Abstract
Background
Acne vulgaris is a multifactorial skin disorder of unknown etiology. Free radical‐mediated reactions have been implicated but their role in eliciting this response and contributing to disease progress remains unexplored. This study was undertaken to investigate the status and contribution of oxidative/nitrosative stress in patients with acne vulgaris. J. Clin. Lab. Anal. 27:45–2, 2013. © 2012 Wiley Periodicals, Inc.
Methods
Sera from 50 acne vulgaris with varying levels of disease activity (mild, moderate, and severe) according to the Global Acne Grading System (GAGS) and 40 age‐ and sex‐matched controls were evaluated for serum levels of oxidative/nitrosative stress markers, including protein oxidation, lipid peroxidation and nitric oxide (NO), superoxide dismutase (SOD), and glutathione (GSH).
Results
Serum analysis showed significantly higher levels of carbonyl contents, malondialdehyde (MDA) and NO, in acne patients compared with healthy controls (P < 0.05). Interestingly, not only there were an increased number of subjects positive for carbonyl contents, but also the levels of these oxidants were significantly increased with the increase of the disease activity (P < 0.05). In addition, a significant correlation was observed between the levels of carbonyl contents and the GAGS scores (r = 0.341, r = 0.355, and r = 0.299, respectively). Furthermore, sera from acne patients had lower levels of SOD and GSH compared with healthy control sera.
Conclusion
These findings support an association between oxidative/nitrosative stress and acne. The stronger response observed in serum samples from patients with higher GAGS scores suggests that markers of oxidative/nitrosative stress may be useful in evaluating the progression of acne and in elucidating the mechanisms of disease pathogenesis.
Keywords: Acne vulgaris, biomarkers, protein oxidation, lipid peroxidation, nitration
INTRODUCTION
Acne vulgaris is one of the most prevalent skin conditions encountered by dermatologists 1. Despite technological advances and an increased degree of sophistication within experimental dermatology, the precise mechanisms of the acne process remain elusive 2. It is considered a multifactorial or complex disease of the pilosebaceous unit 3.
Excessive generation of reactive oxygen species (ROS) has the potential to initiate damage to proteins, lipids, and nucleic acids 4, 5. Antioxidant defense systems, such as superoxide dismutase (SOD) and glutathione (GSH) keep ROS production in check, thereby maintaining an appropriate cellular redox balance. Alterations in this redox balance resulting from elevated ROS levels and/or decreased antioxidant levels can lead to oxidative stress 6. Protein oxidation, which results in functional disruption, is not random but appears to be associated with increased oxidation in specific proteins 7. A number of studies indicate a strong role for increases in protein oxidation as a primary cause of cellular dysfunction observed during aging and age‐related neurodegenerative diseases 8 such as Alzheimer's disease, Parkinson's disease, and some other protein conformational diseases. Inhibition of a wide array of enzyme activities have been reported 9. Modification of structural protein can also lead to loss of function. As for example, fibrinogen on exposure to ROS loses its ability to form a solid clot 10. Oxidation of synovial fluid immunoglobulin causes aggregation and contributes to the etiology of rheumatoid arthritis 11. These disorders could be due to the formation of abnormal protein aggregates as a consequence of ROS damage. Lipid peroxidation, a process of oxidative degeneration of polyunsaturated fatty acids set into motion by ROS, leads to formation of highly reactive aldehydes, such as malondialdehyde (MDA) that can bind covalently to proteins and thus causes structural protein modifications and affects biologic functions 12. The skin is constantly exposed to oxidative injury induced by ROS generated both from endogenous sources and from external pro‐oxidant stimuli 13. Free radical‐mediated damage has been implicated in the pathogenesis of acne vulgaris 14, 15, 16. Like ROS, reactive nitrogen species (RNS) could also play a significant role in the pathogenesis of various diseases and has drawn significant attention in recent years 17, 18. Nitric oxide (NO) is one of the most important and widely studied RNS. The potential role of NO in disease pathogenesis lies largely in the extent of its production and generation of O2 .−, leading to formation of peroxynitrite (ONOO−). ONOO− is a potent nitrating and oxidizing agent that induced modifications of endogenous proteins and nucleic acids, which disturbed the oxidative/nitrosative homeostasis 18.
Increased oxidative stress in acne vulgaris has been reported 15, 16. However, the potential role of oxidative/nitrosative stress, especially the consequences of oxidative modification of proteins, and the disturbances in oxidants–antioxidant systems in the disease progression remains unresolved. Therefore, we hypothesized that overproduction of reactive oxygen and nitrogen species (RONS) leads to a variety of RONS‐mediated modifications of the endogenous proteins, such as increased formation of protein oxidative biomarker carbonyl contents, lipid peroxidative byproducts MDA, and nitrosative biomarker NO, which thus leads to oxidative and nitrosative stress in acne patients. To assess this hypothesis and establish a link between RONS and acne, we examined the levels of biochemical markers of oxidative/nitrosative stress in the sera of acne patients, and analyzed their relationship to the extent of disease activity (mild, moderate, and severe) according to the Global Acne Grading System (GAGS). Our results not only support an association between oxidative/nitrosative stress and acne, but also suggest that oxidative/nitrosative stress markers may be important in the evaluation of acne progression and in the elucidation of the mechanisms of disease pathogenesis.
