Abstract
Background
Newborn screening tests have been designed to identify infants with severe disorders that are relatively prevalent and treatable or controllable. Comparing to other countries, the incidence of these diseases are very high in Turkey where the rate of consanguineous marriage is high.
Methods
In this article, it is aimed to evaluate the development and organization of newborn screening programs in Turkey which include phenylketonuria, congenital hypothyroidism and biotinidase deficiency screenings. The point reached today, limitations of the program, expectations and projects for the future are discussed.
Results
Today, the point reached in screening programs of the country is appreciable. While the screening rate of the live born babies was 4,7% in 1987, this rate reached to 95% by 2008. Predicted target for newborn screening program at the strategic plan of Ministry of Health for 2010–2014 was to enhance this rate above 95% by the end of 2012. It seems that the envisaged goal has been reached.
Conclusion
National newborn screening program appears to be conducted successfully and extensively as a result of political determination and performance of health care workers who are in charge of this program. Nevertheless, limited numbers of the nutrition and metabolism clinics and specialists on these branches have caused some access difficulties, waste of time, and financial loss. Therefore, special planning to improve quality and the number of the clinics would be useful.
Keywords: newborn screening, phenylketonuria, congenital hypothyroidism, biotinidase deficiency
INTRODUCTION
In 1978, the Declaration of Alma‐Ata declared that every individual has equal access to basic healthcare irrespective of race, gender, nationality, ethnic or social origin as a universal birthright 1. Rather than the quantity, quality of health services has been the focus historically in developing countries, ample evidence suggests that quality of care (or the lack of it) must be at the center of every discussion about better health 2.
Although screening may lead to an earlier diagnosis, not all screening tests have been shown to benefit the person being screened; overdiagnosis, misdiagnosis, and creating a false sense of security are some potential adverse effects of screening. In 1968, Wilson and Jungner discussed their Principles and Practice of Screening for Disease, which was published as a World Health Organization (WHO) monograph 3.
Newborn screening aims to identify a selected amount of genetically transmitted diseases. Most of these diseases are autosomal recessive. Although they can pass on a mutated gene, the parents do not usually show symptoms. For that reason, there has not been any previous occurrence of the disease in the family and the disease occurs unexpectedly. Newborn screening tests have been designed to identify infants with severe disorders that are relatively prevalent and treatable or controllable. If early diagnosis has a positive influence in this regard, screening is advisable, as it benefits the child 4, 5.
In this article, we aim to evaluate the development and organization of newborn screening programs in Turkey. Recent developments of the programs, limitations of the programs, expectations, and projects for the future are discussed.
THE DEVELOPMENT OF NEWBORN SCREENING IN THE WORLD AND TURKEY
Historically, screening for phenylketonuria (PKU) was based on a prediction that a dietary treatment would prevent mental retardation 6. After some small, locally organized programs for PKU in the United Kingdom, comprehensive newborn screening was widely promoted by Robert Guthrie, who developed a feasible way to detect PKU using a bacterial inhibition assay for phenylalanine 7. A few years after the use of Guthrie test for PKU in newborn screening, Guthrie enabled the detection of more metabolic disorders by developing bacterial inhibition tests for other metabolites. Examples of these metabolites are leucine for maple syrup urine disease, methionine for homocystinuria, and galactose for galactosemia. Beutler used different methods in newborn screening by developing an assay enzyme applied on a dried blood sample for galactosemia 8. From this point, the number of disease screened in the newborn period began to increase.
Recent technological innovations have changed the newborn screening landscape significantly. In particular, tandem mass spectrometry (MS/MS)—developed by Dave Millington and his group in the early 1990s—offers a wide spectrum of diagnostic possibilities in a single analytical run 9. The system allows screening for more than 30 well‐defined genetic disorders and the analyses can be performed simultaneously. Furthermore, once MS/MS is introduced, including new tests into the program adds only minimal extra cost. A number of European countries have implemented expanded population‐wide newborn screening using MS/MS technology 10. Germany expanded its program in December 2004, but decided to limit screening with MS/MS to eight diseases 11. The Netherlands added 14 diseases in 2007, and Austria has screened for 20 disorders using MS/MS 10. Other countries with expanded newborn screening programs are Belgium, Denmark, Poland, Portugal, and Spain. The United Kingdom and other countries (e.g., Switzerland) are, however, cautious in expanding their programs 12. In 2006, a large‐scale pilot study on newborn screening for medium‐chain acyl‐CoA dehydrogenase deficiency (MCADD) was completed in Britain. A nationwide newborn screening for MCADD using MS/MS was introduced in February 2009 13.
