Abstract
Measles is the leading cause of death in infants, although a vaccine is available for its prevention. At this stage of measles elimination and eradication, it is so important to confirm clinically diagnosed measles cases in the laboratory but, developing countries have troubles in collecting and maintaining the cold chain of the specimens while transporting them to the laboratories. Therefore, filter papers are good candidates for simplification of specimen collection and transportation. In this research, the effects of the temperature, at which the dried specimens were kept, and the time duration the dried specimens were kept before being tested, were studied.
Since there were not enough patients’ oral fluid samples available, a nested reverse transcriptase PCR (RT‐PCR) that detected measles virus (MV) from dried filter papers was set up using MV infected cells diluted in sterile phosphate‐buffered saline (PBS). Dried specimens were stored at −25°C, 4°C, and room temperature for 1 day, 1, 2, and 3 weeks before being tested. This method was then applied to filter paper oral fluids collected from nine clinically diagnosed measles patients in Iran in 2010 which were tested after being kept at room temperature for 1 day, 1 and 3 weeks after preparation.
The results showed that dried oral fluids on filter papers are reliable specimens for the detection of MV RNA using nested RT‐PCR, but the nested RT‐PCR results of low titer viruses dried onto filter papers are not reproducible and reliable. J. Clin. Lab. Anal. 26:215‐222, 2012. © 2012 Wiley Periodicals, Inc.
Keywords: measles, RT‐PCR, filter paper, oral fluids
INTRODUCTION
Measles is a highly contagious viral disease characterized by high fever, coryza, cough, and conjunctivitis, followed by the appearance of a maculopapular rash, and is a leading cause of vaccine‐preventable deaths among young children 1.
Live attenuated measles virus (MV) vaccines have been used to control measles in the industrialized world, and the MV transmission has been interrupted in some countries including Australia 2. The Americas set a goal in 1994 to eliminate measles and rubella transmission by 2000, and successfully achieved regional measles elimination in 2002, although there have been occasional small outbreaks from imported cases since then 3. Still vaccination has been less successful in developing countries. This can be the result of a combination of problems such as insufficient vaccination coverage, problems related to cold chain maintenance, civil wars, and safety issues related to the AIDS pandemic 4, 5, 6.
To this date, measles remains a common disease in many parts of the world. An estimated 10 million cases and 164,000 deaths from measles occur worldwide each year 7. As the level of disease control increases, the role of the laboratory in measles surveillance becomes more and more important 8. Recognition of measles cases is based on clinical case definition 9; however, clinical diagnosis is less accurate during the elimination phase 10 and laboratory confirmation is necessary for effective surveillance. The “gold standard” for laboratory diagnosis of measles is the detection of specific serum immunoglobulin M (IgM) antibodies 11. However, these antibodies may be very low or even absent in patients sampled in an early stage of infection or in immunocompromised patients 5. Seroepidemiological studies, conducted before and after mass vaccinations, can be used for monitoring the effectiveness of vaccination programs 12. Studies have shown the usefulness of reverse transcriptase PCR (RT‐PCR) analysis as an additional tool in the diagnosis of these patients 13.
One of the main problems in the expansion of measles surveillance is that many parts of the world lack the fundamentals for appropriate collection, processing, storage, and shipment of obtained specimens. The development of a simplified procedure and the ability to store and transport specimens at ambient temperature would allow the expansion of measles surveillance to the most remote areas 14.
Previous studies have shown that oral fluid is a rich source of MV, and that the virus can be cultured from the mucus membranes of the nasopharynx, conjunctivae, and mouth of an infected individual a few days before the onset of rash, suggesting that the respiratory tract is the site of virus release 15, 16. Accordingly, oral fluid has been used successfully for the detection of MV‐specific antibodies 17, 18 and also for the detection of MVs by RT‐PCR and their subsequent genetic characterization 16, 19, 20, 21. A major advantage of oral fluid dried onto filter paper is that it provides a new type of specimen transportation that is simple, convenient, and inexpensive. In addition, collecting oral fluids is less invasive compared to collecting blood samples 22. Oral fluid has a minimal risk of transmitting blood‐borne diseases 23. It has also been shown that blood is a less‐optimal specimen for the detection of MV RNA by RT‐PCR compared to samples collected from the respiratory tract 15, 22, where MV RNA remains detectable for up to 2 weeks after the onset of rash 24, and the use of oral fluid sampling can extend the opportunity for RNA detection after rash onset 23.
The technique of collecting specimens (whole blood or oral fluids) onto filter paper for the detection of antibody or viral nucleic acids has already been reported 5, 14, 15, 23, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35).