PATIENTS AND METHODS
Human Subjects
The study group involved 50 patients (30 female and 20 male) with acne vulgaris, as defined by the GAGS criteria 19, and the mean age was 20.3 years. The GAGS score was determined using the disease activity and the patients were divided into the following three groups based on the lower vs. higher GAGS scores: 1 mild acne 2 moderate acne, and 3 severe acne. Patients did not receive any systemic or topical drug therapy for the last three months, did not have any additional systemic disease, had no history of systemic medication use, and had no smoking and other regular exercising habits except their daily activities. The control group comprised 40 healthy subjects (23 female and 17 male; mean age 19.3 years). The mean ages were not significantly different between the groups (P > 0.05). The racial/ethnic and sex compositions of the acne groups were comparable with those of the control group.
The study was approved by Local Ethical Committee, College of Medicine, Qassim University. Venous blood samples from the control subjects and acne patients were collected, and serum from individual subjects was stored in small aliquots at –20°C until analyzed further.
Assay for Protein Oxidation
Protein oxidation in acne patients was determined by carbonyl groups formation in the serum samples of acne patients and normal human subjects as previously described 20, 21 with slight modifications. Briefly, the reaction mixture containing 10 μl of serum samples, 0.5 ml of 10 mM 2,4‐dinitrophenylhydrazine/2.5 M HCl was added and thoroughly mixed. After addition of 250 μM trichloroacetic acid (catalog # T6399, Sigma‐Aldrich, St. Louis, Mo, USA; 20%) and centrifugation, the pellet was collected and washed three times with 1 ml ethanol:ethylacetate (1:1) mixture. The pellet was then dissolved in 1 ml of 6 M guanidine solution and incubated at 30°C for 15 min. After centrifugation, the supernatant was collected and the carbonyl contents were estimated from the absorbance at 370 nm using a molar absorption coefficient of 22,000 M−1cm1. Samples were spectrophotometrically analyzed against a blank of 1 ml of guanidine solution (6 M). Protein concentration was determined in the samples and carbonyl contents were expressed as nanomoles per milligram protein.
Assay for Lipid Peroxidation
Lipid peroxidation was determined in the sera of acne vulgaris patients and healthy controls by measuring the levels of lipid peroxidative end product MDA as described earlier 22. Briefly, 0.2 ml of serum was mixed with 1 ml of 20% trichloroacetic acid. To the mixture, 0.4 ml of 0.67% thiobarbituric acid (TBA) was added, shaken, and kept for 3 min in a boiling water bath. After cooling at room temperature, 1.6 ml of butanol was added and the mixture was shaken. Organic mixture was separated by centrifugation and its absorbance was measured at 532 nm.
Assay for Nitrosative Stress
Nitrosative stress in acne patients was determined by NO estimation using total nitrite assay in the serum samples of acne patients and normal human subjects as described previously 23. Briefly, serum samples were deproteinated using ZnSO4 reagent and total nitrite was determined in the deproteinated serum samples by using Griess reagent (sulfanilamide 17 mM, N‐(1‐naphthyl) ethylene diamine dihydrochloride 0.4 mM, (w/v) 2.5% orthophosphoric acid in 0.1 M phosphate buffer). Absorbance of the reaction mixture was measured at 540 nm. Concentration of NO was determined using sodium nitrite (2.5–30 mM) as standard and the results were expressed as nanomoles per milliliter.
Antioxidants Assays
Determination of GSH
Levels of GSH were determined in the serum samples of acne patients and normal human subject as described elsewhere 24. Briefly, 100 ml of freshly thawed serum was pipetted into Eppendorf tube containing 200 ml of a 10% solution of tricholoroacetic acid, vortexed, and centrifuged at 4000 × g for 10 min at 10°C. To 200 ml of the supernatant, 700 ml of 400 mM Tris‐HCl buffer, pH 8.9, was added followed by the addition of 100 ml of 2.5 mM DTNB (5.5‐dithio‐bis(2‐nitrobenzoic acid)) dissolved in 40 mM Tris‐HCl buffer pH 8.9. After 10 min at room temperature, the extinction of the samples was measured at 412 nm. Blank consisted of DTNB instead of serum; its extinction was subtracted from the test sample extinction before matching it with the standard curve. The concentration of GSH in the samples was read from a standard curve using different concentrations of GSH (1–10 mmol/ml).
Determination of SOD activity
Activity of antioxidant enzyme SOD in the serum samples of acne patients and normal human subject was determined as described earlier 25. Enzyme activity was detected by its ability to inhibit the autoxidation of epinephrine at pH 10.2. Each cuvette contained in a final volume of 3.0:0.50 ml of 1.8 mM epinephrine (freshly prepared); 0.50 ml of 0.6 mM ethylenediaminetertra acetic acid (EDTA); 0.50 ml of 0.30 M sodium carbonate, pH 10.2, and serum samples. The reaction was initiated by the addition of epinephrine, and an increase in absorbance was measured at 480 nm.
Statistical Analysis
All measurements were performed in duplicates and repeated at least two times using age‐ and sex‐matched acne or control samples. Comparisons were performed by Origin 6.1 (Northampton, MA) and Graph Pad Prism‐5 (San Diego, CA) statistical software's using one‐way ANOVA followed by Tukey's post hoc analysis). Data correlations were performed using Spearman's rank correlation coefficient. P values less than 0.05 were considered significant. Values shown are mean ± standard error of the mean (SEM) unless stated otherwise.
