Abstract
Background
Lupus nephritis is one of the most serious manifestations of systemic lupus erythematosus (SLE). Novel biomarkers are necessary to enhance the diagnostic accuracy, prognostic stratification, monitoring of treatment response, and detection of early renal flares.
Methods
Our study was conducted on 90 participants. They were divided into three groups, group I (controls) encompassed 30 ages and sex‐matched healthy personnel. Group II included 30 non‐nephritic SLE patients and finally group III included 30 SLE nephritic patients. Urinary monocyte chemoattractant protein‐1 (UMCP‐1) and hepcidin were evaluated by ELISA technique, compared and correlated in different groups, with each other and with other routine variables and with renal biopsy done to study group (III).
Results
Both UMCP‐1 and hepcidin in group III showed significant increase compared to other two groups (controls and group II) (468 ± 128, 111 ± 12, 252 ± 56 pg/ml, respectively, for UMCP‐1 and 40 ± 12, 11 ± 2, 20 ± 5 ng/ml, respectively, for hepcidin, P < 0.01). Also both UMCP‐1 and hepcidin in group III showed significant increase in diffuse proliferative subgroup compared to focal proliferative and mesangioproliferative subgroups (580 ± 43, 502 ± 46, and 352.6 ± 100 pg/ml, respectively, for UMCP‐1 and 47.8 ± 9.5, 41.4 ± 6, and 32.9 ± 10.8 ng/ml, respectively, for urinary hepcidin, P < 0.05).
Conclusion
UMCP‐1 and hepcidin could be associated with the susceptibility of lupus nephritis.
Keywords: lupus nephritis, urinary biomarkers, monocyte chemoattractant protein‐1, hepcidin
INTRODUCTION
Kidney disease is one of the most serious manifestations of systemic lupus erythematosus (SLE). Glomerulonephritis is one of the commonest and most serious manifestations of SLE 1. Despite the overall improvement in the care of SLE in the past two decades, the prognosis of lupus nephritis remains unsatisfactory. Up to 25% of patients still develop end‐stage renal failure 10 years after onset of renal disease 2. Novel biomarkers are available to enhance the diagnostic accuracy and sensitivity of lupus renal disease, prognostic stratification, monitoring of treatment response, and detection of early renal flares 3. Urine biomarkers appear to be more encouraging than serum biomarkers possibly because they are the direct products or consequences of kidney inflammation or injury. Urinary excretion of hepcidin is greatly enhanced in patients with iron overload, infections, or inflammatory diseases 4 and monocyte chemoattractant protein‐1 (MCP‐1; a leukocyte chemotactic factor that is involved in mediating inflammation and injury in lupus nephritis) 3. The aim of this study is to compare these markers with each other and other usual laboratory investigations.
SUBJECTS AND METHODS
The present study was conducted on 90 Egyptian females. They were divided into three groups; group I (controls) included 30 ages and sex‐matched healthy subjects. Group II encompassed 30 SLE patients without evidence of kidney affection. Finally, group III included 30 SLE patients with evidence of lupus nephritis. SLE patients were recruited from the rheumatology clinic, nephrology clinic, and Internal Medicine Department at Cairo University Hospital in the period from December 2010 to December 2011. They were all females. Their ages ranged from 15 to 47 years, whereas controls’ ages ranged from 18 to 49 years. All participants were subjected to medical history, full clinical examination, and routine laboratory investigations including serum creatinine, glomerular filtration rate (GFR), complement levels (C3, C4), antibodies to double‐stranded DNA, ESR, and albumin/creatinine ratio in urine. Renal biopsy was done to study group III. Specific laboratory investigations including immunoassay of hepcidin 25 and MCP‐1 urinary samples of all groups were done by using ELISA technique.
Statistical Methods
Data were summarized using mean, standard deviation for the quantitative variable. Comparisons between groups were done using analysis of variance (ANOVA) in quantitative variables. P values less than 0.05 were considered as statistically significant. Correlations between measured parameters and renal pathology were done using Spearman correlation coefficient. Statistical package SPSS version 16 was used.
Declaration of Ethics
This study was approved by the review board of Kasr Al‐Aini hospital, and written informed consent was obtained from all patients according to Helsinki guidelines of research ethics.