The purpose of this research was to first, set up a nested RT‐PCR with the highest possible sensitivity for the detection of low titers of MV from specimens dried onto filter papers, and to evaluate the effect of time and temperature on the results, and to eventually use the tested method for the detection of MV using oral fluids dried onto filter papers to overcome the problem of specimen collection and transportation that needs cold chain maintenance. The study was designed to be conducted with dilutions of MV due to lack of positive oral fluid specimens, but a measles outbreak happened during the study, and nine oral fluids were available for further research.
MATERIALS AND METHODS
Virus
The virus used in this study was isolated from a throat swab specimen sent to the national measles laboratory in 2009. The patient was positive for both MV‐specific IgM in serum, and MV‐specific RT‐PCR in throat swab [using Center for Disease Control and Prevention (CDC) MeV 214 and MeV 216 primers]. The specimen was cultured on Vero‐SLAM cells 36, and passaged few times to increase the amount of virus. In order to do so, 10 ml of ready‐to‐use DMEM (Gibco BRL Life Technologies, Gaithersburg, MD) plus 10% fetal bovine serum (Gibco BRL Life Technologies, Gaithersburg, MD) and 200 μg/ml Streptomycin, 200 unit/ml Penicillin, and 3 μg/ml Fungizone were added to a 75 cm3 flask. The flask was kept in a 37°C incubator with 5% CO2 until the cells confluency was 70–80%. The cells were washed with sterile PBS twice to remove the serum and 400 μl of the virus was added to the flask. The flask was then left in the 37°C incubator for 1 hr. After an hour, 10 ml of DMEM, 200 μg/ml Streptomycin, 200 unit/ml Penicillin, and 3 μg/ml Fungizone were added to the flask, and the flask was kept in the 37°C incubator. After 2 days, the specific cytopathic effects (CPE) of MV were observed in the flask, and it was confirmed that the virus was measles using RT‐PCR. The virus was aliquoted into 1.5 ml micro tubes and kept in −70°C freezer until being used.
Virus Titration/TCID50
In order to determine the titer of the virus, a TCID50 test was conducted in two 48‐well plates. Dilutions of 10−1 to 10−12 were prepared from the virus stock using sterile PBS. Six wells were used for each dilution. At first, each well was filled with 1 ml DMEM plus 200 μg/ml Streptomycin, 200 unit/ml Penicillin, and 3 μg/ml Fungizone. A total of 10,000 Vero/SLAM cells/well were added to the plates, and the plates were kept in a 37°C incubator for 24 hr. Then, the wells were washed once with sterile PBS and 200 μl of the viral dilutions were added to each well. The plates were then kept in the 37°C incubator with 5% CO2 for 40 min for the viruses to attach to the cells. At the end, 1,800 μl of DMEM was added to each well and the plates were left in the incubator for a week. Clean filter tips were used for every single dilution step. The plates were checked every day for 7 days for measles‐specific CPE. The titration test was repeated 3 times to make sure that the results were accurate.
Invert log 0.4 = 2.5 → 2 × 109 viral particles/200 μl (since 200 μl of the virus was used in each well) → 109 viral particles/100 μl.
Preparation of Virus Dilutions on Filter Paper
Three dilutions (10−5, 10−6, and 10−7) were chosen based on the TCID50 and nested RT‐PCR results (the three dilutions with the lowest virus titers, which were positive using RT‐PCR after drying them onto filter papers). The dilutions were made using a new stock of virus from the −70°C freezer and sterile PBS. Whatman S&S # 903 papers were used. The papers were left under ultraviolet (UV) light for 40 min before drying the viruses on them. For each dilution 24 dried spots were prepared. For each sample 100 μl of the diluted virus was loaded on a circle of the paper. The papers were divided into three groups, and kept at −25°C, 4°C, and room temperature (25°C), and nested RT‐PCR was conducted on them after 1 day, 1, 2, and 3 weeks. For each dilution, and temperature and time condition, two exact same samples were tested. The filter papers that were supposed to be kept at room temperature were stored open to the atmosphere, but the papers that were kept in −25°C, and 4°C were stored in sealed paper envelopes.
