Abstract
Background
To assess the prognostic significance of four inflammatory markers (TNF‐α, high sensitive C‐reactive protein (hs‐CRP), intercellular cell adhesion molecule‐1 (ICAM‐1), vascular cell adhesion molecule‐1 (VCAM‐1)) in chronic heart failure (CHF) patients with respect to individual outcomes, especially disease exacerbation and mortality.
Methods
Plasma adhesion molecules, ICAM‐1, and VCAM‐1, together with TNF‐α and hs‐CRP were determined in 120 CHF patients and 69 healthy controls. Endothelial function was also estimated by flow‐mediated brachial artery dilatation.
Results
Increased levels of all investigated inflammatory markers were found in CHF patients compared to controls, with the rise more pronounced in New York Heart association (NYHA) functional IV class. Significant correlations were obtained for VCAM‐1 and brain natriuretic peptide (r = 0.191; P = 0.038), as well as, ICAM‐1 and endothelium‐dependent vasodilatation (r = −0.235; P = 0.01). Kaplan–Meier analysis showed disease exacerbation in patients with TNF‐α levels >2.78 pg/ml significantly shorter compared to those with TNF‐α levels <2.78 pg/ml (log‐rank test = 8.270; P = 0.004), while similar association was observed for patients with hs‐CRP levels >4.76 mg/l (log‐rank test = 5.052; P = 0.025) and VCAM‐1 levels >1200 ng/l (log‐rank test = 5.45; P = 0.020) with respect to mortality. Cox regression analysis demonstrated only VCAM‐1 (HR = 4.7; 95% confidence interval (CI): 1.1–18.7; P = 0.030) as independent death predictor, while TNF‐α was associated with disease exacerbation (HR = 8.2; 95%CI: 1.1–23.0; P = 0.045).
Conclusions
VCAM‐1 appears to be useful in risk stratification of CHF patients and in screening, to identify subjects at risk for heart failure related events. J. Clin. Lab. Anal. 27:105–112, 2013. © 2013 Wiley Periodicals, Inc.
Keywords: inflammation, adhesion molecules, heart failure
INTRODUCTION
For more than a decade, chronic heart failure (CHF) has been regarded as a complex cascade of chronic inflammatory reactions, causing gradual cardiac depression and the syndrome of heart failure 1, 2. Inflammation is important in the pathogenesis and progression of many forms of heart failure and biomarkers of inflammation have become the subject of intense inquiry 2. Among them, proinflammatory cytokines (e.g. TNF‐α) and adhesion molecules, intercellular cell adhesion molecule‐1 (ICAM‐1), and vascular cell adhesion molecule‐1 (VCAM‐1) have gained most attention. While their proposed patophysiological role in CHF is well documented 1, the data on prognostic significance of these inflammatory molecules are still limited.
The heart failure cytokine hypothesis proposes that a precipitating event, such as ischemic cardiac injury, triggers innate stress responses, including the production of proinflammatory cytokines TNF‐α, IL‐6, and IL‐1 3, 4. Raised TNF‐α production in CHF seems to be associated with several untowed effects, such as left ventricular dysfunction and remodeling, increased cardiac myocyte apoptosis, reduced skeletal muscle blood flow, and endothelial dysfunction 2, 4, 5. Moreover, studies to date have shown that increased circulating TNF‐α levels were associated with increased mortality in heart failure patients 6, 7, 8. Several TNF‐α‐mediated mechanisms may cause endothelial dysfunction 2, 4, 9, 10. TNF‐α activates NF‐κB with subsequent expression of endothelial adhesion molecules 2, 4, 11. The role of adhesion molecules, VCAM‐1, ICAM‐1, and E‐selectin, in the initiation of the inflammatory process is well established 12. VCAM‐1, which is involved in the vessel wall invasion of monocytes and T lymphocytes, can also contribute to reduction of endothelium‐dependent NO‐mediated vasodilation, in response to hormonal agonists and shear stress. Until now, elevated levels of adhesion molecules, VCAM‐1 and ICAM‐1, have been found in CHF patients in comparison with controls 13, 14. It should be noted that only VCAM‐1 concentrations are decreasing after heart transplantation 13.
The severity of endothelial dysfunction has been shown to have prognostic value for cardiovascular events. Several studies have shown soluble VCAM‐1 as independent predictor of death in patients with coronary artery disease 15, while others confirmed soluble ICAM‐1 as the predictor of peripheral artery disease and myocardial infarction 16, 17. However, clinical data on the prognostic value of adhesion molecules in patients with CHF are limited and inconsistent 18, 19, 20. In the present study, we investigated the prognostic significance of four inflammatory molecules, TNF‐α, high sensitive C‐reactive protein (hs‐CRP), ICAM‐1, and VCAM‐1 with regard to individual outcomes, such as death, disease exacerbation, and episodes of unstable angina pectoris in patients with CHF.