RESULTS
Role of Protein Oxidation in the Progress of Acne Vulgaris
Protein oxidation typically results in an increase in carbonyl contents, therefore, increase in protein carbonyl content is considered to be a biomarker of protein oxidation 21. The data showed significant increase in serum protein carbonyl contents (P < 0.05) in 50 acne vulgaris patients compared with 40 normal subjects of the same age group. The average carbonyl contents ( ±SEM) in all studied subjects of acne serum proteins and normal human serum (NHS) proteins were 2.65 ± 0.13 and 2.06 ± 0.12 nmol/mg proteins, respectively (Fig. 1B). In addition, we have also determined the role of protein carbonylation in the progress of acne vulgaris. For that carbonyl contents, these were estimated in accordance with the disease activity in mild (n = 20), moderate (n = 23), and severe (7) acne patients. The average carbonyl contents ( ±SEM) in the patients sera with mild, moderate, and severe groups were 2.11 ± 0.10, 2.73 ± 0.15, and 3.10 ± 0.13 nmol/mg protein, respectively (Fig. 1A). Our results showed that carbonyl contents were significantly increased in moderate or severe patients as compared with healthy controls (P < 0.05), whereas mild acne patients showed no change in the carbonyl contents, when compared with normal human subjects (P > 0.05).
Figure 1.
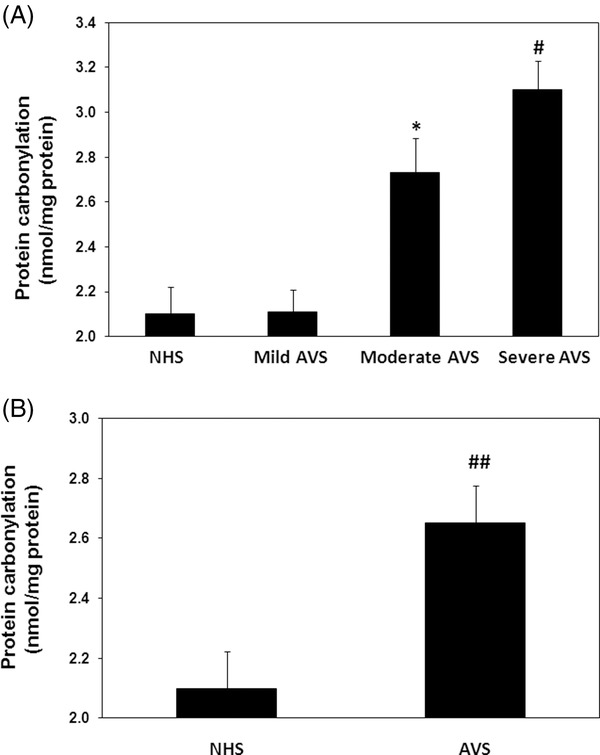
Protein oxidation in acne vulgaris (AVS). (A) Levels of protein carbonyl contents in the sera of AVS patients with mild (n = 20), moderate (n = 23), and those with severe (n = 7) scores and in normal human sera (NHS, n = 40). *P < 0.01 vs. mild AVS; # P < 0.05 vs. moderate AVS; # P < 0.001 vs. mild AVS. (B) Levels of protein carbonyl contents in the sera of all studied AVS patients (n = 50) and in NHS(n = 40). ## P < 0.001 vs. NHS. Histograms show the mean ± SEM. Comparison analysis was performed using one‐way ANOVA followed by Tukey's post hoc test.
Effect of Lipid Peroxidation in the Progress of Acne Vulgaris
The degree of lipid peroxidation in the progress of acne vulgaris was analyzed by lipid peroxidative end products, MDA. Our data showed that MDA levels were significantly increased with the increase of the disease activity (P < 0.05). The average MDA levels ( ±SEM) in the patients sera with mild (n = 20), moderate (n = 23), or severe (n = 07) groups were 1.66 ± 0.01, 1.77 ± 0.07, and 2.00 ± 0.13 nmol/ml, respectively (Fig. 2A). Our results also demonstrated that MDA level was significantly high in acne vulgaris patients when compared with normal human subjects (P < 0.05). The average MDA (±SEM) in the sera of acne patients and normal human subjects was 1.72 ± 0.05 and 1.47 ± 0.03 nmol/ml, respectively (Fig. 2B).
Figure 2.
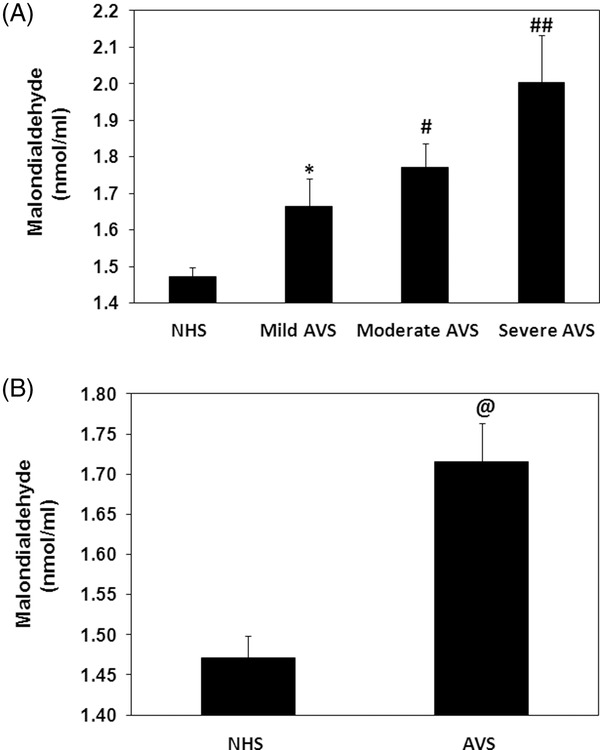
Lipid peroxidation in acne vulgaris (AVS). (A) Levels of malondialdehyde (MDA) in the sera of AVS patients with mild (n = 20), moderate (n = 23), and severe (n = 7) scores and in normal human sera (NHS, n = 40). *P < 0.01 vs. NHS; # P < 0.05 vs. mild AVS; ## P < 0.05 vs. moderate AVS; # P < 0.001 vs. mild AVS. (B) Levels of MDA in the sera of all studied AVS patients (n = 50) and NHS (n = 40). *P < 0.01 vs. NHS. Histograms show the mean ± SEM. Comparison analysis was performed using one‐way ANOVA followed by Tukey's post hoc test.