Preparation of Oral Fluids on Filter Paper
Nine oral fluid samples that were proven positive using serology (ELISA) and RT‐PCR were chosen from the specimens sent to the national measles laboratory for the research. These specimens were collected using Oracol swabs (M.M. Malvern Medical Development Limited, Saliva Collection System, Worcester, United Kingdom), and after being centrifuged, they were kept in the −70°C freezer until the day of use. Whatman S&S # 903 papers were used. The papers were left under UV for 40 min before drying the oral fluids on them. Three circles were used for each patient's sample and 100 μl of the oral fluid was dried on each circle. The papers were kept at room temperature, and open to the atmosphere, and were tested after 1 day, 1 and 3 weeks.
Extraction of RNA from Filter Paper
Total RNA was extracted from viral dilutions and oral fluids dried onto filter papers using QIAamp Viral RNA Mini Kit (QIAGEN GMBH, Germany) according to the manufacturer's guideline with minor modifications (CDC Inter‐country laboratory training workshop on MV
____________________________________________________________________________________
detection and genotyping handbook, Tunisia, 19–23 April, 2010). Briefly, a circle of the dried specimen was excised from the filter paper using a sterile scissor. The circle was cut into four pieces and placed in a 1.5‐ml Eppendorf tube. An amount of 560 μl of prepared viral lysis buffer (AVL) containing carrier RNA from the kit, and 150 μl of sterile 1 × PBS was added to the tube. The tube was pulse‐vortexed for 15 sec and then incubated at room temperature for 10 min. Following incubation, the sample was spun at 10,391.81 g‐value (13,000 rpm) for 2 min. An amount of 600 μl of the liquid was removed to a new tube, and 600 μl of ethanol was added to it. The tube was pulse‐vortexed for 15 sec, and further extraction steps were carried out according Step 5 to the manufacturer's guidelines, below.
Sensitivity of Nested RT‐PCR
The positive control used for the nested RT‐PCR was a synthetic RNA of a measles N‐gene with a 220 base insert in the 3′ variable region (MeV‐N3in). The positive control was sent to us by CDC. Concentration of the RNA was 1 × 109 copies RNA/μl. Dilutions of the positive control were made (10−1 to 10−9) using distilled water. The 10−9 dilution had only 1 copy RNA/μl. RT‐PCR was then conducted on these dilutions to detect the sensitivity of the RT‐PCR. Extraction was done using QIAamp Viral RNA Mini Kit (QIAGEN GMBH, Germany), and RT‐PCR was done using QIAGEN OneStep RT‐PCR kit (GIAGEN GMBH, Germany), and MeV 214 and MeV 216 primers were used (CDC recommended primers).
Nested RT‐PCR
The nested RT‐PCR reactions were performed using the QIAGEN OneStep RT‐PCR kit (QIAGEN GMBH, Germany). The primers used in the first round were MeV 216, 5′‐ TGG AGC TAT GCC ATG GGA GT‐3′ (forward primer), and MeV 214, 5′‐TAA CAA TGA TGG AGG GTA GG‐3′ (reverse primer) (CDC Inter‐country laboratory training workshop on MV detection and genotyping handbook, Tunisia, 19–23 April, 2010). The second round primers were MV61, 5′‐ CTT GTT TCA GAG ATT GCA ATG CAT‐3′ (forward primer), and MV63, 5′‐CTG GCC CTC GGC CTC TCG CAC‐3′ (reverse primer) (All four primers used in this study have been recommended by CDC). Reactions in both rounds were carried out in 50 μl volume according to the manufacturer's recommendations. PCR cycling conditions for the first round were the same as recommended by the manufacturer. Cycling conditions of the second round were 94°C for 10 min followed by 30 cycles of 94°C for 30 sec, 55°C for 35 sec, and 72°C for 55 sec, and a final extension at 72°C for 10 min. Ten microliters of the extracted RNA was used in the first round, and 5 μl of the first round product was used for the second round. PCR products were visualized by agarose gel electrophoresis using 10 μl of the nested reaction product and ethidium bromide staining.
RESULTS
TCID50 Results and Calculation
According to the TCID50 results and based on the Reed–Muench method, the titer of the initial virus stock was 109/100 μl (TCID50 = 10−9.4). So, the dilutions 10−5, 10−6, and 10−7 used in the research, each contain 10,000, 1,000, and 100 infectious units, respectively.
PCR Sensitivity
Dilutions 10−1 to 10−9 of the control RNA were used to determine the sensitivity of the RT‐PCR used in the research. The PCR result of all the dilutions was positive in only one round (not nested) using 5 μl of the dilutions. This means the PCR was able to detect five copy RNAs/μl with only one round (Fig. 1). Dilutions 10−1 to 10−9 of dried specimens of Vero/SLAM cells infected with MV were tested using the nested RT‐PCR, and the sensitivity of the nested RT‐PCR was 100 infectious units/filter paper.