METHODS
Selection of Study Participants
One hundred and twenty consecutive patients with CHF and angiographically confirmed cardiovascular disease (74 men and 46 women, mean age 59.1± 5.9 years) were enrolled in the study. Patients were consecutively recruited during the dispensary check‐ups at Medical Center “Bezanijska Kosa" between the years of 2008 and 2009. The diagnosis of CHF was based on patient's history, physical examination, electrocardiography, chest radiology, echocardiography, and coronary angiography. Major inclusion criteria were left ventricular ejection fraction lower than 45% and CHF in a steady state for a 2‐week period of time. Patients with severe comorbidity, renal failure, liver disease, and severe disturbances in lung function were excluded, as well as, patients with the presence of infection or inflammatory diseases such as sepsis, malignancy, arthritis, or connective tissue disease—in order to avoid the possible effects of these comorbid conditions on cytokine production. Exclusion criterion for the study group was acute or decompensated heart failure as well. Since all New York Heart association (NYHA) functional classes III and IV patients were on diuretics and dietary sodium restriction, important clinical manifestations of CHF secondary to tissue congestion were suppressed. Furthermore, acute events such as infection, arrhythmia or discontinuation of therapy, which could precipitate manifestations of acute heart failure, were not present in these patients. The age‐ and sex‐matched control‐group consisted of 69 healthy subjects (mean age 58.5±5.6 years), without any acute or chronic disease and any symptoms that could be related to the cardiovascular system. The study was approved by Ethic committee of Faculty of Medicine, University of Belgrade, and all enrolled patients gave their written informed consent.
Noninvasive Assessment of Endothelium‐Dependent and ‐Independent Flow‐Mediated Dilation (FMD) of the Brachial Artery
Endothelium‐dependent and ‐independent FMD was performed using the 13.0 MHz linear array transducer (Vivid 7, GE Medical Systems, Little Chalfont, England, UK). After the resting period of 15 min in the supine position, the transducer was placed 4–5 cm above the elbow in the longitudinal section for the scanning of right brachial artery and afterwards the basal diameter of brachial artery and flow velocity were measured. Further on, the sphygmomanometer cuff was placed on the upper arm, and inflated at 250 mmHg for 5 min, and deflated abruptly. The second scan was performed 60 to 90 sec after the cuff release. After 10 min of resting interval, 5 mg of sublingual Nitroglycerin was administered, and brachial artery was scanned within the next 5 min. Diameter measurements were taken at the end of the diastole and calculated at least three times. Endothelium‐dependent and ‐independent vasodilations were defined as the percent change in diameter compared to baseline (maximum diameter‐baseline diameter/baseline diameter ×100).
Echocardiography
All patients underwent two‐dimensional echocardiography after endothelial function assessment. Echocardiography data included left ventricular dimensions and volumes, left ventricular ejection fraction (LVEF) (biplane modified Simpson's), global and regional wall motion, and valve anatomy and function.
Long‐Term Clinical Follow‐Up
Over a mean follow‐up period of 13.1 months, patients underwent a monthly physical examination that was always performed by the same cardiologist, blinded for the obtained laboratory results of the markers of oxidative stress. In addition, patients were contacted regularly by telephone to evaluate the incidence of adverse cardiovascular events, which included all‐cause mortality, nonfatal myocardial infarction, new episodes of unstable angina pectoris, and hospitalization for CHF exacerbation. The decision whether the primary adverse cardiovascular event had occurred was made only after reviewing the patient's medical records, evaluated by cardiologists, in case of death, nonfatal myocardial infarction, angina pectoris, and/or hospitalization. No patient was lost during the follow‐up.
Biochemical Analysis
Blood samples from CHF patients were taken during outpatient visit. Serum levels of glucose, creatinine, cholesterol, triglycerides, proteins, albumin, fibrinogen, and hs‐CRP were determined using commercially available kits. Neurohormonal status was assessed from plasma brain natriuretic peptide (BNP) levels, using the rapid Triage BNP assay (Biosite Inc, San Diego, CA). Fasting plasma and serum samples were collected from each patient for the measurement of proinflammatory markers, including TNF‐α, ICAM‐1, and VCAM‐1. Commercially available ELISA kits for TNF‐α, ICAM‐1, VCAM‐1 (Bender MedSystems, Mercure group, Austria) were used. The intra‐ and interassay coefficients of variation were <8% for all assays in our laboratory.
Statistical Analysis
Statistical analysis was performed using the Statistical Package for the Social Sciences software (IBM Statistics SPSS, version 19.0) and Microcal Origin software (version 5.0). For each variable, values were expressed as mean ± SD (standard deviation). The differences between groups were tested using analysis of variance (ANOVA) method, after normal distribution of parameters had been established using Kolmogorov–Smirnov test. The association between different variables and oxidative stress markers were determined by using the Pearson correlation coefficient. A P‐value <0.05 was considered statistically significant. Receiver operator characteristic (ROC) analysis was performed to assess the sensitivity and specificity of variables in predicting outcome. Event‐free survival was estimated using the Kaplan–Meier method and compared between groups using the log‐rank test. Stepwise Cox hazard regression analyses were used to determine which of the different variables (age, diabetes mellitus, hemoglobin, NYHA class, BNP, ejection fraction (EF), systolic blood pressure, endothelium‐dependent and ‐independent FMD, as well as, VCAM‐1, ICAM‐1, hs‐CRP, and TNF‐α) were significantly associated with individual outcomes.