PKU is the first disease screened in the newborn period in Turkey. It is an autosomal recessive disorder with the phenylalanine hydroxylase locus on chromosome 12q24.1. More than 500 different mutations have been described, including deletions, insertions, missense mutations, splicing defects, and nonsense mutations. The average incidence of this disorder is approximately 1:15,000 live births 14. Compared to other countries, Turkey has a high incidence of PKU, which may be caused by high rate of consanguineous marriages (24.1%) 15. In Turkey, in 2001, the incidence of hyperphenylalaninemia was 1:4,172 whereas a PKU incidence was of 1:5,049 16.
In Turkey, in 1983, an important scientific project, supported by The Scientific and Technological Research Council of Turkey, was initiated by Hacettepe University Medical Faculty, Department of Child Health and Nutrition‐Metabolic Diseases Unit. The results of this project showed that PKU incidence was higher among the Turkish newborns than expected. Afterwards, the Ministry of Health of Turkey expanded the organization of the newborn screening program that was started in 1986 17.
During the first years of the program, the screening covered only some centers of province. In 1993, the provinces of Istanbul (Istanbul University) and İzmir (Dokuz Eylül University), (9, Eylül University) were included in the program. In 1994, screening program was transformed to the “National Phenylketonuria Screening Program” encompassing the whole country 18.
Sivas Cumhuriyet University was included in the program by screening the province of Sivas in 2001 and provinces of Malatya, Tokat, and Erzincan in 2002. The remaining 74 provinces have been screened by Hacettepe University in coordination with Ministry of Health, Maternal and Child Health and Family Planning General Directorate (currently known as Department of Child and Adolescent Health, DCAH).
Since this is a very large‐scale program at the country level, it requires political determination. The political support for this area was included in the policy documents of the government and development of mother and child health. In this regard, preventive healthcare services and health protection have been particularly emphasized 19, 20.
While the screening program was conducted by the collaboration of these four universities, several problems emerged. These problems were as follows:
Standardization problems as screening was performed by different laboratories of the institutions.
Control and organization problems as the institutions where the screenings were carried out had autonomous structure and administrative problems with the same reason.
Sample transportation difficulties from the field to the laboratories resulted in delays.
The infrastructure and capacity problems as the laboratories were not specialized for screening.
The preservation, evaluation, and documentation of the data.
Therefore, the process of conducting screening and surveillance by one center,The Ministry of Health, came into question in order to access the babies with “suspicious” screening tests in a faster way. Two laboratories of Refik Saydam National Public Health Agency of Turkey (currently known as Public Health Institution of Turkey, PHIT), situated in Ankara and Istanbul, have been privatized for this purpose. With the advisory decision of the Scientific Committee consisting of academicians, congenital hypothyroidism (CH) screening was added to PKU screening on December 25, 2006. In this manner, the program has transformed into “National Newborn Screening Program” by the coordination of DCAH and PHIT 21.
Although the prevalence of CH varies by race and ethnicity, it is 1 in 3,000 to 4,000 live births in the world 22. In a study conducted in our country, the incidence of permanent CH was reported to be 1 in 2,736 live births 23. Afterwards, the screening for biotinidase deficiency was added to the program in October 2008 with the recommendation of Scientific Committee.
Biotinidase deficiency may cause severe neurological defects, dermatological signs, metabolic disorders, coma, and death if untreated in the early neonatal period 24, 25, 26. The estimated incidence of the disease is about 1 in 60,000 27. A pilot study conducted in Turkey showed that the incidence of biotinidase deficiency (1:11,000) was higher than other countries 28.
