Abstract
Background
Tobacco‐specific carcinogen 4‐(methylnitrosamino)‐1‐(3‐pyridyl)‐1‐butanol (NNAL) was measured in all participants aged 6 years and older from the Centers for Disease Control and Prevention's National Health and Nutrition Examination Survey 2007–2008. The suitability of using creatinine or specific gravity for urinary NNAL correction in exposure assessment is examined in this study.
Methods
Effects of both specific gravity and creatinine correction on urinary NNAL among smokers were investigated with multiple linear regression models using either normalization or the fitting of creatinine and specific gravity in the model as covariates.
Results
When log‐scaled NNAL was normalized by either creatinine or specific gravity, R 2 was slightly higher for creatinine than for specific gravity (R 2 = 0.1694 and 0.1439, for creatinine and specific gravity, respectively). When log‐scaled NNAL was normalized by both factors, the R 2 was improved (R 2 = 0.2068). When specific gravity or creatinine was included as a covariate separately in the models, they were highly significant factors (P < 0.001, R 2 = 0.2226 and 0.1681 for creatinine and specific gravity, respectively). However, when both were included in the model as covariates, creatinine remained highly significant (P < 0.001), whereas the significance of specific gravity was eliminated (P = 0.4294).
Conclusion
This study confirms significant relationships between NNAL concentrations and both urine creatinine and specific gravity. We conclude that creatinine is the more influential and preferred variable to account for urine dilution in tobacco‐specific nitrosamine exposure assessment.
Keywords: tobacco‐specific nitrosamine, 4‐(methylnitrosamino)‐1‐(3‐pyridyl)‐1‐butanol (NNAL), creatinine, specific gravity
INTRODUCTION
The tobacco‐specific carcinogen NNK, 4‐(methylnitrosamino)‐1‐(3‐pyridyl)‐1‐butanone, is a major harmful constituent of tobacco. In the body NNK is metabolized to NNAL, 4‐(methylnitrosamino)‐1‐(3‐pyridyl)‐1‐butanol, which can be isolated and quantified in human urine 1, 2. NNAL is a potent pulmonary carcinogen in rodents 3, 4, 5, 6. NNAL is a biomarker exclusively specific to the exposure of tobacco‐specific carcinogen NNK. It has not been detected in nontobacco users unless they were exposed to secondhand smoke. Since NNAL is a trace component of cigarette smoke, the primary origin of NNAL in urine of cigarette smokers is the metabolism of NNK. As a consequence, urinary NNAL is an important indicator of a person's direct exposure to tobacco nitrosamine carcinogens.
To accurately account for intra‐ and interpersonal variation in urine concentration caused by fluctuations in fluid intake, physical activity, temperature, etc., the urinary NNAL concentrations should be corrected. The reason is that uncorrected values may lead to under‐ or overestimation if the urine sample is overly diluted or overly concentrated, respectively. Conventional methods of urine metabolite normalization include creatinine and specific gravity 7, 8. Each has its limitations, but each can also be useful when used properly. Muscat et al. found creatinine and specific gravity are interchangeable to correct urinary cotinine 9. When meat intake or muscle mass has a large variation in the population, specific gravity seems to be more appropriate 10.
Creatinine correction is most useful for biomarkers that undergo a similar process of renal elimination 11. In the kidney, blood flows into the glomerulus and is filtered into the Bowman's capsule. Compounds bound for excretion are moved from the Bowman's capsule into the renal tubule, while other components are reabsorbed back into the blood in the tubular system. Metabolites may also passively diffuse or be actively transported from the blood into the tubule to be excreted. Creatinine is cleared from the body primarily through filtration, whereas some (15–20%) may be actively secreted by the tubules. Creatinine is not known to be reabsorbed by the renal tubules 8, 12.