Effect of Nitrosative Stress in Acne Vulgaris Progress
Nitrosative stress in acne patients was demonstrated by the estimation of NO using nitrite assays in the sera of studied subjects. Our data showed that NO level was significantly increased with the increase of the acne activity (P < 0.05). The average NO levels (±SEM) in the patients sera with mild (n = 20), moderate (n = 23), or severe (n = 07) groups were 5.92 ± 0.37, 8.31 ± 0.91 and 10.80 ± 1.59 nmol/ml, respectively (Fig. 3A). Our results also demonstrated that NO level was significantly high in acne patients when compared with normal human subjects (P < 0.05). The average NO (±SEM) in the sera of acne patients (50 independent assays) and normal human subjects (40 independent assays) was 1.72 ± 0.05 and 1.47 ± 0.03 nmol/ml, respectively (Fig. 3B).
Figure 3.
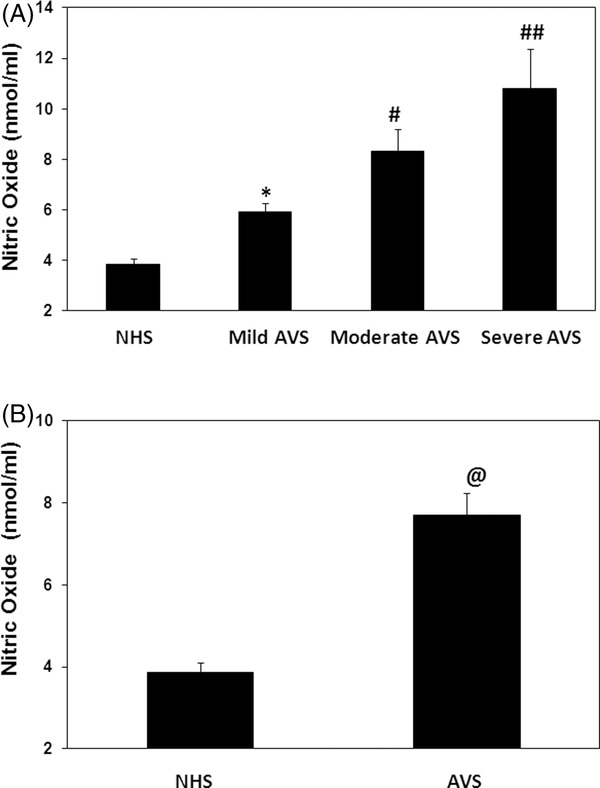
Nitric oxide in acne vulgaris (AVS). (A) Levels of nitric oxide in the sera of AVS patients with mild (n = 20), moderate (n = 23), and those with severe (n = 7) scores and in normal human sera (NHS, n = 40). *P < 0.05 vs. NHS; # P < 0.05 vs. mild AVS; ## P < 0.05 vs. severe AVS. (B) Levels of nitric oxide in the sera of all studied AVS patients (n = 50) and NHS (n = 40). @ P < 0.01 vs. NHS. Each bar shows the mean ± SEM. Comparison analysis was performed using one‐way ANOVA followed by Tukey's post hoc test.
Effect of Antioxidant System in the Progress of Acne Vulgaris
To validate our hypothesis that oxidative stress may be involved in acne vulgaris, we assessed the antioxidant potential with the following parameters in patient's sera as a function of disease activity.
Correlation of GSH with disease activity
As evident from Figure 4A, the levels of serum GSH were significantly reduced in mild or severe acne patients compared with normal subjects of the same age group (P < 0.05). The average GSH levels (±SEM) in mild and severe acne patients were 3.65 ± 0.12 and 3.59 ± 0.33 nmol/ml, respectively, whereas the average GSH ( ±SEM) in normal human controls was 4.55 ± 0.18 nmol/ml. Our results also demonstrated that mild acne patients showed insignificant decreased in the serum GSH when compared with normal human subjects (P > 0.05). As far as GSH level in all studied acne cases was concerned, our data showed that GSH level was significantly reduced in acne patients when compared with normal human subjects (P < 0.05). The average GSH (±SEM) in the sera of acne patients (50 independent assays) and normal human subjects (40 independent assays) was 3.99 ± 0.11 and 4.55 ± 0.18 nmol/ml, respectively (Fig. 4B).
Figure 4.