RESULTS
The controls’ ages (group I) ranged from 17 to 47 years with mean value of 33.59 ± 7.75, group II patients’ ages ranged from 15 to 45 years with mean value of 31.23 ± 5.15, whereas group III patients’ ages ranged from 18 to 49 years with a mean value of 39.57 ± 11.88 years. Regarding laboratory data, there was significantly higher albumin/creatinine ratio in lupus nephritis patients (group III) compared to controls (group I) and SLE patients with no evidence of nephritis (group II) (2267 ± 865, 15 ± 6, and 17 ± 6 mg/g, respectively, P < 0.01). Creatinine was significantly higher in group III compared to other two groups (controls and group II) (1.9 ± 0.7, 0.9 ± 0.2, and 0.9 ± 0.2 mg/dl, respectively, P < 0.01). GFR was significantly lower in group III compared to other two groups (32 ± 9, 109 ± 16, and 108 ± 15 ml/min, respectively, P < 0.01). C3 and C4 in group III showed significant decrease compared to other two groups (45 ± 12, 119 ± 20, and 67 ± 9 mg/dl, respectively, for C3 and 7 ± 3, 34 ± 5, and 10 ± 3 mg/dl, respectively, for C4, P < 0.01). ESR in group III showed significant increase compared to other two groups (100 ± 5, 7 ± 4, and 93 ± 10, respectively, P < 0.01). Urinary MCP‐1 (UMCP‐1) in SLE patients with evidence of lupus nephritis (group III) showed significant increase compared to other two groups (468 ± 128, 111 ± 12, and 252 ± 56 pg/ml, respectively, P < 0.01). In addition, urinary hepcidin in group III showed significant increase compared to other two groups (40 ± 12, 11 ± 2, and 20 ± 5 ng/ml, respectively, P < 0.01) (Table 1). Both UMCP‐1 and hepcidin in group III showed significant increase in diffuse proliferative subgroup compared to focal proliferative and mesangioproliferative subgroups (580 ± 43, 502 ± 46, and 352.6 ± 100 pg/ml, respectively, for UMCP‐1 and 47.8 ± 9.5, 41.4 ± 6, and 32.9 ± 10.8 ng/ml, respectively, for urinary hepcidin, P < 0.05). Correlations between both UMCP‐1 and hepcidin with each of creatinine, albumin/creatinine ratio, GFR, C3, and C4 in groups II and III revealed significant positive correlations regarding s.creatinine and albumin/creatinine ratio (r = 0.593, 0.652, respectively, with MCP‐1 and r = 0.645, 0.548, respectively, with hepcidin, P = 0.000), whereas there was a significant negative correlation regarding GFR, C3, and C4 (r = −0.693, −0.720, −0.674, respectively, with MCP‐1 and r = −0.704, –0.662, −0.618, respectively, with hepcidin, P = 0.000). There was a significant positive correlation between UMCP‐1 and hepcidin in lupus nephritis patients (group III) (r = 0.516, P = 0.003) (Fig. 1).
Table 1.
Statistical Comparison Between Mean Value of Each of UMCP‐1 and Hepcidin in All Studied Groups
| Parameter mean ± SD | Group I (controls) | Group II (SLE) | Group III (lupus nephritis) | P value |
|---|---|---|---|---|
| UMCP‐1 (pg/ml) | 111 ± 12 | 252 ± 56 | 468 ± 128 | <0.01 |
| Urinary hepcidin (ng/ml) | 11 ± 2 | 20 ± 5 | 40 ± 12 | <0.01 |
Figure 1.
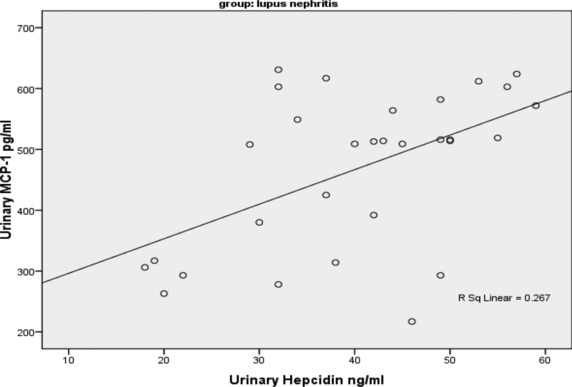
Correlation between UMCP‐1 and urinary hepcidin in group III (r = 0.516, P = 0.003).