RESULTS
General Characteristics of CHF Patients and Controls
Baseline patients’ characteristics, together with results on plasma concentration of inflammatory markers, are presented in Table 1. The study population comprised 120 consecutive NYHA class I–IV CHF patients (74 males and 46 female) with ischemic cardiomyopathy, average age of 59 ± 1 years, mean LVEF 35 ± 9%, and mean body mass index 28 ± 5 kg/m2. Among the CHF patients, no significant differences between groups were observed in terms of age, body mass index, heart rate, and biochemical profile. With the increase of NYHA class, LVEF revealed progressive deterioration of the myocardial function, while the concentration of BNP was significantly elevated. The degree of endothelium‐dependent NO‐mediated vasodilation and endothelium‐independent nitroglycerin (NTG)‐mediated vasodilation of brachial artery decreased with the progression of CHF.
Table 1.
General Characteristics, Echocardiographic, and Inflammatory Parameters in Chronic Heart Failure (CHF) Patients and Controls
| NYHA | |||||
|---|---|---|---|---|---|
| Controls | I | II | III | IV | |
| (n = 69) | (n = 11) | (n = 71) | (n = 28) | (n = 10) | |
| Risk factors | |||||
| Age | 58.4 ± 5.5* | 57.9 ± 4.4 | 57.7 ± 5.8 | 62.1 ± 5.6 | 61.6 ± 5.5 |
| Sex (M/F) | 40/29 | 5/6 | 48/23 | 14/14 | 7/3 |
| LVEF (%) | 66.8 ± 3.9 | 43.3 ± 2.8a | 38.7 ± 7.0a,b | 30.1 ± 7.4a,b,c | 21.7 ± 5.9a,b,c,d |
| FMD‐NO (%) | 9.1 ± 5.4a | 7.0 ± 4.9 | 5.0 ± 5.2d | 4.1 ± 3.8 | 1.5 ± 1.8 d,e |
| FMD‐NTG (%) | 15.5 ± 6.3 | 13.2 ± 4.9 | 12.5 ± 7.0 | 10.1 ± 5.8 | 10.7 ± 4.3 |
| BNP (pg/ml) | 13.2 ± 28.2a | 76.7 ± 86.7 | 117 ± 126a | 362 ± 222a,b | 878 ± 718a,b,c |
| Markers of inflammation | |||||
| Hs‐CRP (mg/l) | 1.5 ± 1.6 | 3.1 ± 3.7 | 4.1 ± 6.8a | 4.2 ± 4.5a | 17.9 ± 17.4 a,b,c,d |
| TNFα (pg/ml) | 1.9 ± 1.9 | 4.6 ± 5.7 | 4.7 ± 4.0a | 4.6 ± 4.9a | 7.8 ± 13.4a,b |
| ICAM‐1 (ng/L) | 281 ± 188 | 374 ± 170 | 362 ± 142a | 359 ± 134a | 420 ± 76.1a |
| VCAM‐1 (ng/l) | 860 ± 341 | 1046 ± 279 | 1116 ± 301a | 1346 ± 579a,d | 1245 ± 307 a,d |
LVEF, left ventricular ejection fraction; FMD‐NO‐endothelium dependent NO mediated vasodilation; FMD‐NTG‐endothelium independent NTG mediated vasodilation; BNP, brain natriuretic peptide; Hs‐CRP, high sensitive‐C‐reactive protein; TNF‐α, tumor necrosis factor‐alpha; ICAM‐1, intercelular adhesion molecule‐1; VCAM‐1, vascular cell adhesion molecule‐1.
*Values are mean ± SD.
Statistically significant difference compared to controls (P < 0.05).
Statistically significant difference compared to NYHA I patients (P < 0.05).
Statistically significant difference compared to NYHA II patients (P < 0.05).
Statistically significant difference compared to NYHA III patients (P < 0.05).
Markers of Inflammation in Patients with Various Degrees of CHF
Results obtained in this study have shown that patients with NYHA I class, hs‐CRP, and TNF‐α concentrations did not significantly differ from values obtained in controls. However, in NYHA III/IV patients, hs‐CRP, and TNF‐α levels were significantly elevated in comparison with healthy subjects, with rise more pronounced in patients with most severe disease (Table 1). Concerning the relationship between markers of inflammation and myocardial dysfunction, only plasma hs‐CRP significantly correlated with LVEF (r = −0.189, P = 0.039) (data not shown). Correlation was also observed between hs‐CRP and endothelium‐dependent NO‐mediated vasodilation (r = −0.203, P = 0.026) (data not shown).
Regarding the adhesion molecules, levels of both ICAM‐1 and VCAM‐1 did not significantly differ in NYHA I patients when compared to values obtained in controls. VCAM‐1 levels were significantly higher in NYHA III/IV in comparison with NYHA I/II patients (P < 0.05 and P < 0.01, respectively). On the other hand, ICAM‐1 levels were only slightly elevated in NYHA II‐IV groups, with rise most pronounced in patients with the most severe stage of disease (Table 1). Further analysis showed significant correlations between VCAM‐1 and BNP (r = 0.191, P = 0.038), as well as, ICAM‐1 and endothelium‐dependent vasodilatation (r = −0.235, P = 0.01) (data not shown).
Prognostic Significance of Inflammatory and Adhesion Markers in CHF Patients
The prognostic significance of four inflammatory markers with respect to individual outcomes, specifically disease exacerbation and mortality in CHF patients was tested. During a median follow‐up of 13.1 months, 13 patients were hospitalized due to CHF exacerbation, while 11 patients died due to cardiac reasons. Statistical analysis showed significant correlations of VCAM‐1 with both repeated hospitalizations (r = 0.185; P < 0.04) and a total number of cardiovascular events (r = 0.22; P < 0.02) (data not shown). ROC analysis was performed to assess sensitivity and specificity of variables (TNF‐α, hs‐CRP, ICAM‐1, and VCAM‐1) in predicting individual outcomes.