Figure 1.
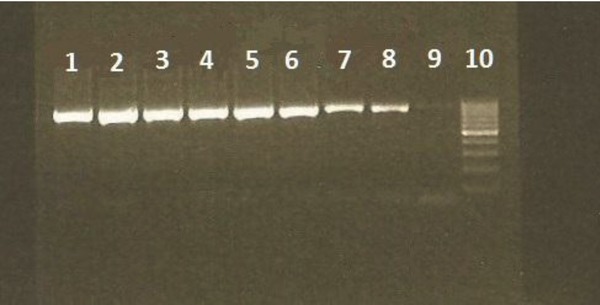
PCR results of the positive control dilutions. Lane 1–9: 10−1 to 10−9 dilutions of the positive control, respectively, and lane 10: ladder (the negative control was in the lower part of the gel in a different row, which can not be seen in this picture).
Dried Diluted Viruses Nested RT‐PCR Results
Seventy‐two diluted specimens of three viral dilutions were dried onto filter papers. The specimens were divided into three groups and kept at −25°C, 4°C, and room temperature (eight samples of each dilution were kept in each temperature). Two samples of each dilution were then tested with nested RT‐PCR after 1 day, 1, 2, and 3 weeks (Fig. 2). Thirty‐five of the 72 samples were positive. Table 1 shows details of the results of the nested RT‐PCR on the 72 specimens.
Figure 2.
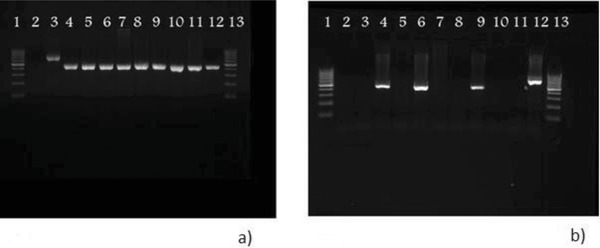
Comparison of dried viral dilutions PCR results with different time durations (a) after a day, and (b) after 3 weeks. (a) lane 1: ladder, lane 2: negative control, lane 3: positive control, lanes 4–6: 10−7 dilutions kept at −25°C, 4°C and room temperature (25°C), respectively, lanes 7–9: 10−6 dilutions kept at −25°C, 4°C, and room temperature (25°C), respectively, lanes 10–12: 10−5 dilutions kept at −25°C, 4°C, and room temperature (25°C), respectively, and lane 13: ladder. (b) lane 1: ladder, lanes 2–4: 10−5 dilutions kept at room temperature, 4°C and −25°C, respectively, lanes 5–7: 10−6 dilutions kept at room temperature, 4°C, and −25°C, respectively, lanes 8–10: 10−7 dilutions kept at room temperature, 4°C, and −25°C, respectively, lane 11: negative control, lane 12: positive control, and lane 13: ladder.
Table 1.
Measles Virus Detection by Nested RT‐PCR on Viruses Dried on Filter Paper
| Virus dilution | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 10−5 | 10−6 | 10−7 | ||||||||||
| 1 day | 1 week | 2 weeks | 3 weeks | 1 day | 1 week | 2 weeks | 3 weeks | 1 day | 1 week | 2 weeks | 3 weeks | |
| Room temp. | + | + | + | − | + | − | − | − | + | − | − | − |
| (25°C) | + | − | − | − | + | − | − | − | + | − | − | − |
| 4°C | + | + | + | − | + | − | − | + | + | + | − | + |
| + | + | − | − | − | − | − | − | + | − | − | − | |
| −25°C | + | + | + | + | + | + | + | + | + | − | + | − |
| + | + | + | − | − | − | − | + | + | − | + | − | |
Two filter papers for each condition.
Dried Oral Fluids Nested RT‐PCR
Nine oral fluids from patients with positive measles, sent to the national measles laboratory, were tested. Three samples were tested for each patient. The samples were all kept at room temperature, but were tested using RT‐PCR after a day, 1 and 3 weeks (Fig. 3). All 27 samples were positive. Table 2 shows details of the results of the nested RT‐PCR on the 27 dried oral fluids.
Figure 3.