Urinary creatinine has been used to estimate biomarker excretion rates because it is excreted at a reasonably constant rate, and thus it is useful for normalizing spot urine samples within demographic groups 8, 13. In addition to normalizing for dilution due to water excretion, there are other advantages of creatinine correction: (i) creatinine excretion is a time surrogate, so that creatinine‐corrected values are in actuality excretion rates; (ii) creatinine‐corrected values can be used to calculate doses at steady state (multiplied by expected creatinine excretion/body weight, or other formula); and (iii) creatinine‐corrected values are intrinsically adjusted for lean body mass, and thus they are somewhat proportional to the internal dose of biomarker in most nonfat tissues. As such it has become the standard means of correcting for urine concentration variability in many studies 13, 14, 15, 16, 17, 18, 19. However, Barr et al. (8) noted previous research that indicated creatinine levels are different between persons based on muscle mass, gender, age, diet, and renal function. Because of these differences in creatinine formation and excretion, normalization of biomarker excretion rates across different demographic groups may be compromised.
Specific gravity is a measure of the relative density of a substance to a reference material, usually water. Previous studies have indicated a strong correlation between specific gravity and creatinine, and they have shown that specific gravity can be a useful replacement for creatinine when correcting for urinary metabolites 7, 20. Thus specific gravity has become another accepted means when urinary biomarker concentration is normalized. However, the use of creatinine or specific gravity to adjust metabolites seems to be biomarker‐dependent. Gaines et al. indicated that creatinine‐corrected urinary biomarkers show more accuracy than specific gravity corrected results, whereas Newman et al. concluded that interpersonal specific gravity variability is less than creatinine and may be a more appropriate method of correction 21, 22, 23.
The following formula is used when the concentration is normalized by specific gravity (SG), where [Analyte]normalized is the normalized concentration of the analyte in the sample, [Analyte]measured is the measured concentration of the analyte in the sample, SGavg is the average of specific gravity in all samples, and SG is the specifc gravity in the sample:
This formula was first used by Levine and Fahy in 1945 24 when they found the mass of total dissolved solids to be inversely log10‐linearly proportional to SG − 1 in spot urine samples. Later, on the basis of the work of Araki 25, Vij and Howell 26 proposed a modified SG correction formula, as shown above. This formula has been used to correct urinary tobacco exposure biomarkers 11, urinary cadmium 10, urinary arsenic 27, 1,6‐hexamethylene diamine 11, urinary protein and albumin 22, and other toxic substances in urine 28, 29.
The primary goal of this study was to examine the suitability of using creatinine concentrations or specific gravity for urinary NNAL correction in exposure assessment. Our objective was to determine the best method for correcting NNAL to account for variability in interpersonal urine concentrations in National Health and Nutrition Examination Survey (NHANES) samples by using creatinine and specific gravity, alone or together, in ratio normalization or as covariates in multiple linear regression models.
MATERIALS AND METHODS
Study Design
NHANES is a large national survey conducted by the National Center for Health Statistics of the Centers for Disease Control and Prevention (CDC). NHANES is unique in that it combines interviews and physical examinations 30. This survey is designed to assess the health and nutritional status of adults and children in the United States. Urinary NNAL measurements have been included in the NHANES protocol beginning in 2007. The sampling design for NHANES is based on a complex, multistage probability strategy that includes selection of primary sampling units (counties), household segments within the counties, and sample patients from selected households. The sampling is designed to represent the U.S. population on the basis of gender, race/ethnicity, and age. The detailed information regarding the sample design and the sample size is stated elsewhere 31. The unweighted sample size for NHANES 2007–2008 is 9,762 examined participants. As stated, data are collected through both household interviews and standardized physical examinations, which are conducted in a mobile examination center. Urine specimens were collected from each participant aged 6 years or older to analyze urinary total NNAL (free NNAL plus NNAL‐glucuronide)32, 33. The NNAL measurement protocol was reviewed and approved by CDC's Institutional Review Board, and informed written consent was obtained from all participants in the NHANES study.
Demographic Variables
In NHANES 2007–2008, 9,762 participants reported sociodemographic data, among which 6,943 participants (6 years and older) provided urine samples for NNAL measurement. After the exclusion of invalid samples and nonreportable laboratory results, N = 6,384 was used in the data analysis. Sociodemographic data including age, gender, and race/ethnicity were derived from self‐reported questionnaire data. In this report, we have focused on all participants in gender groups of male and female, and in age groups of 6–11, 12–19, 20–44, 45–64, and ≥65 years. The race/ethnicity variable was also categorized into four groups consisting of non‐Hispanic white (NHW), non‐Hispanic black (NHB), Mexican American (MA), and other (OTH) participants.