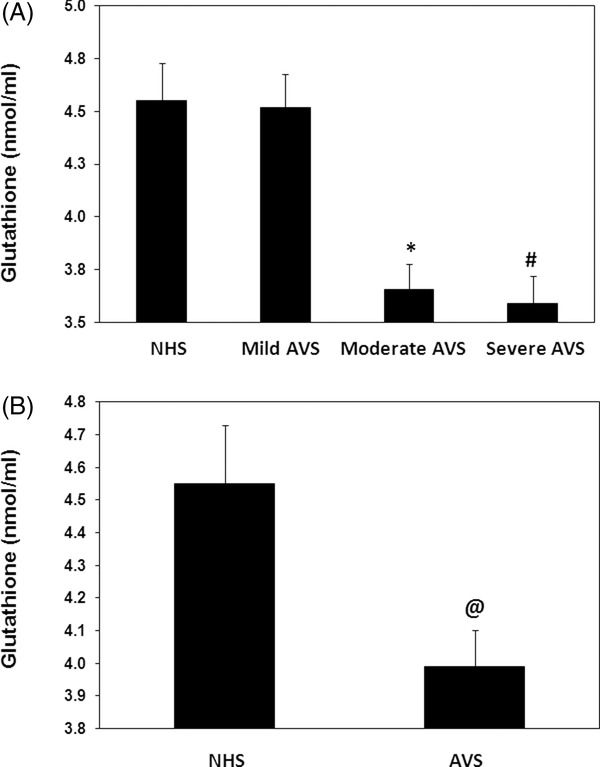
Antioxidant glutathione in acne vulgaris (AVS). (A) Levels of glutathione (GSH) in the sera of AVS mild scores (n = 20), moderate scores (n = 23), and those with severe scores (n = 7) and in normal human sera (NHS, n = 40). *P < 0.001 vs. mild AVS; # P < 0.001 vs. mild AVS. (B) Levels of GSH in the sera of all studied AVS patients (n = 50) and NHS (n = 40). @ P < 0.05 vs. NHS. Histograms show the mean ± SEM. Comparison analysis was performed using one‐way ANOVA followed by Tukey's post hoc test.
Disease progress related decrease of SOD activity
To provide further support to our hypothesis and assess the week antioxidant system of acne patients, activity of SOD was determined in the serum samples of studied subjects. As shown in Figure 5A, SOD activity was significantly decreased with the increase of the acne activity (P < 0.05). The average SOD activity (±SEM) in the patient's sera with mild (n = 20), moderate (n = 23), or severe (n = 07) groups was 37.38 ± 0.48, 36.75 ± 0.27 and 35.67 ± 0.32 kU/l, respectively (Fig. 3A). Our results also demonstrated that SOD activity was significantly reduced in acne patients when compared with normal human subjects (P < 0.05). The average SOD activity (±SEM) in the sera of acne patients (50 independent assays) and normal human subjects (40 independent assays) was 37.19 ± 0.33 and 38.95 ± 0.40 nmol/ml, respectively (Fig. 5B).
Figure 5.
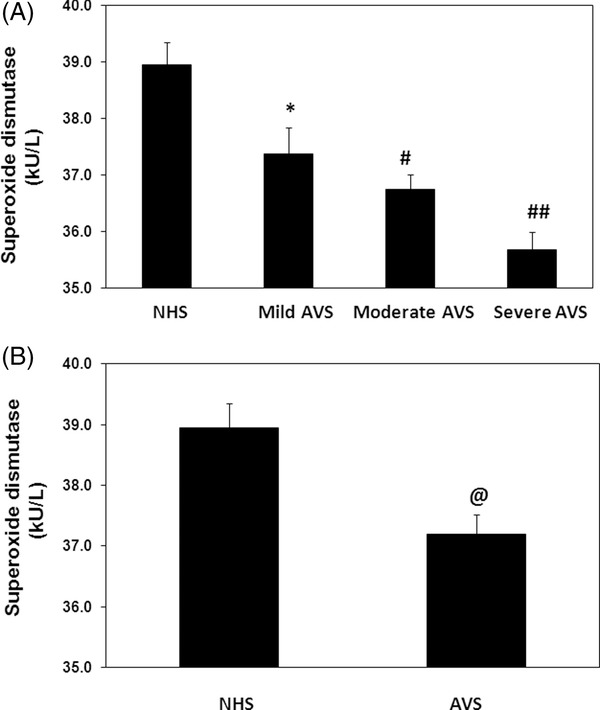
Activity of superoxide dismutase (SOD) in acne vulgaris (AVS). (A) Activity of antioxidant enzyme SOD in the sera of AVS patients with mild (n = 20), moderate (n = 23), and severe (n = 07) scores and normal human sera (NHS, n = 40). *P < 0.05 vs. NHS; # P < 0.05 vs. mild AVS; ## P < 0.05 vs. moderate AVS. (B) Activity of SOD in the sera of all studied AVS patients (n = 50) and NHS (n = 40). @ P < 0.05 vs. NHS. Each bar shows the mean ± SEM. Comparison analysis was performed using one‐way ANOVA followed by Tukey's post hoc test.
DISCUSSION
Acne vulgaris is a skin disease caused by the changes in pilosebaceous units. It is common in adolescence and may proceed into adulthood, affecting roughly 33% of people aged between 15 and 44 years 26. Despite the research, the etiology and pathogenesis of acne is not completely understood and a single, primary cause that has not been identified 2, 27. A few previous studies have proposed the role of oxidative stress in the aetiopathogenesis of acne 14, 15, 16. The generation of ROS by neutrophils resulting in tissue injury is proposed to be responsible for one of the pathogenic conditions in acne vulgaris, and inhibition of ROS production has been reported to be of therapeutic benefit 28, 29. In the present study, we have demonstrated for the very first time that the roles of biochemical markers of protein oxidation, lipid peroxidation, or nitrosative stress in the progress of acne vulgaris.