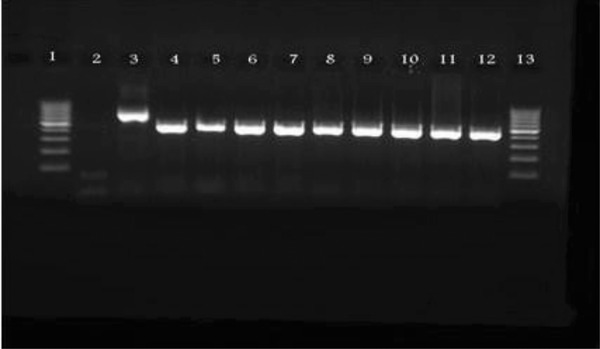
PCR results of dried oral fluids kept at room temperature for 3 weeks (lane 1: ladder, lane 2: negative control, lane 3: positive control, lane 4: specimen 1, lane 5: specimen 2, lane 6: specimen 3, lane 7: specimen 4, lane 8: specimen 5, lane 9: specimen 6, lane 10: specimen 7, lane 11: specimen 8, lane 12: specimen 9, and lane 13: ladder).
Table 2.
Nested RT‐PCR Results of Dried Oral Fluids
| Nested RT‐PCR results | |||
|---|---|---|---|
| Sample number | 1 day | 1 week | 3 weeks |
| 1 | + | + | + |
| 2 | + | + | + |
| 3 | + | + | + |
| 4 | + | + | + |
| 5 | + | + | + |
| 6 | + | + | + |
| 7 | + | + | + |
| 8 | + | + | + |
| 9 | + | + | + |
All nine oral fluid samples were partially sequenced, and the genotype of all the samples was D4. (The sequences are available at NCBI. GenBank HQ395685.1, HQ596509.1, HQ596510.1, HQ596512.1, HQ596514.1, HQ596515.1, HQ668021.1, HQ711619.1, and HQ668022.1.) D4 is the circulating genotype of MV in Iran, and only sporadic cases of D8 and H1 have been reported. (Available on NCBI website—submitted by Virology Department, School of Public Health, Tehran University of Medical Sciences, Tehran, Iran, which is the National Measles Laboratory in Iran.)
DISCUSSION
RT‐PCR has been used to detect MV RNA from a variety of clinical specimens 13, 24, 37, 38, 39, 40. The use of blood samples dried onto filter paper was then introduced for the detection of MV, being useful both for specific IgM 5, 32, 41, and PCR 5, 14, 33. Measles‐specific IgM can be detected from dried blood spots with lower sensitivity and specificity than with serum, 95% and 96%, respectively 41) findings from the Netherlands show that measles‐nucleic acid stays stable in dried blood long enough to allow detection of MV by PCR, even after storage for 25 weeks at 25°C 5. However, several groups have shown that blood has some drawbacks for the detection of MV RNA. The virus in serum stays detectable by RT‐PCR for a shorter period compared to respiratory secretions, and this is influenced by the MV‐specific IgG response 24. Similarly, a study of dried blood samples on filter papers showed that the number of MV positives obtained by RT‐PCR was indirectly proportional to the presence of MV‐specific IgM 5. Another study has shown that blood is a less‐optimal specimen for the detection of MV using RT‐PCR compared to respiratory secretions, which have detectable amounts of MV RNA for up to 2 weeks after rash onset 24.
Oral fluid has two major advantages. First, the sampling method is noninvasive, and avoids handling of blood that has safety and waste disposal problems. Filter paper blood spots have the advantage of easy sample collection by heel or finger prick, which requires less medical training and is usually more acceptable for both parents and infants than venepuncture 42. However, the most important advantage of filter paper blood sample is the ease of storage and transport. Oral fluids require a cold chain, but filter paper blood spots can be stored at ambient temperature and shipped by regular mail 5. Being able to use dried oral fluids on filter papers, makes oral fluids the most suitable specimen for the detection of MV using PCR. A study has already shown that oral fluids collected onto filter papers are suitable specimens for the detection of MV RNA by RT‐PCR and subsequent genotyping and phylogenetic analysis of the virus 15.