ORGANIZATION OF THE NEWBORN SCREENING PROGRAM IN TURKEY
In this program, it has been stated that the blood samples ideally should be taken at the 48th–72nd h after birth. However, with the aims of reaching many more infants and registering these babies as soon as possible, blood samples have been taken “at the last moment before leaving the health institution.” If the blood sample is taken before adequate feeding, the parents should be advised to apply at the nearest health facility within the first week of life for a new blood sample.
In newborns hospitalized in NICU, specimens should be collected on the screening cards at 24–36 h after birth, unless the infant receives blood. Repeat specimens are obtained on cards at 14 and 30 days of age or upon discharge, if discharge is prior to 14 or 30 days of age. Alternatively, if there is a 72‐h window of opportunity during the first 30 days that the infant is not being transfused or receiving total parenteral nutrition, the post‐72 h repeat specimen should be obtained at that time. This specimen would be in place of the 14‐ or 30‐day specimen whichever is closer. Important information such as prematurity, antibiotic use, and blood transfusion should be noted on filter papers.
It should be noticed that CH screening cannot preclude the clinical suspicion of hypothyroidism (e.g., protracted jaundice) on health staff examination at or after neonatal period. For these babies,whether screened or not, blood should be absolutely taken for T4 and Thyroid stimulating hormone (TSH) measurements 29.
Blood‐spot samples, taken by health facilities in the province for neonatal screening program, should be collected by the Provincial Health Directorate. Then all the samples should be gathered in one package and sent to Ankara and Istanbul laboratories of PHIT periodically (two times per week), depending on the study speed of the laboratories. Data entry for the blood samples includes the information available on the paper and application made on a web browser of Ministry of Health. Each blood sample paper has a special barcode number, which ensures that each baby has a single record 30. Selection of the laboratory center and posting day of the blood samples should be planned according to the cities’ geographic location and the expected number of live births.
Screening Methods
At the beginning of the program, bacterial inhibition method was used to screen PKU. Currently, fluorescent immunoassay method has been used for this purpose. Colorimetric method for biotinidase deficiency and HPLC for CH have been used. The results are entered in a database via the web application. Registered data are announced and published by the Provincial Health Directorates. At this stage, healthcare workers in charge of this program immediately reach the babies whose screening results are suspicious (Figs. 1, 2, 3). The need for further diagnostic tests and the decision whether or not to treat the infant are at the clinician's discretion.
Figure 1.
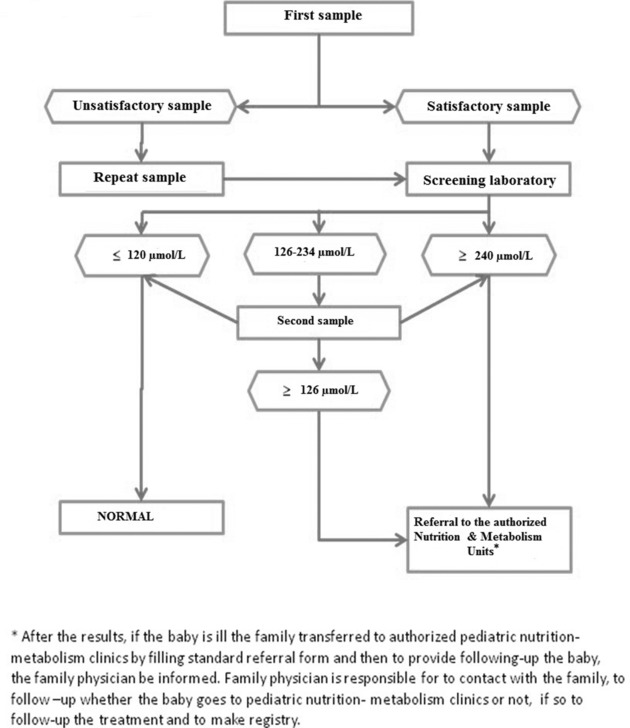
The algorithm of newborn screening for PKU.
Figure 2.