DISCUSSION
Renal biopsy is the gold standard for providing information on the histological classes of lupus nephritis and the relative degree of activity and chronicity in the glomeruli. However, it is invasive, this necessitated discovery of novel biomarkers to enhance the diagnostic accuracy and sensitivity of lupus renal disease, prognostic stratification, monitoring of treatment response, and detection of early renal flares 5, 6, 7. MCP‐1, chemokine (C‐C motif) ligand 2 (CCL2) recruits monocytes, memory T cells, and dendritic cells to sites of tissue injury, infection, and inflammation. A number of recent cross‐sectional studies have confirmed that levels of urine MCP‐1 are elevated in patients with active lupus nephritis compared to those with inactive renal disease or healthy controls. MCP‐1 levels were higher in patients with proliferative (WHO class III or IV) than membranous (class V) nephritis 1. Hepcidin is a peptide hormone produced by the liver. It was discovered in 2000, and appears to be the master regulator of iron homeostasis in humans and other mammals. Hepcidin functions to increase iron storage in cells, thereby preventing an organism from losing too much iron. In humans, HAMP is the gene that encodes for hepcidin. A urine proteomic evaluation of lupus nephritis demonstrated that hepcidin may be a biomarker for SLE renal flare 8. The present study was chosen to implement a battery of novel laboratory investigations to assess the possibility of prediction of lupus nephritis among SLE patients and to use these novel markers as an alternative to renal biopsy.
In comparing demographic data (age and sex) of different groups, it was statistically insignificant. UMCP‐1 in group III showed significant increase compared to other two groups (468 ± 128, 111 ± 12, and 252 ± 56 pg/ml, respectively, the difference among the three groups was highly significant, P < 0.001). The data obtained in this study confirmed with those reported by Singh et al. 9 who implemented a similar clinical trial and reported that baseline mean UMCP‐1 levels in lupus nephritis flare, nonrenal flare, and stable SLE patients were 2.32 ± 1.06, 0.171 ± 0.03, and 0.213 ± 0.026 ng/mg creatinine, respectively, P < 0.001. Furthermore they stated that mean UMCP‐1 levels correlated significantly with severity of lupus nephritis (P = 0.0358). In addition, Kiani et al. 10 and Merks et al. 11 supported these findings and elicited that levels of urine MCP‐1 are elevated in patients with active lupus nephritis compared to those with inactive renal disease or healthy controls. Also this is in consistent with Rosa et al. 12 who found that UMCP‐1 is a biomarker of active LN, and MCP‐1 is made by infiltrating interstitial leukocytes in a number of glomerular diseases. UMCP‐1 was significantly greater in patients with moderate–severe interstitial inflammation than patients with mild or no interstitial inflammation.
A limited amount of work has been done on hepcidin evaluation in lupus nephritis patients, parallel to MCP‐1 assay, in our study urinary hepcidin showed significant increase in patients with moderate–severe interstitial inflammation (group III) as compared with patients with mild or no inflammation (controls and group II) (40 ± 12, 11 ± 2, and 20 ± 5 ng/ml, respectively, P < 0.01). In agreement with these findings, Zhang et al. 8 found that hepcidin 25 returned to baseline 4 months after the flare and reported that the urine level of hepcidin 25 changed according to lupus nephritis activity, and leukocytes (probably monocytes) infiltrating the renal interstitium of patients with SLE and active nephritis expressed hepcidin. These findings suggested that hepcidin from infiltrating renal monocytes may contribute to urinary hepcidin expression in lupus nephritis so that they can provide a beginning proteomic analysis aimed at predicting impending renal relapse, relapse severity, and the potential for recovery after SLE nephritis flare. Additionally, these data matched the opinion of Zhang and Rovin 13 who held an assay of hepcidin by a different methodology, investigated whether inflammatory cytokines relevant to the pathogenesis of lupus nephritis and other glomerular diseases regulate hepcidin expression by human monocytes. Hepcidin expression was examined by real‐time PCR and enzyme immunoassay, both techniques revealed that monocyte hepcidin mRNA increased during adherence to the tissue culture wells, reaching a level of 150‐fold higher than baseline within 12 hr of plating. Both UMCP‐1 and hepcidin in group III showed significant increase in diffuse proliferative subgroup compared to focal proliferative and mesangioproliferative subgroups (AUC 0.915, 0.927, respectively). It falls within the interest of Brunner et al. 14 to elicit the differential increase in levels of urinary biomarkers that formed a pattern reflective of specific histologic features seen in active LN. Correlations between both UMCP‐1 and hepcidin with each of creatinine, albumin/creatinine ratio, GFR, C3, and C4 in groups II and III revealed significant positive correlations regarding s.creatinine and albumin/creatinine ratio (r = 0.593, 0.652, respectively, with MCP‐1 and r = 0.645, 0.548, respectively, with hepcidin, P = 0.000), whereas there was a significant negative correlation regarding GFR, C3, and C4 (r = −0.693, −0.720, −0.674, respectively, with MCP‐1 and r = −0.704, – 0.662, −0.618, respectively, with hepcidin, P = 0.000). Furthermore, there was a significant positive correlation between UMCP‐1 and hepcidin in lupus nephritis patients (group III) (r = 0.516, P = 0.003). Further studies on larger populations are required to validate our reports and to evaluate the potential utility of UMCP‐1 and urinary hepcidin measurements in the monitoring and progression of lupus nephritis.