Since none of the studies conducted before have tried to determine the lowest titers of the virus that can be detected from filter papers using a nested RT‐PCR, the purpose of this study was to first, set up an RT‐PCR that would detect measles RNA from low‐titer specimens dried on filter papers, to evaluate the effect of time and temperature on the results, and to subsequently apply the tested method to filter paper oral fluids collected from nine actual patients. To do so, 72 samples that were prepared from the same virus stock, and contained 10,000, 1,000, and 100 viral particles were tested. The samples were kept at different temperatures (−25°C, 4°C, and 25°C), and were tested after a day, 1, 2, and 3 weeks. The samples kept at room temperature were open to the atmosphere, and the samples kept at 4°C and −25°C were kept in sealed paper envelopes. Thirty‐five of 72 (48.6%) of the samples were positive. The factor of time had a clear effect on the results, and decreased the number of positive samples. The decrease of temperature also had the anticipated effect on the results, and increased the number of positive samples. The only problem observed in the research was the nonreproducibility and the nonreliability of the method when the dried specimens had low titers of virus. This was most probably because the extraction of viral RNA from filter papers was not successful 100%, and some of the viral particles were eliminated during the process, and since very low titers of the virus were used, there was not enough RNA available to be amplified by the nested RT‐PCR. As shown in the Results, two specimens with the same amount of dried virus that were kept under the exact same conditions of time and temperature did not give the same result, and one was positive and the other was negative (dilution 10−5 after a week). In addition, there were times (like the results of 3 weeks) where a sample was positive (dilution 10−6), but samples with higher titers were negative (dilution 10−5), or when a dilution kept in room temperature was positive (dilution 10−6 after a day), but the samples of the same dilution kept at −25°C and 4°C were negative. These results are all the exact opposite of the predicted results. This study proves for the first time that this procedure is not reproducible and reliable when low titers of the virus have been dried onto filter papers. The method of keeping the samples (open to the atmosphere or sealed in a paper envelope) did not seem to have an impact on the results. The next step could be preparing dilutions of low titer MV using a healthy person's oral fluids instead of sterile PBS to see if it has an effect on the results, and whether oral fluids help obtaining positive results from low amounts of virus.
In the second part of the research, nine oral fluids from patients with measles were dried onto filter papers and tested. Three samples were dried from each patient's oral fluid, and all of the samples were kept at room temperature, and open to the atmosphere. These samples were then tested after a day, 1 and 3 weeks. All the samples were positive in all the tests. This shows that the procedure used in this research is applicable for patient's oral fluids that have high titers of virus in them.
In conclusion, this study showed that after collecting oral fluid from patients and drying it on filter papers, the papers can be posted to the laboratory at ambient temperature without needing a cold chain. The positive samples also remain positive for at least 3 weeks at room temperature as proven in this study. By comparing the results of the two different sections of the study, it can be concluded that while the mentioned procedure is not reliable for detecting low titers of the virus, oral fluid is a perfect sample for this method due to its high titers of virus. On the other hand it appears that the stability of MV in oral fluid is much higher than the stability of MV‐infected cells, thus the results remain positive in oral fluids for a longer time and in room temperature. Oral fluid spots provide a simple, noninvasive approach to specimen collection and a relatively inexpensive way of specimen transport. Oral fluid sample can be reconstituted for IgM ELISA, thus enables the combination of serology and RT‐PCR for surveillance of measles. Oral fluid spots offer the ability of expanding the measles surveillance to remote areas.
ACKNOWLEDGMENTS
The authors thank Zahra Saadatmand and Ghazal Sadat Fateminasab for assistance with specimen collection and processing.
Grant sponsor: School of Public Health, Tehran University of Medical Sciences; Grant number: 10685.
REFERENCES
- 1. Bellini WJ, Rota JS, Rota PA. Virology of measles‐virus. J Infect Dis 1994;170:S15–S23. [DOI] [PubMed] [Google Scholar]
- 2. Chibo D, Riddell M, Catton M, Lyon M, Lum G, Birch C. Studies of measles viruses circulating in Australia between 1999 and 2001 reveals a new genotype. Virus Res 2003;91:213–221. [DOI] [PubMed] [Google Scholar]
- 3. CDC . Progress towards measles elimination. MMWR 2004;53:304–306. [PubMed] [Google Scholar]
- 4. Cutts FT, Henao‐Restrepo AM, Olive JM. Measles elimination: Progress and challenges. Vaccine 1999;17:S47–S52. [DOI] [PubMed] [Google Scholar]
- 5. De Swart RL, Nur Y, Abdallah A, Kruining H, El Mubarak HS, Ibrahim SA, Van den Hoogen B, Groen J, Osterhaus A. Combination of reverse transcriptase PCR analysis and immunoglobulin M detection on filter paper blood samples allows diagnostic and epidemiological studies of measles. J Clin Microbiol 2001;39:270–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wild TF. Measles vaccines, new developments and immunization strategies. Vaccine 1999;17:1726–1729. [DOI] [PubMed] [Google Scholar]
- 7. CDC . Available at: http://wwwnc.cdc.gov/travel/content/in-the- news/measles.aspx, October 23, 2010.