Regarding CHF exacerbation, the significant area under the curve (AUC) of 0.743 (95% CI 0.63–0.86; P = 0.005) (area under the ROC curve) was obtained only for TNF‐α with optimal cut‐off value of 2.78 pg/ml, sensitivity of 84.6%, and specificity of 65%. Kaplan–Meier analysis has shown that exacerbation‐free survival was significantly shorter in patients with TNF‐α levels >2.78 pg/ml vs. those with TNF‐α levels less than 2.78 pg/ml (log‐rank test = 8.270, P = 0.004; Fig. 1). In the univariate Cox regression analysis, NYHA class, LVEF, and TNF‐α were associated with higher risk for disease exacerbation. However, only NYHA class and TNF‐α were recognized as predictors when these three variables were included in multivariate analysis (NYHA class: HR‐hazad ratio = 3.5; 95% CI: 1.8–6.8; P = 0.001 and TNF‐α: HR = 8.2; 95% CI: 1.1–23.0; P = 0.045) (Supporting Information Table S1).
Figure 1.
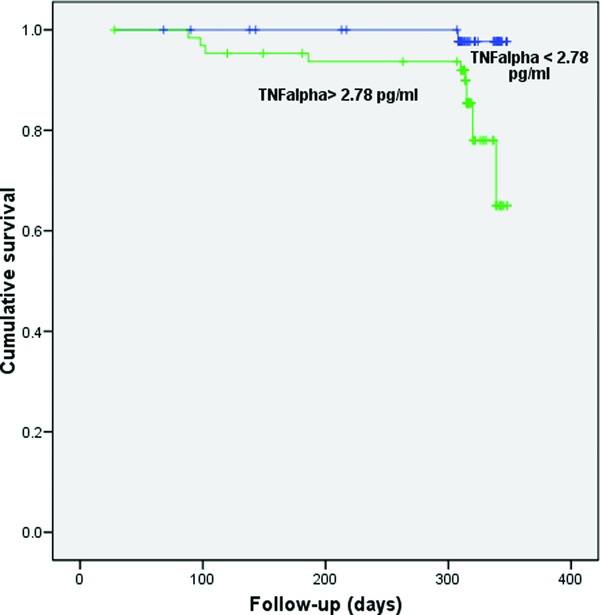
Kaplan–Meier survival plot for patients stratified by tumor necrosis factor‐alpha (TNF‐α) (cut‐off level 2.78 pg/ml) with respect to disease exacerbation.
Prognostic significance with respect to all‐cause mortality was obtained for hs‐CRP and VCAM‐1. ROC analysis has shown that optimal cut‐off value of hs‐CRP for predicting death was 4.76 mg/l (AUC = 0.695; sensitivity 62.5%, specificity 78.6%) and for VCAM‐1 was 1200 ng/l (AUC = 0.682; sensitivity 63.6%, specificity 65%) (data not shown). In Kaplan–Meier analysis, significantly shorter survival was demonstrated in patients with hs‐CRP levels greater than 4.76 mg/L in comparison with those with hs‐CRP levels less than 4.76 mg/l (log‐rank test = 5.052, P = 0.025; Fig. 2). Similar findings were also obtained for patients with VCAM‐1 levels greater than 1200 ng/l (log‐rank test = 5.45, P = 0.020; Fig. 3). When examined variables were included in the model for analysis of predictors of all‐cause mortality, NYHA class, hs‐CRP, and VCAM‐1 were recognized as significant in univariate analysis. However, Cox regression analysis demonstrated that only NYHA class (HR = 2.5; 95% CI: 1.2–5.5, P = 0.018) and VCAM‐1 (HR = 4.7; 95% CI: 1.1–18.7, P = 0.030) were independent predictors of death (Supporting Information Table S2).
Figure 2.
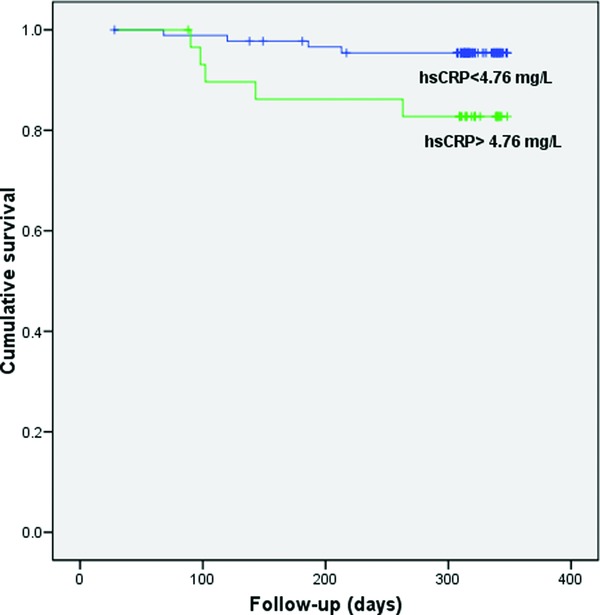
Kaplan–Meier survival plot for patients stratified by high sensitive C‐reactive protein (hs‐CRP) (cut‐off level 4.76 mg/l) with respect to all‐cause mortality.