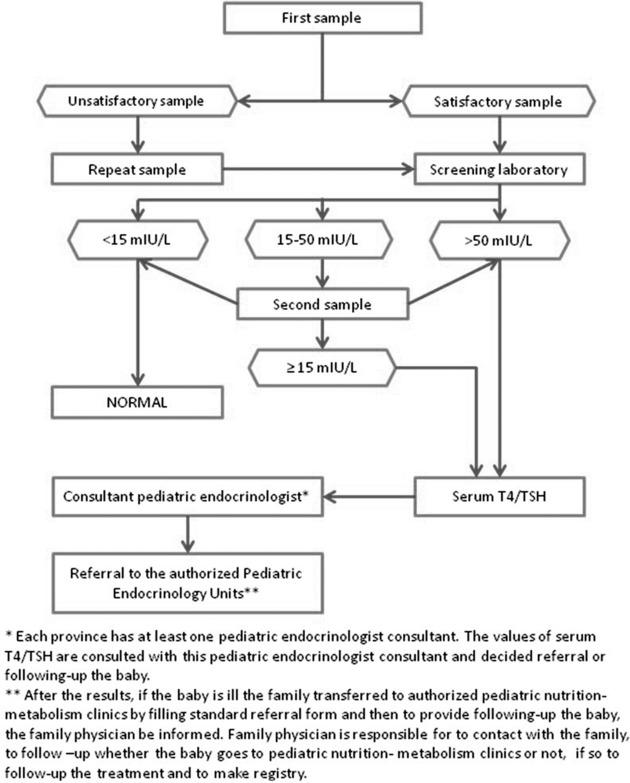
The algorithm of newborn screening for CH.
Figure 3.

The algorithm of newborn screening for biotinidase deficiency.
Currently, there are 14 nutrition and metabolism clinics and 49 pediatric endocrinology clinics nationwide in Turkey 31. Babies are referred to the related clinic or specialist according to their results of screening by the representative of the Provincial Health Directorates. The results of the detailed diagnostic tests of these infants are entered in a different database for close follow‐up of them. Additionally, consultant academicians are assigned to consult CH cases in each province. In this way, these patients can be treated and followed‐up in their own settings. Unfortunately, the same practice cannot be achieved for PKU and biotinidase deficiency due to the limited number of nutrition and metabolism clinics.
RECENT NEWBORN SCREENING PROGRAM IN TURKEY
Today, the point reached in screening programs of the country is appreciable. Turkey, with 72 million people and an area of 780,000 km2, is one of the largest countries in European region of the WHO. Approximately 1.3 million babies are born throughout the world each year. While the screening rate of the liveborn babies was 4.7% in 1987, this rate reached to 95% by 2008 (Fig. 4, 32).
Figure 4.
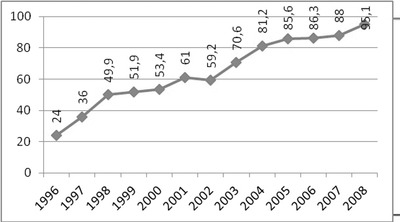
Newborn screening rates in Turkey by years.
According to data of the last Demographic and Health Survey (2008) of Turkey, which is conducted every 5 years, 92% of the families (between 2003 and 2008) have responded positively when asked whether blood‐spot samples were taken from their babies or not 33. Predicted target for newborn screening program of the strategic plan of Ministry of Health for 2010–2014 is to increase this rate above 95% by the end of 2012. It seems that the envisaged goal has been reached 34.
In a recent survey covering the EU member states (potential), candidate member states including Turkey and EFTA countries, in total 40 countries, the status and function of newborn screening programs were evaluated. The survey revealed that actual practice was often organized but not regulated by guidelines. The authors reported that material for informing patients focused on treatment rather than explanation of necessity of confirmatory diagnosis. It was also stated that training of professionals was rarely regulated by guidelines 35, 36.
FUTURE OF NEWBORN SCREENING PROGRAM IN TURKEY
National newborn screening program appears to be conducted successfully and extensively as a result of political determination and performance of healthcare workers who are in charge of this program. Nevertheless, limited numbers of the nutrition and metabolism clinics and specialists on these branches have caused some access difficulties, waste of time, and financial loss. Therefore, special planning to improve quality and the number of the clinics would be useful.