The oxidative modification of proteins, lipids, and nucleic acids has been implicated in the etiology of numerous disorders and diseases 30, 31, 32, 33. Oxidatively modified serum proteins can serve as important in vivo biomarkers of oxidative stress. Proteins are better candidates in detecting oxidative stress, due to the accessibility of serum protein for sampling, their relatively long half‐lives, and their well‐defined biochemical pathways. Since extracellular fluids contain only small amounts of antioxidant enzymes, it has been proposed that the major extracellular antioxidants are proteins 34. The in vitro oxidation of amino acid residues leads to protein degradation, aggregation, and crosslinking. In contrast, significant evidence for the presence of ROS‐mediated protein damage in vivo and its possible clinical significance is not currently available. The oxidation of a protein typically results in an increase in carbonyl contents. This increase is due to the oxidation of Lys, Arg, Pro, or other amino acid residues. In short, protein carbonyl groups are the biomarker of oxidative stress 21. In human plasma, all amino acids in the protein are susceptible to oxidative modification by oxidants such as hydroxyl radicals and hypochlorous acid 21. Present data showed total serum protein carbonyl contents were significantly increased (P < 0.05) in acne vulgaris patients as compared to normal subjects. Our data pointed out that mainly serum protein carbonyl contents were significantly increased in moderate or severe acne patients as compared with the total serum protein present in normal human subjects, whereas mild acne patients showed negligible increase in the carbonyl contents as compared with healthy controls (P > 0.05). This data clearly indicated that serum proteins in acne patients were oxidatively modified and this oxidative modification may have a role in disease progression.
Lipid peroxidation is another important parameter and a hallmark of the oxidative stress. MDA, an end product of lipid peroxidation induced by ROS and is considered to be the suitable biomarker for the determination of lipid peroxidation 35. Increased lipid peroxidation has previously been detected in acne vulgaris patients 12, but the significance of lipid peroxidation with disease activity or in the initiation or development of acne remains largely unexplored. In this study, when the acne patients were divided into three groups on their GAGS scores, such as mild, moderate, and severe acne groups, all the groups showed higher serum levels of MDA than were observed in healthy controls, but the levels were much greater in the groups with higher GAGS scores as MDA levels: control < mild < moderate < severe. This clearly suggests an ongoing involvement of lipid peroxidation in acne patients. In contrast to this, it also indicates that there is a close association between lipid peroxidation and disease activity, i.e. the greater the lipid peroxidation stress, the higher the acne activity.
NO is a diffusible messenger known to display a variety of physiological functions, including vasorelaxation, bronchodilator, inhibition of platelet aggregation, and neurotransmission. Additionally, it appears to be involved in the macrophage‐dependent killing of intracellular parasites and possibly cancer cells, indicating the potential of this free radical to mediate cytotoxic and pathological effects. When produced in excess, NO can have a multitude of potentially toxic effects, which are highly dependent on its concentration and the particular microenvironment in which it is produced 36. NO has been reported to inhibit mitochondrial respiration and ribonucleotide reductase and to damage DNA 37 and bring about protein modification 38. There is a increasing evidence that NO may be involved in the pathogenesis of various diseases including acne vulgaris 15. Serum nitrite/nitrate level, which is an index of NO production, was well reported to correlate with disease activity in various diseases 38. Therefore, we hypothesized that increased serum level of NO thus presents another important potential mechanism in the pathogenesis of acne vulgaris and it may be correlated with disease activity. Our results provide evidences that NO level was significantly increased with the increase of the acne activity in patient groups with mild, moderate, or severe as NO: control < mild < moderate < severe acne patients. The increased levels of NO observed in the acne patients in the present study provide solid evidence of the involvement of nitrosative stress in the progress acne vulgaris.
Humans have both enzymatic and nonenzymatic antioxidant defense systems. SOD, a major enzyme and first line of defense against oxygen‐derived free radicals, controls ROS production by catalyzing the dismutation of the O2.− into hydrogen peroxide (H2O2), which is further converted into water by catalase, and thereby, an appropriate cellular redox balance is maintained. Alterations in this normal balance, as a result of elevated ROS production and/or decreased antioxidant levels, can lead to a state of oxidative stress 39. Another important well‐known parameter for antioxidant defense system is GSH. GSH together with its related enzymes, comprises a system that maintains the intracellular reducing environment and acts as primary defense against excessive generation of harmful ROS 40. The oxygen radical scavenging activity of GSH directly facilitates ROS neutralization and the repair of ROS‐induced damage 40. In addition to its antioxidant activity, GSH has many physiological functions including detoxification of xenobiotics, modulation of redox‐regulated signal transduction, regulation of cell proliferation, and immune responses 41. Now, it is well established that GSH deficiency contributes to oxidative stress, which plays a key role in various pathological conditions 40, 41.
The enhanced protein oxidation, lipid peroxidation, and nitration observed in acne patients in this study drew our attention to evaluating the SOD activity and serum GSH levels in these subjects. Clearly, the SOD activity or GSH levels was significantly lower in the acne patients as compared with the healthy controls, but the groups of patients with severe or moderate acne showing even greater reductions in the SOD activity or GSH levels in comparison with acne patients with mild disease activity. The decreased serum activities of SOD or GSH levels suggest that the antioxidant balance is compromised in acne that may lead to increased ROS levels and, thus, contribute to increased oxidative stress. In addition, these findings also indicate that oxidative stress is increased in acne vulgaris and is associated with increased disease activity.