Figure 3.
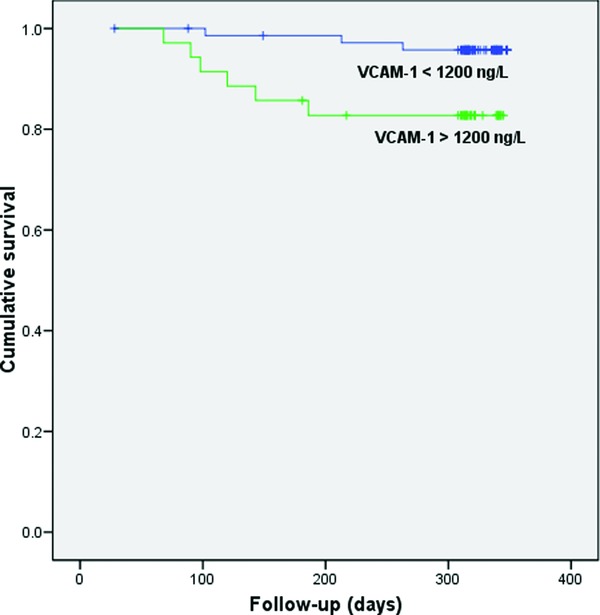
Kaplan–Meier survival plot for patients stratified by vascular cell adhesion molecule‐1 (VCAM‐1) (cut‐off level 1200 ng/l) with respect to all‐cause mortality.
DISCUSSION
Results obtained in this investigation showed that inflammatory markers and mediators may be related to the prognosis of CHF, with special emphasis on prognostic significance of plasma VCAM‐1. Thus, high plasma levels of VCAM‐1 were a significant predictor of mortality, while TNF‐α was the predictor of disease exacerbation in our cohort of stable CHF patients. Furthermore, association between adhesion molecules and parameters of both myocardial and endothelial dysfunction further implicate their important role in pathogenesis and progression of heart failure.
Until now, in CHF, only one study has shown VCAM‐1 to be a predictor of total number of adverse cardiac events in end‐stage disease, in small group of patients with LVEF less than 30% 20. Our data, for the first time, demonstrated that circulating VCAM‐1 gradually increases with the severity of CHF, negatively correlates with BNP, and provides important prognostic information. By using receiver operating characteristic analysis and defining cut‐off value, the data presented here indicated that VCAM‐1 makes a substantial contribution to risk prediction of death in relatively large cohort of medium‐risk CHF patients. It is important to point out the similar prognostic significance of plasma concentration of VCAM‐1 in other diseases characterized by endothelial dysfunction and chronic vascular inflammation. Thus, high VCAM‐1 levels were shown to be strong predictors of mortality in patients with coronary artery disease 16 as well as, predictor of cardiovascular mortality in patients with type 2 diabetes and systemic lupus erythematosus 21, 22. Vascular inflammation plays a critical role in angiotensin II induced remodeling of arteries. Mice deficient in macrophage colony‐stimulating factor (a monocyte chemotactic factor) exhibit reduced inflammation. When infused with angiotensin II, these animals show attenuated vascular remodeling (both media/lumen ratio and medial thickness) and VCAM‐1 expression in comparison with angiotensin II infused wild‐type controls 23.
Besides its well‐established role in inflammatory conditions, the role of VCAM‐1‐mediated signaling in endothelial cells has emerged relatively recently. The study of Cook‐Mills has shown that VCAM‐1 signaling is mediated by nicotinamide adenine dinucleotide phosphate (NADPH) oxidase production of reactive oxygen species and subsequent activation of matrix metalloproteinases, further implying molecular interplay between oxidative stress and inflammation in endothelial activation and remodelling 24. Important role in signaling was also shown for another adhesion molecule, ICAM‐1 in increasing endothelial activation and augmentation of atherosclerotic plaque formation 25. In that way, accelerated inflammation, vascular dysfunction and plaque growth interact and stimulate each other. Finally, both increased activity and redox perturbations of these cellular networks could determine the course of the underlying vascular disease. Our results on significant association of ICAM‐1 and endothelium NO‐dependent vasodilation, support this assumption. Besides activated endothelium, it seems that high plasma ICAM‐1 levels in CHF patients, observed in our and several other studies, can also reflect increased expression on the failing myocardium itself 2, 9. However, results of studies that investigated the use of ICAM‐1 as a biomarker of cardiovascular disease prognosis are contradictory 16, 17. In contrast to our and several other studies, only the investigation of Tsutamoto et al. reported relationship of soluble ICAM‐1 with clinical outcomes in CHF 2, 18, 19.