The next step is to integrate new diseases to the screening panel. Epidemiological studies are needed to determine which diseases are appropriate for screening nationwide. For this reason, PHIT plans to conduct a new project. In the scope of this program, the screening procedure will be done on 100,000 blood samples coming to the screening laboratories. The possible diseases that can be added to panel will be screened by MS/MS and by this way the diseases will have greater chance to be determined. This will shed light onto the future orientation of screening program. Of course, the improvement efforts should be continued.
ACKNOWLEDGMENTS
We would like to express our sincere thanks to all members of the Scientific Committee for their significant contribution in the establishment and development of National newborn Screening Program in Turkey. The skillful technical assistance of all laboratory workers of Refik Saydam National Public Health Agency (currently called as Public Health Institution of Turkey) is greatly appreciated.
REFERENCES
- 1. Declaration of Alma‐Ata International Conference on Primary Health Care , Alma‐Ata, USSR, 6–12 September 1978. [PubMed]
- 2. Health Promotion Glossary World Health Organization 1998 Division of Health Promotion , Education and Communications (HPR) Health Education and Health Promotion Unit (HEP) WHO/HPR/HEP/98.1 p 3.
- 3. Wilson JMG, Jungner F. Principles and Practice of Screening for Disease (Public Health Papers No. 34). Geneva: World Health Organization; 1968. p. 26–27. [Google Scholar]
- 4. Dhondt JL. Expanded newborn screening: Social and ethical issues. J Inherit Metab Dis 2010;33(Suppl 2):S211–S217. [DOI] [PubMed] [Google Scholar]
- 5. Wieser B. Public accountability of newborn screening: Collective knowing and deciding. Soc Sci Med 2010;70:926–933. [DOI] [PubMed] [Google Scholar]
- 6. Bickel H, Gerrard J, Hickmans E. Influence of phenylalanine intake on phenylketonuria. Lancet 1953;265(6790):812–813. [DOI] [PubMed] [Google Scholar]
- 7. Guthrie R, Susi A. A simple phenylalanine method for detecting phenylketonuria in large populations of newborn infants. Pediatrics 1963;32:338–343. [PubMed] [Google Scholar]
- 8. Beutler E, Baluda MC. A single spot screening test for galactosemia. J Lab Clin Med 1966;68:137–141. [PubMed] [Google Scholar]
- 9. Pollitt RJ. International perspectives on newborn screening. J Inherit Metab Dis 2006;29:390–396. [DOI] [PubMed] [Google Scholar]
- 10. Bodamer OA, Hoffmann GF, Lindner M. Expanded newborn screening in Europe 2007. J Inherit Metab Dis 2007;30:439–444. [DOI] [PubMed] [Google Scholar]
- 11. Hess R. Beschluss über eine Änderung der Richtlinie des Bundesausschusses der Ärzte und Krankenkassen über die Früherkennung von Krankheiten bei Kindern bis zur Vollendung des 6. Lebensjahres (Kinder‐Richtlinien) zur Einführung des erweiterten Neugeborenen‐Screenings vom 21 Dezember 2004. Dt Ärzteblatt 200 5;102:A1158–A1163. [Google Scholar]
- 12. Loeber GJ. Neonatal screening in Europe; the situation in 2004. J Inherit Metab Dis 2007;30:430–438. [DOI] [PubMed] [Google Scholar]
- 13. UK Newborn Screening Programme Centre . Available from: http://newbornbloodspot.screening.nhs.uk/mcadd
- 14. Kaye CI, Committee on Genetics . Newborn screening fact sheets. Pediatrics 2006;118:934–963. [DOI] [PubMed] [Google Scholar]
- 15. Tezcan S. Reproductive Health of Turkish Women: DHS 1998–2008 Approaches for Perinatal Period Congress; Problems & Solutions. February 10–13, 2011, Antalya, Available from: www.perinatolojikongresi.com/11.02.2011/I.oturum/sebehat_tezcan.ppt
- 16. Özalp İ, Coşkun T, Tokatlı A, et al. Newborn PKU screening in Turkey: at present and organization for future. Turk J Pediatr. 2001;43:97–101. [PubMed] [Google Scholar]
- 17. Özalp İ. Yenidoğanda fenilketonüri ve hiperfenilalaninemilerin taranması. Katkı Pediatri Dergisi 2000;21:176–177. [Google Scholar]
- 18. Ministry of Health of Turkey , Mother and Child Health and Family Planning General Directorate, March 29, 1994 and curriculum 4190/1319.
