. All factors in this study, such as Crea, age, gender, BUN, and UA, were only accounted for 37.6% of variation of Cys‐C levels. So there were some other factors associated with variation of Cys‐C.
CONFLICT OF INTEREST
Authors declared no conflicts of interest during this work.
ACKNOWLEDGMENTS
This study was supported by grants from National Natural Science Foundation of China (#30900658) and Sichuan University Young Scientist Funds (#2008095).
Authors’ contribution: Dong‐Dong Li, Meng‐Na Zou, and Xin Hu contributed equally to this work.
REFERENCES
- 1. Xu H, Lu Y, Teng D, Wang J, Wang L, Li Y. Assessment of glomerular filtration rate in renal transplant patients using serum cystatin C . Transplant Proc 2006;38:2006–2008. [DOI] [PubMed] [Google Scholar]
- 2. Yashiro M, Kamata T, Segawa H, Kadoya Y, Murakami T, Muso E. Comparisons of cystatin C with creatinine for evaluation of renal function in chronic kidney disease. Clin Exp Nephrol 2009;13:598–604. [DOI] [PubMed] [Google Scholar]
- 3. Willems D, Wolff F, Mekhali F, Gillet C. Cystatin C for early detection of renal impairment in diabetes. Clin Biochem 2009;42:108–110. [DOI] [PubMed] [Google Scholar]
- 4. El‐Shafey EM, El‐Nagar GF, Selim MF, El‐Sorogy HA, Sabry AA. Is serum cystatin C an accurate endogenous marker of glomerular filtration rate for detection of early renal impairment in patients with type 2 diabetes mellitus? Ren Fail 2009;31:355–359. [DOI] [PubMed] [Google Scholar]
- 5. Narvaez‐Sanchez R, Gonzalez L, Salamanca A, Silva M, Rios D, Arevalo S, Gastelbondo R, Sanchez J. Cystatin C could be a replacement to serum creatinine for diagnosing and monitoring kidney function in children. Clin Biochem 2008;41:498–503. [DOI] [PubMed] [Google Scholar]
- 6. Stevens LA, Schmid CH, Greene T, Li L, Beck GJ, Joffe MM, Froissart M, Kusek JW, Zhang YL, Coresh J, Levey AS. Factors other than glomerular filtration rate affect serum cystatin C levels. Kidney Int 2009;75:652–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Erlandsen EJ, Randers E, Kristensen JH. Reference intervals for serum cystatin C and serum creatinine in adults. Clin Chem Lab Med 1998;36:393–397. [DOI] [PubMed] [Google Scholar]
- 8. Ichihara K, Saito K, Itoh Y. Sources of variation and reference intervals for serum cystatin C in a healthy Japanese adult population. Clin Chem Lab Med 2007;45:1232–1236. [DOI] [PubMed] [Google Scholar]
- 9. Galteau MM, Guyon M, Gueguen R, Siest G. Determination of serum cystatin C: Biological variation and reference values. Clin Chem Lab Med 2001;39:850–857. [DOI] [PubMed] [Google Scholar]
- 10. Finney H, Newman DJ, Price CP. Adult reference ranges for serum cystatin C, creatinine and predicted creatinine clearance. Ann Clin Biochem 2000;37:49–59. [DOI] [PubMed] [Google Scholar]
- 11. Sinton TJ, Cowley DM, Bryant SJ. Reference intervals for calcium, phosphate, and alkaline phosphatase as derived on the basis if multi‐analyzer profiles. Clin Chem 1986;32:76–79. [PubMed] [Google Scholar]
- 12. Norlund L, Fex G, Lanke J, Von Schenck H, Nilsson JE, Leksell H, Grubb A. Reference intervals for the glomerular filtration rate and cell‐proliferation markers: Serum cystatin C and serum beta 2‐microglobulin/cystatin C‐ratio. Scand J Clin Lab Invest 1997;57:463–470. [DOI] [PubMed] [Google Scholar]
- 13. Weinert LS, Prates AB, do Amaral FB, Vaccaro MZ, Camargo JL, Silveiro SP. Gender does not influence cystatin C concentrations in healthy volunteers. Clin Chem Lab Med 2010;48:405–408. [DOI] [PubMed] [Google Scholar]