CONCLUSIONS
Our results clearly show significant increases in oxidative/nitrosative stress in acne patients, suggesting there is an imbalance between RONS production and antioxidant defense mechanisms in acne vulgaris. In this study, the increased levels of protein carbonylation and lipid peroxidation observed in the acne patients also suggest that oxidative modification of endogenous proteins. More importantly, the results of this study, for the first time, provide evidence of a strong association between serum levels of protein oxidation, lipid peroxidation or NO, and acne disease activity, suggesting that oxidative/nitrosative stress markers may be useful in evaluating acne disease activity, and would therefore be helpful for predicting the progression of the disease.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interests.
ACKNOWLEDGEMENTS
This work was supported in part by Research Deanship Grants # SR‐D‐011–952, SR‐D‐012–1298 and funds from College of Medicine, Qassim University. ZR also thanks Professor A. Alghasham (Dean, College of Medicine) for providing research facilities.
Grant sponsor: Deanship of Scientific Research, Qassim University.
REFERENCES
- 1. Sathish D, Shayeda, Rao YM . Acne and its treatment options – A review. Curr Drug Deliv 2011; 8:634–639. [DOI] [PubMed] [Google Scholar]
- 2. Simpson RC, Grindlay DJ, Williams HC. What's new in acne? An analysis of systematic reviews and clinically significant trials published in 2010–11. Clin Exp Dermatol 2011;36:840–843. [DOI] [PubMed] [Google Scholar]
- 3. Del Rosso JQ. Combination topical therapy in the treatment of acne. Cutis 2006;78:5–12. [PubMed] [Google Scholar]
- 4. Tabak O, Gelisgen R, Erman H, et al. Oxidative lipid, protein, and DNA damage as oxidative stress markers in vascular complications of diabetes mellitus. Clin Invest Med 2011;34:E163–E171. [DOI] [PubMed] [Google Scholar]
- 5. Shacter E. Quantification and significance of protein oxidation in biological samples. Drug Metab Rev 2000;32:307–326. [DOI] [PubMed] [Google Scholar]
- 6. Grimsrud PA, Xie H, Griffin TJ, Bernlohr DA. Oxidative stress and covalent modification of protein with bioactive aldehydes. J Biol Chem 2008;283:21837–21841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Poon HF, Vaishnav RA, Gelchell TV, Getchell ML, Butterfield DA. Quantitative proteomics analysis of differential protein expression and oxidative modification of specific proteins in the brains of old mice. Neurobiol Aging 2006;27:1010–1019. [DOI] [PubMed] [Google Scholar]
- 8. Tabner BJ, Turnbull S, El‐Agnaf O, Allsop D. Production of reaction oxygen species from aggregating proteins implicated in Alzheimer's disease. Parkinson's disease and other neurodegenerative disease. Curr Top Med Chem 2001;1:507–517. [DOI] [PubMed] [Google Scholar]
- 9. Arutyunova EI, Danshina PV, Domnina LV, Pleten AP, Muronetz VI. Oxidation of glyceraldehydes‐3‐phosphate dehydrogenase enhances its binding to nucleic acids. Biochem Biophys Res Commun 2003;307:547–552. [DOI] [PubMed] [Google Scholar]
- 10. Shacter E, Williams JA, Levine RL. Oxidative modification of fibrinogen inhibits thrombin‐catalyzed clot formation. Free Radic Biol Med 1995;18:815–821. [DOI] [PubMed] [Google Scholar]
- 11. Chou C. Binding of rheumatoid and lupus synovial fluids and sera‐derived human IgG rheumatoid factor to degalactosylated IgG . Arch Med Res 2002;33:541–544. [DOI] [PubMed] [Google Scholar]
- 12. Januszewski AS, Alderson NL, Jenkins AJ, Thorpe SR, Baynes JW. Chemical modification of proteins during peroxidation of phospholipids. J Lipid Res 2005;46:1440–1449. [DOI] [PubMed] [Google Scholar]
- 13. Prasad A, Pospišil P. Two‐dimensional imaging of spontaneous ultra‐weak photon emission from the human skin: Role of reactive oxygen species. J Biophotonics 2011;4:840–849. [DOI] [PubMed] [Google Scholar]
- 14. Ikeno H, Tochio T, Tanaka H, Nakata S. Decrease in glutathione may be involved in pathogenesis of acne vulgaris. J Cosmet Dermatol 2011;10:240–244. [DOI] [PubMed] [Google Scholar]
- 15. Sarici G, Cinar S, Armutcu F, Altinyazar C, Koca R, Tekin NS. Oxidative stress in acne vulgaris. J Eur Acad Dermatol Venereol 2010;24:763–767. [DOI] [PubMed] [Google Scholar]
- 16. Arican O, Kurutas EB, Sasmaz S. Oxidative stress in patients with acne vulgaris. Mediators Inflamm 2005;2005:380–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nishikawa T, Naruse K, Kobayashi Y, et al. Involvement of nitrosative stress in experimental periodontitis in diabetic rats. J Clin Periodontol 2012;39:342–349. [DOI] [PubMed] [Google Scholar]
- 18. Rocha BS, Gago B, Pereira C, et al. Dietary nitrite in nitric oxide biology: A redox interplay with implications for pathophysiology and therapeutics. Curr Drug Targets 2011;12:1351–1363. [DOI] [PubMed] [Google Scholar]