Among proinflammatory cytokines, TNF‐α is the cytokine that has been studied in greatest detail in CHF. In clinical trials of heart failure with severe systolic dysfunction, elevated TNF‐α was associated with increased mortality 6, 7. Moreover, recent data of Dunley et al. showed TNF‐α as useful biomarker for risk assessment in heart failure patients with both preserved and reduced ejection fraction 8. However, attempts to use TNF‐α as a therapeutic target in patients with severe systolic dysfunction have resulted in an increase in all‐cause mortality 26, 27, 28, further implicated their complex role in the inflammatory processes. Investigating association between TNF‐α plasma levels and individual outcomes, we found prognostic significance of increased TNF‐α for heart failure exacerbation, but not for death. Specifically, the best cut‐off value of TNF‐α for predicting disease exacerbation was 2.78 pg/ml as determined by using receiver operating characteristic analysis. These results are biologically plausible in the light of the role that TNF‐α has in the progression of vascular disease by increasing oxidative stress, downregulating endothelial nitric oxide synthase bioactivity and inducing endothelial cell apoptosis, as well as, myocardial dysfunction in the course of CHF. Discrepancy between our and study of Dunlay et al. 8 may be due to shorter follow‐up period in our study and consequent smaller prevalence of mortality. However, in contrast to Dunlay et al. 8, we excluded patients with severe comorbidity, as well as with the presence of infection or inflammatory diseases in order to avoid the possible effects of these conditions on TNF‐α production.
Finally, our results suggested that C‐reactive protein might be a good predictor of cardiac death and are in accordance with pervious data 29, 30, 31. Moreover, we found significant relationship of hs‐CRP with both LVEF and endothelium NO‐dependent vasodilation. Indeed, it has been shown that hs‐CRP exerts direct adverse effects on the vascular endothelium by reducing NO release and increasing endothelin‐1 production, as well as by inducing expression of endothelial adhesion molecules 32. Moreover, CRP has been evaluated in atherosclerotic plaques and appears to be involved in foam cell formation, promotes monocyte chemotaxis and facilitates LDL (low‐density lipoprotein) uptake by macrophages in vitro 33, 34. These findings suggest that C‐reactive protein may be more than an inflammatory marker of increased cardiovascular risk, playing a causal role in vascular disease and could therefore be a target of therapy. However, elevated levels of C‐reactive protein lack specificity, since acute and chronic infection, cigarette smoking, acute coronary syndromes, and active inflammatory states frequently affect C‐reactive protein concentrations 2.
The data presented here indicated that VCAM‐1 makes a substantial contribution to risk prediction of death over established indicators. We suggest that VCAM‐1 level may be a useful parameter for both monitoring and planning management of CHF patients, especially in the stable CHF patients. Thus, it may be useful in targeting medium‐risk patients who could benefit from aggressive cardiovascular preventive therapy. Still, the question arises whether VCAM‐1 could be used in routine evaluation of CHF patients, since methods used for determination of inflammatory markers were not standardized and cross‐sectional comparative studies cannot be optimally conducted. However, based on novel antigen‐recognition imaging modalities for diagnosing regions of vascular inflammation and atherosclerosis 35, it is plausible that the profile of changes in inflammatory biomarkers might help to identify the specific inflammatory disturbances in any given patient and thereby might aid the selection of appropriate therapy. Moreover, in the future, small molecule compounds, drug therapies, and gene therapy can be delivered to inflamed blood vessels using VCAM‐1 molecular‐targeted microbubble/contrast agent carriers.
DISCLOSURE
The author(s) declare that they have no competing interests, including specific financial interests and relationships and affiliations relevant to the subject of this manuscript.
Supporting information
Disclaimer: Supplementary materials have been peer‐reviewed but not copyedited.
Table 1. Univariate and Multivariate Predictors of CHF Exacerbations over 13.1 months follow‐Up Period HR, hazard ratio from stepwise COX regression analysis; CI, confidence interval; NYHA New York Heart association functional class; BNP‐ brain natriuretic peptide; EF‐ ejection fraction; FMD‐NO‐endothelium dependent NO mediated vasodilation; FMD‐NTG‐endothelium independent NTG mediated vasodilation; Hs‐CRP‐ high sensitive‐C‐reactive protein; TNFα‐tumor necrosis factor‐alpha; ICAM‐1‐intercelular adhesion molecule‐1; VCAM‐1, vascular cell adhesion molecule‐1; *according to EF all patients were divided in three groups: EF 45–35% (group I), EF 35–25% (group II) and EF <25% (group III) Variables with P < 0.05 on univariate analysis are shown and were included in the multivariable model
Table 2. Univariate and Multivariate Predictors of All‐Cause Mortality over 13.1 months Follow‐Up period HR, hazard ratio from stepwise COX regression analysis; CI, confidence interval; NYHA– New York Heart association functional class; BNP‐ brain natriuretic peptide; EF‐ ejection fraction; FMD‐NO‐endothelium dependent NO mediated vasodilation; FMD‐NTG‐endothelium independent NTG mediated vasodilation; Hs‐CRP‐ high sensitive‐C‐reactive protein; TNFα‐tumor necrosis factor‐alpha; ICAM‐1‐intercelular adhesion molecule‐1; VCAM‐1‐vascular cell adhesion molecule‐1; *according to EF all patients were divided in three groups: EF 45–35% (group I), EF 35–25% (group II) and EF <25% (group III) Variables with P < 0.05 on univariate analysis are shown and were included in the multivariable model
ACKNOWLEDGMENTS
This work was supported by a Grant 175052 from the Serbian Ministry of Science and Education.
Grant sponsor: the Serbian Ministry of Science and Education; Grant number 175052.