- 14. Al Wakeel JS, Memon NA, Chaudhary A, Mitwalli AH, Tarif N, Isnani A, Hammad D. Normal reference levels of serum cystatin C in Saudi adults. Saudi J Kidney Dis Transpl 2008;19:361–370. [PubMed] [Google Scholar]
- 15. Groesbeck D, Köttgen A, Parekh R, Selvin E, Schwartz GJ, Coresh J, Furth S. Age, gender, and race effects on cystatin C levels in US adolescents. Clin J Am Soc Nephrol 2008;3:1777–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Knight EL, Verhave JC, Spiegelman D, Hillege HL, de Zeeuw D, Curhan GC, de Jong PE. Factors influencing serum cystatin C levels other than renal function and the impact on renal function measurement. Kidney Int 2004;65:1416–1421. [DOI] [PubMed] [Google Scholar]
- 17. Fehrman‐Ekholm I, Seeberger A, Bjork J, Sterner G. Serum cystatin C: A useful marker of kidney function in very old people. Scand J Clin Lab Invest 2009;69:606–611. [DOI] [PubMed] [Google Scholar]
- 18. Torner A, Odar‐Cederlof I, Kallner A, Akner G. Renal function in community‐dwelling frail elderly. Comparison between measured and predicted glomerular filtration rate in the elderly and proposal for a new cystatin C‐based prediction equation. Aging Clin Exp Res 2008;20:216–225. [DOI] [PubMed] [Google Scholar]
- 19. Armangil D, Yurdakok M, Canpolat FE, Korkmaz A, Yigit S, Tekinalp G. Determination of reference values for plasma cystatin C and comparison with creatinine in premature infants. Pediatr Nephrol 2008;23:2081–2083. [DOI] [PubMed] [Google Scholar]
- 20. Zappitelli M, Parvex P, Joseph L, Paradis G, Grey V, Lau S, et al. Derivation and validation of cystatin C‐based prediction equations for GFR in children. Am J Kidney Dis 2006;48:221–230. [DOI] [PubMed] [Google Scholar]
- 21. Takuwa S, Ito Y, Ushijima K, Uchida K. Serum cystatin‐C values in children by age and their fluctuation during dehydration. Pediatr Int 2002;44:28–31. [DOI] [PubMed] [Google Scholar]
- 22. Fischbach M, Graff V, Terzic J, Bergere V, Oudet M, Hamel G. Impact of age on reference values for serum concentration of cystatin C in children. Pediatr Nephrol 2002;17:104–106. [DOI] [PubMed] [Google Scholar]
- 23. Reinhard M, Erlandsen EJ, Randers E. Biological variation of cystatin C and creatinine. Scand J Clin Lab Invest 2009;69:831–836. [DOI] [PubMed] [Google Scholar]
- 24. Keevil BG, Kilpatrick ES, Nichols SP, Maylor PW. Biological variation of cystatin C: Implications for the assessment of glomerular filtration rate. Clin Chem 1998;44:1535–1539. [PubMed] [Google Scholar]
- 25. Muntner P, Vupputuri S, Coresh J, Uribarri J, Fox CS. Metabolic abnormalities are present in adults with elevated serum cystatin C . Kidney Int 2009;76:81–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ficociello LH, Rosolowsky ET, Niewczas MA, Maselli NJ, Weinberg JM, Aschengrau A, Eckfeldt JH, Stanton RC, Galecki AT, Doria A, Warram JH, Krolewski AS. High‐normal serum uric acid increases risk of early declining renal function in type 1 diabetes: Results of 6‐year follow‐up. Diabetes Care 2010;33:1337–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rosolowsky ET, Ficociello LH, Maselli NJ, Niewczas MA, Binns AL, Roshan B, Warram JH, Krolewski AS. High‐normal serum uric acid is associated with impaired glomerular filtration rate in nonproteinuric patients with type 1 diabetes. Clin J Am Soc Nephrol 2008;3:706–713. [DOI] [PMC free article] [PubMed] [Google Scholar]