- 8. Featherstone D, Brown D, Sanders R. Development of the global measles laboratory network. J Infect Dis 2003;187:S264–S269. [DOI] [PubMed] [Google Scholar]
- 9. Wharton M, Chorba TL, Vogt RL, Morse DL, Buehler JW. Case definitions for public health surveillance. MMWR Recomm Rep 1990;39:1–43. [PubMed] [Google Scholar]
- 10. Ramsay M, Brugha R, Brown D. Surveillance of measles in England and Wales: Implications of a national saliva testing programme. Bull World Health Organ 1997;75:515–521. [PMC free article] [PubMed] [Google Scholar]
- 11. Grandien M, Osterhaus A, Rota PA, Smaron MF, Wild TF, Nascimento J, Elnageh MM, Leduc J, Pervikov Y, Scott RM. Laboratory diagnosis of measles infection and monitoring of measles immunization—Memorandum from a who meeting. Bull World Health Organ 1994;72:207–211. [PMC free article] [PubMed] [Google Scholar]
- 12. Salimi V, Mokhtari Azad T, Hamkar R, Gouya MM, Esteghamati AAR, Varshochiyani S. Seroepidemiology of measles in 5–25 year old age group before measles/rubella mass vaccination campaign in Tabriz (2003). Med J T U Med Sci 2006;28:77–82. [Google Scholar]
- 13. El Mubarak HS, Van De Bildt MWG, Mustafa OA, Vos HW, Mukhtar MM, Groen J, El Hassan AM, Niesters HGM, Ibrahim SA, Zijlstra EE, Wild TF, Osterhaus A, De Swart RL. Serological and virological characterization of clinically diagnosed cases of measles in suburban Khartoum. J Clin Microbiol 2000;38:987–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Katz RS, Premenko‐Lanier M, McChesney, MB , Rota PA, Bellini WJ. Detection of measles virus RNA in whole blood stored on filter paper. J Med Virol 2002;67:596–602. [DOI] [PubMed] [Google Scholar]
- 15. Chibo D, Riddell MA, Catton MG, Birch CJ. Applicability of oral fluid collected onto filter paper for detection and genetic characterization of measles virus strains. J Clin Microbiol 2005;43:3145–3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Oliveira SA, Siqueira MM, Camacho LAB, Castro‐Silva R, Bruno BF, Cohen BJ. Use of RT‐PCR on oral fluid samples to assist the identification of measles cases during an outbreak. Epidemiol Infect 2003;130:101–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Brown DWG, Ramsay MEB, Richards AF, Miller E. Salivary diagnosis of measles—A study of notified cases in the United Kingdom, 1991–3. Brit Med J 1994;308:1015–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Helfand RF, Kebede S, Alexander JP, Alemu W, Heath JL, Gary HE, Anderson LJ, Beyene H, Bellini WJ. Comparative detection of measles‐specific IgM in oral fluid and serum from children by an antibody‐capture IgM EIA . J Infect Dis 1996;173:1470–1474. [DOI] [PubMed] [Google Scholar]
- 19. Jin L, Brown DWG, Ramsay MEB, Rota PA, Bellini WJ. The diversity of measles virus in the United Kingdom, 1992–1995. J Gen Virol 1997;78:1287–1294. [DOI] [PubMed] [Google Scholar]
- 20. Jin L, Vyse A, Brown DWG. The role of RT‐PCR assay of oral fluid for diagnosis and surveillance of measles, mumps and rubella. Bull World Health Organ 2002;80:76–77. [PMC free article] [PubMed] [Google Scholar]
- 21. Nigatu W, Jin L, Cohen BJ, Nokes DJ, Etana M, Cutts FT, Brown DWG. Measles virus strains circulating in Ethiopia in 1998–1999: Molecular characterisation using oral fluid samples and identification of a new genotype. J Med Virol 2001;65:373–380. [DOI] [PubMed] [Google Scholar]
- 22. WHO . Measles and Rubella laboratory manual. Available at: http://www.who.int/immunization_monitoring/laboratory_ measles_resources/en/
- 23. WHO . Measles and Rubella laboratory network: 2007 meeting on use of alternative sampling techniques for surveillance. Wkly Epidemiol Rec 2008;83:225–232. [PubMed] [Google Scholar]