- 19. Doshi A, Zaheer A, Stiller MJ. A comparison of current acne grading systems and proposal of a novel system. Int J Dermatol 1997;36:416–418. [DOI] [PubMed] [Google Scholar]
- 20. Levine RL, Williams J, Stadtman ER, Shacter E. Carbonyl assays for determination of oxidatively modified proteins. Methods Enzymol 1994;233:346–357. [DOI] [PubMed] [Google Scholar]
- 21. Dalle‐Dome I, Rossi R, Giustarini D, Milzani A, Colombo R. Protein carbonyl groups as biomarkers of oxidative stress. Clin Chim Acta 2003;29:23–38. [DOI] [PubMed] [Google Scholar]
- 22. Thayer WS. Serum lipid peroxides in rats treated chronically with adriamycin. Biochem Pharmacol 1984;33:2259–2263. [DOI] [PubMed] [Google Scholar]
- 23. Ding AH, Nathan CF, Stuchr DJ. Release of reactive nitrogen intermediates and reactive oxygen intermediate from mouse peritoneal macrophages. Comparison of activating cytokines and evidence for independent production. J Immunol 1988;141:2407–2412. [PubMed] [Google Scholar]
- 24. Dutta P, Seirafi J, Halpin D, Pinto J, Rivlin R. Acute ethanol exposure alters hepatic glutathione metabolism in riboflavin deficiency. Alcohol 1995;12:43–47. [DOI] [PubMed] [Google Scholar]
- 25. Misra HP, Fridovich I. The role of superoxide anion in the autooxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem 1972;247:3170–3175. [PubMed] [Google Scholar]
- 26. Lolis MS, Bowe WP, Shalita AR. Acne and systemic disease. Med Clin North Am 2009;93:1161–1181. [DOI] [PubMed] [Google Scholar]
- 27. Johnson BA, Nunley JR. Use of systemic agents in the treatment of acne vulgaris. Am Fam Physician 2000;62:1823–1836. [PubMed] [Google Scholar]
- 28. Akamatsu H, Horio T. The possible role of reactive oxygen species generated by neutrophils in mediating acne inflammation. Dermatology 1998;196:82–85. [DOI] [PubMed] [Google Scholar]
- 29. Basak PY, Gultekin F, Kilinc I, Delibas N. The effect of benzoyl peroxide and benzoyl peroxide/erythromycin combination on the antioxidative defense system in papulopustular acne. Eur J Dermatol 2002;12:53–57. [PubMed] [Google Scholar]
- 30. Rasheed Z, Al‐Shobaili HA, Alzolibani AA, et al. Immunological functions of oxidized human immunoglobulin G in type 1 diabetes mellitus: Its potential role in diabetic smokers as a biomarker of elevated oxidative stress. Dis Markers 2011;31:47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Al‐Shobaili HA, Al Robaee AA, Alzolibani A, Khan MI, Rasheed Z. Hydroxyl radical modification of immunoglobulin g generated cross‐reactive antibodies: Its potential role in systemic lupus erythematosus. Clin Med Insights Arthritis Musculoskelet Disord 2011;4:11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rasheed Z. Hydroxyl radical damaged immunoglobulin G in patients with rheumatoid arthritis: Biochemical and immunological studies. Clin Biochem 2008;41:663–669. [DOI] [PubMed] [Google Scholar]
- 33. Vera‐Ramirez L, Ramirez‐Tortosa M, Perez‐Lopez P, Granados‐Principal S, Battino M, Quiles JL. Long‐term effects of systemic cancer treatment on DNA oxidative damage: The potential for targeted therapies. Cancer Lett 2012;327:134–141. [DOI] [PubMed] [Google Scholar]
- 34. Lee HP, Pancholi N, Esposito L, et al. Early induction of oxidative stress in mouse model of Alzheimer disease with reduced mitochondrial superoxide dismutase activity. PLoS One 2012;7:e28033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Michel F, Bonnefont‐Rousselot D, Mas E, Drai J, Thérond P. Biomarkers of lipid peroxidation: Analytical aspects. Ann Biol Clin (Paris) 2008;66:605–620. [DOI] [PubMed] [Google Scholar]
- 36. Brito C, Naviliat M, Tiscornia AC, et al. Peroxynitrite inhibits T lymphocyte activation and proliferation by promoting impairment of tyrosine phosphorylation and peroxynitrite‐driven apoptotic death. J Immunol 1999;162:3356–3366. [PubMed] [Google Scholar]
- 37. Nguyen T, Brunson D, Crespi CL, Penman BW, Wishnok JS, Tannenbaum SR. DNA damage and mutation in human cells exposed to nitric oxide in vitro. Proc Natl Acad Sci USA 1992;89:3030–3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Oates JC, Christensen EF, Reilly CM, Self SE, Gilkeson GS. Prospective measure of serum 3‐nitrotyrosine levels in systemic lupus erythematosus: Correlation with disease activity. Proc Assoc Am Physicians 1999;111:611–621. [DOI] [PubMed] [Google Scholar]
- 39. Valdivia A, Pérez‐Alvarez S, Aroca‐Aguilar JD, Ikuta I, Jordán J. Superoxide dismutases: A physiopharmacological update. J Physiol Biochem 2009;65:195–208. [DOI] [PubMed] [Google Scholar]
- 40. Pastore A, Federici G, Bertini E, Piemonte F. Analysis of glutathione: implication in redox and detoxification. Clin Chim Acta 2003;333:19–39. [DOI] [PubMed] [Google Scholar]
- 41. Santangelo F, Witko‐Sarsat V, Drueke T, Descamps‐Latscha B. Restoring glutathione as a therapeutic strategy in chronic kidney disease. Nephrol Dial Transplant 2004;19:1951–1955. [DOI] [PubMed] [Google Scholar]