REFERENCES
- 1. Anker SD, von Haehling S. Inflamatory mediators in chronic heart failure: An overview. Heart 2004;90:464–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Braunwald E. Biomarkers in heart failure. N Engl J Med 2008;358:2148–2159. [DOI] [PubMed] [Google Scholar]
- 3. Seta Y, Shan K, Bozkurt B, Oral H, Mann DL. Basic mechanisms in heart failure: The cytokine hypothesis. J Card Fail 1996;2:243–249. [DOI] [PubMed] [Google Scholar]
- 4. Kofler S, Nickel T, Weis M. Role of cytokines in cardiovascular diseases: A focus on endothelial responses to inflammation. Clin Sci 2005;108:205–213. [DOI] [PubMed] [Google Scholar]
- 5. Von Haehling S, Jankowska EA, Anker SD. Tumour necrosis factor‐alpha and the failing heart: Pathophysiology and therapeutic implications. Basic Res Cardiol 2004;99:18–28. [DOI] [PubMed] [Google Scholar]
- 6. Deswal A, Peterson NJ, Feldman AM, Young JB, White BG, Mann DL. Cytokines and cytokine receptors in advanced heart failure: An analysis of the cytokine database from the Vesnarinone Trial (VEST). Circulation 2001;103:2055–2059. [DOI] [PubMed] [Google Scholar]
- 7. Rodriguez‐Reyna TS, Arrieta O, Castillo‐Martinez L, Guevara P, Rebollar V, Granados J. Tumour necrosis factor alpha and troponin T as predictors of poor prognosis in patients with stable heart failure. Clin Invest Med 2005;28:23–29. [PubMed] [Google Scholar]
- 8. Dunlay SM, Weston SA, Redfield MM, Killian JM, Roger VL. Tumor necrosis factor‐alpha and mortality in heart failure: A community study. Circulation 2008;118:625–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Devaux B, Scholz D, Hirche A, Klövekorn WP, Schaper J. Upregulation of cell adhesion molecules and the presence of low grade inflammation in human chronic heart failure. Eur Heart J 1997;18:470–479. [DOI] [PubMed] [Google Scholar]
- 10. Madge LA, Pober JS. TNF signaling in vascular endothelial cells. Exp Mol Pathol 2001;70:317–325. [DOI] [PubMed] [Google Scholar]
- 11. True AL, Rahman A, Malik AB. Activation of NF‐κB induced by H2O2 and TNF‐α and its effects on ICAM‐1 expression in endothelial cells. Am J Physiol Lung Cell Mol Physiol 2000;279:302–311. [DOI] [PubMed] [Google Scholar]
- 12. Muller WA. Mechanisms of transendothelial migration of leukocytes. Circ Res 2009;105:223–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Andreassen AK, Nordøy I, Simonsen S, et al. Levels of circulating adhesion molecules in congestive heart failure and after heart transplantation. Am J Cardiol 1998;81:604–608. [DOI] [PubMed] [Google Scholar]
- 14. Tousoulis D, Homaei H, Ahmed N, et al. Increased plasma adhesion molecule levels in patients with heart failure who have ischemic heart disease and dilated cardiomyopathy. Am Heart J 2001;141:277–280. [DOI] [PubMed] [Google Scholar]
- 15. Blankenberg S, Rupprecht HJ, Bickel C, et al. Circulating cell adhesion molecules and death in patients with coronary artery disease. Circulation 2001;104:1336–1342. [DOI] [PubMed] [Google Scholar]
- 16. Pradhan AD, Rifai N, Ridker PM. Soluble intercellular adhesion molecule‐1, soluble vascular adhesion molecule‐1, and the development of symptomatic peripheral arterial disease in men. Circulation 2002;106:820–825. [DOI] [PubMed] [Google Scholar]
- 17. Ridker PM, Hennekens CH, Roitman‐Johnson B, Stampfer MJ, Allen J. Plasma concentration of soluble intercellular adhesion molecule 1 and risks of future myocardial infarction in apparently healthy men. Lancet 1998;351:88–92. [DOI] [PubMed] [Google Scholar]
- 18. Tsutamoto T, Hisanaga T, Fukai D, et al. Prognostic value of plasma soluble intercellular adhesion molecule‐1 and endothelin‐1 concentration in patients with chronic congestive heart failure. Am J Cardiol 1995;76:803–808. [DOI] [PubMed] [Google Scholar]
- 19. Yin WH, Chen JW, Jen HL, et al. The prognostic value of circulating soluble cell adhesion molecules in patients with chronic congestive heart failure. Eur J Heart Fail 2003;5:507–516. [DOI] [PubMed] [Google Scholar]
- 20. Chiang MC, Yin WH, Jen HL, et al. Vascular cell adhesion molecule‐1 as a prognostic marker in patients with end‐stage heart failure. Acta Cardiol Sin 2004;20:94–104. [Google Scholar]
- 21. Jager A, van Hinsbergh VW, Kostense PJ, et al. Increased levels of soluble vascular cell adhesion molecule 1 are associated with risk of cardiovascular mortality in type 2 diabetes: the Hoorn study. Diabetes 2000;49:485–491. [DOI] [PubMed] [Google Scholar]