- 24. Riddell MA, Chibo D, Kelly HA, Catton MG, Birch CJ. Investigation of optimal specimen type and sampling time for detection of measles virus RNA during a measles epidemic. J Clin Microbiol 2001;39:375–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Abe K, Konomi N. Hepatitis C virus RNA in dried serum spotted onto filter paper is stable at room temperature. J Clin Microbiol 1998;36:3070–3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cassol S, Salas T, Gill MJ, Montpetit M, Rudnik J, Sy CT, O'Shaughnessy MV. Stability of dried blood spot specimens for detection of human immunodeficiency virus DNA by polymerase chain reaction. J Clin Microbiol 1992;30:3039–3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Comeau AM, Pitt J, Hillyer GV, Landesman S, Bremer J, Chang BH, Lew J, Moye J, Grady GF, McIntosh K. Early detection of human immunodeficiency virus on dried blood spot specimens: Sensitivity across serial specimens. J Pediatr 1996;129:111–118. [DOI] [PubMed] [Google Scholar]
- 28. Condorelli F, Scalia G, Stivala A, Gallo R, Marino A, Battaglini CM, Castro A. Detection of immunoglobulin‐g to measles‐virus, rubella‐virus, and mumps‐virus in serum samples and in microquantities of whole‐blood dried on filter paper. J Virol Methods 1994;49:25–36. [DOI] [PubMed] [Google Scholar]
- 29. El Mubarak HS, Yuksel S, Mustafa OM, Ibrahim SA, Osterhaus A, de Swart RL. Surveillance of measles in the Sudan using filter paper blood samples. J Med Virol 2004;73:624–630. [DOI] [PubMed] [Google Scholar]
- 30. Fischler B, Rodensjo P, Nemeth A, Forsgren M, Lewensohn‐Fuchs I. Cytomegalovirus DNA detection on Guthrie cards in patients with neonatal cholestasis. Arch Dis Child 1999;80:F130–F134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gupta BP, Jayasuryan N, Jameel S. Direct detection of hepatitis B virus from dried blood spots by polymerase chain reaction amplification. J Clin Microbiol 1992;30:1913–1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Helfand RF, Keyserling HL, Williams I, Murray A, Mei J, Moscatiello C, Icenogle J, Bellini WJ. Comparative detection of measles and rubella IgM and IgG derived from filter paper blood and serum samples. J Med Virol 2001;65:751–757. [DOI] [PubMed] [Google Scholar]
- 33. Mosquera MD, Echevarria JE, Puente S, Lahulla F, de Ory F. Use of whole blood dried on filter paper for detection and genotyping of measles virus. J Virol Methods 2004;117:97–99. [DOI] [PubMed] [Google Scholar]
- 34. Nakano JH, Miller DL, Foster SO, Brink EW. Microtiter determination of measles hemagglutination inhibition antibody with filter papers. J Clin Microbiol 1983;17:860–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pitcovski J, Shmueli E, Krispel S, Levi N. Storage of viruses on filter paper for genetic analysis. J Virol Methods 1999;83:21–26. [DOI] [PubMed] [Google Scholar]
- 36. Ono N, Tatsuo H, Hidaka Y, Aoki T, Minagawa H, Yanagi Y. Measles viruses on throat swabs from measles patients use signaling lymphocytic activation molecule (CDw 150) but not CD46 a cellular receptor. J Virol 2001;75:4399–4401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Godec MS, Asher DM, Swoveland PT, Eldadah ZA, Feinstone SM, Goldfarb LG, Gibbs CJ, Carleton Gajdusek D. Detection of measles virus genomic sequences in SSPE brain tissue by the polymerase chain reaction. J Med Virol 1990;30:237–244. [DOI] [PubMed] [Google Scholar]
- 38. Matsuzono Y, Narita M, Ishiguro N, Togashi T. Detection of measles‐virus from clinical‐samples using the polymerase chain‐reaction. Arch Pediat Adol Med 1994;148:289–293. [DOI] [PubMed] [Google Scholar]
- 39. Rota PA, Khan AS, Durigon E, Yuran T, Villamarzo YS, Bellini WJ. Detection of measles‐virus rna in urine specimens from vaccine recipients. J Clin Microbiol 1995;33:2485–2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Shimizu H, McCarthy CA, Smaron MF, Burns JC. Polymerase chain‐reaction for detection of measles‐virus in clinical‐samples. J Clin Microbiol 1993;31:1034–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Riddell MA, Leydon JA, Catton MG, Kelly HA. Detection of measles virus‐specific immunoglobulin M in dried venous blood samples by using a commercial enzyme immunoassay. J Clin Microbiol 2002;40:5–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hutse V, Van Hecke K, De Bruyn R, Samu O, Lernout T, Muyembe JJ, Brochier B. Oral fluid for the serological and molecular diagnosis of measles. Int J Infect Dis; in Press, corrected proof. [DOI] [PubMed]