Laboratory Methods
During the physical examinations in the mobile examination center, urine specimens were collected from participants, aliquoted, and stored frozen until shipped to the CDC's National Center for Environmental Health. We measured total NNAL using a previously described method, with additional modifications 1, 34. Briefly, 5 ml urine samples were spiked with 13C6‐labeled NNAL internal standard, from Toronto Research Chemicals Inc. (Toronto, Canada), and hydrolyzed overnight with β‐glucuronidase, purchased from Sigma‐Aldrich (St. Louis, MO, USA). The hydrolysate was then further processed by the sample cleanup method and analyzed by high‐performance liquid chromatography atmospheric‐pressure ionization tandem mass spectrometry (HPLC–API MS/MS). NNAL was quantified based on the peak area ratio of the native ion to isotope‐labeled internal standard. This method of measuring NNAL has a limit of detection of 0.6 pg/ml, based on the variance from the repetitive analyses of a low‐spiked urine sample (2 pg/ml). We have confirmed that NNAL remains stable in urine for at least several years during long‐term storage at −70°C 35. Serum cotinine was measured using a previously described method 36. Urinary creatinine concentrations of all participants aged 6 years and older were determined using an enzymatic (creatinase) method implemented on a Beckman Synchron CX3 Clinical Analyzer by Beckman Coulter (Brea, CA, USA). Additional details about the NNAL, cotinine, and creatinine methods are available at the NHANES Web site (CDC, NHANES 2007–2008).
Specific gravity was measured by a digital refractometer ATAGO PAL‐10S from ATAGO (Bellevue, WA, U.S.A), with automatic temperature compensation. A drop of urine sample (∼0.3 ml) was placed on the quartz window and the reading was recorded. The refractometer was calibrated to 1.000 with HPLC grade water once for each run. Since there were 24 samples in each run, the refractometer was calibrated once every 24 samples. A blank and two quality‐control pools were included in each analytical run for both NNAL and SG measurements. Reported results met the accuracy and precision specifications of the quality control/quality assurance program of the Division of Laboratory Sciences, National Center for Environmental Health, CDC 37.
Statistical Analysis
In NHANES 2007–2008, 1,343 participants aged 12 years and older reported the use of cigarettes exclusively in the last 5 days, which is 84% of all participants who reported the use of tobacco products in the past 5 days (N = 1,551, all tobacco forms). Some of the people who used other forms of tobacco, such as pipes (N = 15, 0.9%), cigars (N = 115, 7.2%), chewing tobacco (N = 90, 5.6%), and snuff (N = 39, 2.4%), reported smoking cigarettes in addition to using other forms of tobacco products. Since the majority of tobacco users were cigarette smokers in NHANES 2007–2008, the term smoker is used interchangeably with tobacco user throughout this article. Smokers were separated from nonsmokers based on a serum cotinine cutoff point of 10 ng/ml 38. By using this cutoff point, 24.42% of NHANES 2007–2008 participants were smokers and 75.58% were nonsmokers.
In univariate analyses, we calculated geometric mean concentrations of urinary creatinine and specific gravity among nonsmokers and smokers, including subcategories such as gender, race/ethnicity, and age. Both urinary creatinine and specific gravity were log10‐transformed to reduce the skewness in their distributions. A quadratic term of age was found to be statistically significant in a preliminary analysis. For this reason, both a linear and a quadratic term of age were included in the model. Two sets of multiple linear regression models were developed to examine NNAL correction by creatinine, specific gravity, and both creatinine and specific gravity. In the first set of the three models, the dependent variables are NNAL corrected by creatinine, NNAL corrected by gravity, and NNAL corrected by the both the creatinine and the gravity, where the independent variables are age, age square, race, and gender. NNAL correction was calculated by normalization using established formulas. NNAL‐creatinine correction (NNAL‐CR) was generated as NNAL microgram per gram creatinine:
where [NNAL] was in nanogram per milliliter, [CR] was in milligram per deciliter, and a factor of 100 was used to convert deciliter to milliliter.