CONFLICT OF INTEREST
None declared.
ACKNOWLEDGMENTS
We thank Kasr Al‐Aini Hospital outpatient clinic employees for their assistance and guidance in this research. We also thank our patients for their willing participation in our research.
REFERENCES
- 1. Mok CC. Prognostic factors in lupus nephritis. Lupus 2005;14(1):39–44. [DOI] [PubMed] [Google Scholar]
- 2. Mok CC. Update on emerging drug therapies for systemic lupus erythematosus. Expert Opin Emerg Drugs 2010;15:53–70. [DOI] [PubMed] [Google Scholar]
- 3. Rovin BH, Birmingham DJ, Nagaraja HN, Yu CY, Hebert LA. Biomarker discovery inhuman SLE nephritis. Bull NYU Hosp Jt Dis 2007;65(3):187–193. [PubMed] [Google Scholar]
- 4. Nemeth E, Valore EV, Territo M, et al. Hepcidin, aputative mediator of anemia of inflammation, is a type II acute‐phase protein. Blood 2003;101(7):2461–2463. [DOI] [PubMed] [Google Scholar]
- 5. Gillespie E. Interferon‐regulated biomarkers of disease activity in SLE. Lupus 2010;19:1.20511279 [Google Scholar]
- 6. Weening JJ, D'Agati VD, Schwartz MM, et al. Classification of glomerulonephritis in systemic lupus erythematosus revisited. Kidney Int 2004;65: 521–530. [DOI] [PubMed] [Google Scholar]
- 7. Binder E, Edelbauer M. Use of biomarkers in the management of children with lupus. Curr Rheumatol Rep 2013;15(3):312. [DOI] [PubMed] [Google Scholar]
- 8. Zhang X, Jin M, Wu H, et al. Biomarkers of lupus nephritis determined by serial urine proteomics. Kidney Int 2008;74(6):799–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Singh RG, Usha FR, Rathore SS, Behura SK, Singh NK. Urinary MCP‐1 as diagnostic and prognostic marker in patients with lupus nephritis flare. Lupus 2012;21(11):1214–1218. [DOI] [PubMed] [Google Scholar]
- 10. Kiani AN, Johnson K, Chen C, et al. Urine osteoprotegerin and monocyte chemoattractant protein‐1 in lupus nephritis. J Rheumatol 2009;36(10):2224–2230. [DOI] [PubMed] [Google Scholar]
- 11. Marks SD, Shah V, Pilkington C, Tullus K. Urinary monocyte chemoattractant protein‐1 correlates with disease activity in lupus nephritis. Pediatr Nephrol 2010;25(11):2283–2288. [DOI] [PubMed] [Google Scholar]
- 12. Rosa RF, Takei K, Araújo NC, Loduca SM, Szajubok JC, Chahade WH. Monocyte chemoattractant‐1 as a urinary biomarker for the diagnosis of activity of lupus nephritis in Brazilian patients. J Rheumatol 2012;39(10):1948–1954. [DOI] [PubMed] [Google Scholar]
- 13. Zhang X, Rovin BH. Hepcidin expression by human monocytes in response to adhesion and pro‐inflammatory cytokines. Biochim Biophys Acta 2010;12:1262–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Brunner HI, Bennett MR, Mina R, et al. Association of noninvasively measured renal protein biomarkers with histologic features of lupus nephritis. Arthritis Rheum 2012;64(8):2687–2697. [DOI] [PMC free article] [PubMed] [Google Scholar]