- 22. Gustafsson JT, Simard JF, Gunnarsson I, et al. Risk factors for cardiovascular mortality in patients with systemic lupus erythematosus, a prospective cohort study. Arthritis Res Ther 2012;14(2):R46 (1–11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. De Ciuceis C, Amiri F, Brassard P, Endemann DH, Touyz RM, Schiffrin EL. Reduced vascular remodeling, endothelial dysfunction, and oxidative stress in resistance arteries of angiotensin II‐infused macrophage colony‐stimulating factor‐deficient mice: evidence for a role in inflammation in angiotensin‐induced vascular injury. Arterioscler Thromb Vasc Biol 2005;25:2106–2113. [DOI] [PubMed] [Google Scholar]
- 24. Cook‐Mills JM. VCAM‐1 signals during lymphocyte migration: Role of reactive oxygen species. Mol Immunol 2002;39:499–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lawson C, Wolf S. ICAM‐1 signaling in endothelial cells. Pharmacol Rep 2009;61:22–32. [DOI] [PubMed] [Google Scholar]
- 26. Chung ES, Packer M, Lo KH, Fasanmade AA, Wilkerson JT. Randomized, double‐blind, placebo‐controlled, pilot trial of infliximab, a chimeric monoclonal antibody to tumor necrosis factor‐α, in patients with moderate‐to‐severe heart failure. Circulation 2003;107:3133–3140. [DOI] [PubMed] [Google Scholar]
- 27. Mann DL, McMurray JJ, Packer M, et al. Targeted anticytokine therapy in patients with chronic heart failure: Results of the Randomized Etanercept Worldwide Evaluation (RENEWAL). Circulation 2004;109:1594–1602. [DOI] [PubMed] [Google Scholar]
- 28. Anker SD, Coats AJ. How to recover from renaissance? The significance of the results from recover, renaissance, renewal and attach. Int J Cardiol 2002;86:123–130. [DOI] [PubMed] [Google Scholar]
- 29. Kaneko K, Kanda T, Yamauchi Y, et al. C‐reactive protein in dilated cardiomyopathy. Cardiology 1999;91:215–219. [DOI] [PubMed] [Google Scholar]
- 30. Anand IS, Latini R, Florea VG, et al. C‐reactive protein in heart failure: Prognostic value and the effect of valsartan. Circulation 2005;112:1428–1434. [DOI] [PubMed] [Google Scholar]
- 31. Yin WH, Chen JW, Jen HL, et al. Independent prognostic value of elevated high‐sensitivity C‐reactive protein in chronic heart failure. Am Heart J 2004;147:931–938. [DOI] [PubMed] [Google Scholar]
- 32. Venugopal SK, Deveraj S, Jialal I. Effect of C‐reactive protein on vascular cells: Evidence for a proinflammatory, proatherogenic role. Curr Opin Nephrol Hypertens 2005;14:33–37. [DOI] [PubMed] [Google Scholar]
- 33. Torzewski M, Rist C, Mortensen RF, et al. C‐reactive protein in the arterial intima: Role of C‐reactive protein receptor‐dependent monocyte recruitment in atherogenesis. Arterioscler Thromb Vasc Biol 2000;20:2094–2099. [DOI] [PubMed] [Google Scholar]
- 34. Zwaka TP, Hombach V, Torzewski J. C‐reactive protein mediated low density lipoprotein uptake by macrophages: Implications for atherosclerosis. Circulation 2001;103:1194–1197. [DOI] [PubMed] [Google Scholar]
- 35. Nahrendorf M, Jaffer FA, Kelly KA, et al. Noninvasive vascular cell adhesion molecule‐1 imaging identifies inflammatory activation of cells in atherosclerosis. Circulation 2006;114:1504–1511. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Disclaimer: Supplementary materials have been peer‐reviewed but not copyedited.
Table 1. Univariate and Multivariate Predictors of CHF Exacerbations over 13.1 months follow‐Up Period HR, hazard ratio from stepwise COX regression analysis; CI, confidence interval; NYHA New York Heart association functional class; BNP‐ brain natriuretic peptide; EF‐ ejection fraction; FMD‐NO‐endothelium dependent NO mediated vasodilation; FMD‐NTG‐endothelium independent NTG mediated vasodilation; Hs‐CRP‐ high sensitive‐C‐reactive protein; TNFα‐tumor necrosis factor‐alpha; ICAM‐1‐intercelular adhesion molecule‐1; VCAM‐1, vascular cell adhesion molecule‐1; *according to EF all patients were divided in three groups: EF 45–35% (group I), EF 35–25% (group II) and EF <25% (group III) Variables with P < 0.05 on univariate analysis are shown and were included in the multivariable model
Table 2. Univariate and Multivariate Predictors of All‐Cause Mortality over 13.1 months Follow‐Up period HR, hazard ratio from stepwise COX regression analysis; CI, confidence interval; NYHA– New York Heart association functional class; BNP‐ brain natriuretic peptide; EF‐ ejection fraction; FMD‐NO‐endothelium dependent NO mediated vasodilation; FMD‐NTG‐endothelium independent NTG mediated vasodilation; Hs‐CRP‐ high sensitive‐C‐reactive protein; TNFα‐tumor necrosis factor‐alpha; ICAM‐1‐intercelular adhesion molecule‐1; VCAM‐1‐vascular cell adhesion molecule‐1; *according to EF all patients were divided in three groups: EF 45–35% (group I), EF 35–25% (group II) and EF <25% (group III) Variables with P < 0.05 on univariate analysis are shown and were included in the multivariable model