- 19. Republic of Turkey 58th Cabinet Emergency Action Plan, Health Policies, no. 36, p. 99.
- 20. Ninth Development Plan 2007–2013, 7.3.2. Activation of Health System, item 611, p. 88.
- 21. Ministry of Health of Turkey , Mother and Child Health and Family Planning General Directorate, December 25, 2006 and curriculum 4911/130.
- 22. Fisher DA. Second International Conference on Neonatal Thyroid Screening: Progress report. J Pediatr 1983;102:653–654. [DOI] [PubMed] [Google Scholar]
- 23. Yordam N, Calikoglu AS, Hatun S, et al. Screening for congenital hypothyroidism in Turkey. Eur J Pediatr 1995;154:614–616. [DOI] [PubMed] [Google Scholar]
- 24. Sweetman L. Two forms of biotin‐responsive multiple carboxylase deficiency. J Inherit Metab Dis 1981;4:53–54. [DOI] [PubMed] [Google Scholar]
- 25. Moss J, Lane MD. The biotin‐dependent enzymes. Adv Enzymol 1971;35:321–442. [DOI] [PubMed] [Google Scholar]
- 26. Wolf B. Disorders of biotin metabolism: Treatable neurological syndromes In: Rosenberg R, Prusiner SB, Di Mauro S, Barchi RL, Kunkel LM, editors. The Molecular and Genetic Basis of Neurological Disease. Stoneham, MA: Butterworth; 1993. [Google Scholar]
- 27. Wolf B. Worldwide survey of neonatal screening for biotinidase deficiency. J Inherit Metab Dis 1991;14:923–927. [DOI] [PubMed] [Google Scholar]
- 28. Baykal T, Huner G, Sarbat G, Demirkol M. Incidence of biotinidase deficiency in Turkish newborns. Acta Paediatr. 1998;87:1102–1103. [DOI] [PubMed] [Google Scholar]
- 29. Ministry of Health of Turkey , Newborn Screening Program. Available from: http://www.saglik.gov.tr/ACSAB z/dosya/1‐71556/h/yenidogan‐topuk‐kani‐ornegi‐toplama‐klavuzu.pdf.
- 30. Ministry of Health of Turkey , Newborn Screening Web Application. Available from: http://yenineonatal.rshm.gov.tr/Login.
- 31. Ministry of Health of Turkey , Newborn Screening Program, Available from: http://www.saglik.gov.tr/ACSAB/dosya/1‐71542/h/ntp‐genelge.pdf.
- 32. Ministry of Health of Turkey , Turkey Health Transformation Program. Available from: http://www.saglik.gov.tr/EN/dosya/2‐1217/h/htp2009jan.pdf.
- 33. Hacettepe University Institute of Population Studies . Fertility, Reproductive Health and Ageing in Turkey, Ankara. 2010, p. 143–145. [Google Scholar]
- 34. Ministry of Health of Turkey , Strategic Plan 2010–2014; p 94. Available from: http://www.sgb.saglik.gov.tr/content/files/spflash/flashbrosur/index.html.
- 35. Loeber JG, Burgard P, Cornel MC, et al. Newborn screening programmes in Europe; arguments and efforts regarding harmonization. Part 1. From blood spot to screening result. J Inherit Metab Dis. 2012;35:603–611. [DOI] [PubMed] [Google Scholar]
- 36. Burgard P, Rupp K, Lindner M, et al. Newborn screening programmes in Europe; arguments and efforts regarding harmonization. Part 2. From screening laboratory results to treatment, follow‐up and quality assurance. J Inherit Metab Dis. 2012;35:613–625. [DOI] [PubMed] [Google Scholar]