NNAL‐specific gravity correction (NNAL‐SG) was calculated by the normalization of NNAL by specific gravity using:
and NNAL correction by both creatinine and specific gravity (NNAL‐CR‐SG) was calculated by using the SG‐corrected‐creatinine ratio normalization technique:
| (1) |
where [NNAL] is the NNAL concentration, SGavg is the average SG, and [CR] is the creatinine concentration.
In the second set of models (five models in Table 3), NNAL correction was examined by adding log‐scaled creatinine and/or specific gravity in the models as a covariate or as covariates. Forward selection was carried out from a base model with significant demographic variables to build model 1. The dependent variable was NNAL, and the independent variables were age, age square, race, and gender for model 1. Sequentially, log(creatinine) and log(SG) were entered into model 1 as independent variables one at a time to create model 2 and model 3. Log(SG) was added to model 1 as independent variable for model 4, and then lastly log(creatinine) was added to model 4 for model 5. The percentage change of beta coefficients was compared between models.
Table 3.
Beta Coefficients and R 2 from the Multiple Regression Models for Urinary NNAL (Picogram per Milliliter) Adjusting by Log‐Scaled Creatinine and Specific Gravity as Covariates Among Tobacco Users* in NHANES 2007–2008
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| R 2 | 0.0716 | 0.2226 | 0.2275 | 0.1681 | 0.2275 | |||||
| Beta | P‐ | Beta | P‐ | Beta | P‐ | Beta | P‐ | Beta | P‐ | |
| coefficient | value | coefficient | value | coefficient | value | coefficient | value | coefficient | value | |
| Age | 0.0083 | <0.0001 | 0.0124 | <0.0001 | 0.0127 | <0.0001 | 0.0113 | <0.0001 | 0.0127 | <0.0001 |
| Age square | −0.0003 | 0.0003 | −0.00035 | <0.0001 | −0.00035 | <0.0001 | −0.0004 | <0.0001 | −0.0004 | <0.0001 |
| Race/ethnicity | −0.0896 | 0.0043 | −0.113 | 0.0006 | −0.1136 | 0.0009 | −0.1152 | 0.0013 | −0.1136 | 0.0009 |
| Log(creatinine) | 0.726 | <0.0001 | 0.7637 | <0.0001 | 0.7637 | <0.0001 | ||||
| Log(gravity) | −6.2809 | 0.4294 | 57.4876 | <0.0001 | −6.2809 | 0.4294 | ||||
| Percent change of beta coefficient | Comparing model 2 with model 1 | Comparing model 3 with model 2 | Comparing model 4 with model 1 | Comparing model 5 with model 4 | ||||||
| Age (linear) | 49% | 2% | 36% | 12% | ||||||
| Race | 26% | 0% | 29% | −1% | ||||||
| Dependent | Independent | |
|---|---|---|
| Model 1 | NNAL | Age + age square + race + gender |
| Model 2 | NNAL | Age + age square + race + gender + log creatinine |
| Model 3 | NNAL | Age + age square + race + gender + log creatinine+ log specific gravity |
| Model 4 | NNAL | Age + age square + race + gender + log specific gravity |
| Model 5 | NNAL | Age + age square + race + gender + log specific gravity + log creatinine |
*Tobacco users/smokers defined as having serum cotinine concentrations >10 ng/ml.
All statistical analyses were performed by using SUDAAN (release 10.0) Proc DESCRIPT and Proc REGRESS from RTI (Research Triangle Park, NC), with graphical analyses performed by using SAS (version 9.3; SAS Institute, Cary, NC). Analyses incorporated sampling weights to adjust for unequal probabilities of selection.
RESULTS
The distribution of both creatinine and specific gravity (unweighted) in NHANES 2007–2008 is skewed to the left, as shown in Figure 1. When plotted on a log scale, the data remained skewed for both creatinine and specific gravity (not shown). A high correlation (r = 0.80, P < 0.001) was observed between weighted creatinine and weighted specific gravity (log scale). Unweighted and weighted creatinine and specific gravity (original and log‐scaled) are plotted in Figure 2 to demonstrate their correlation.
Figure 1.
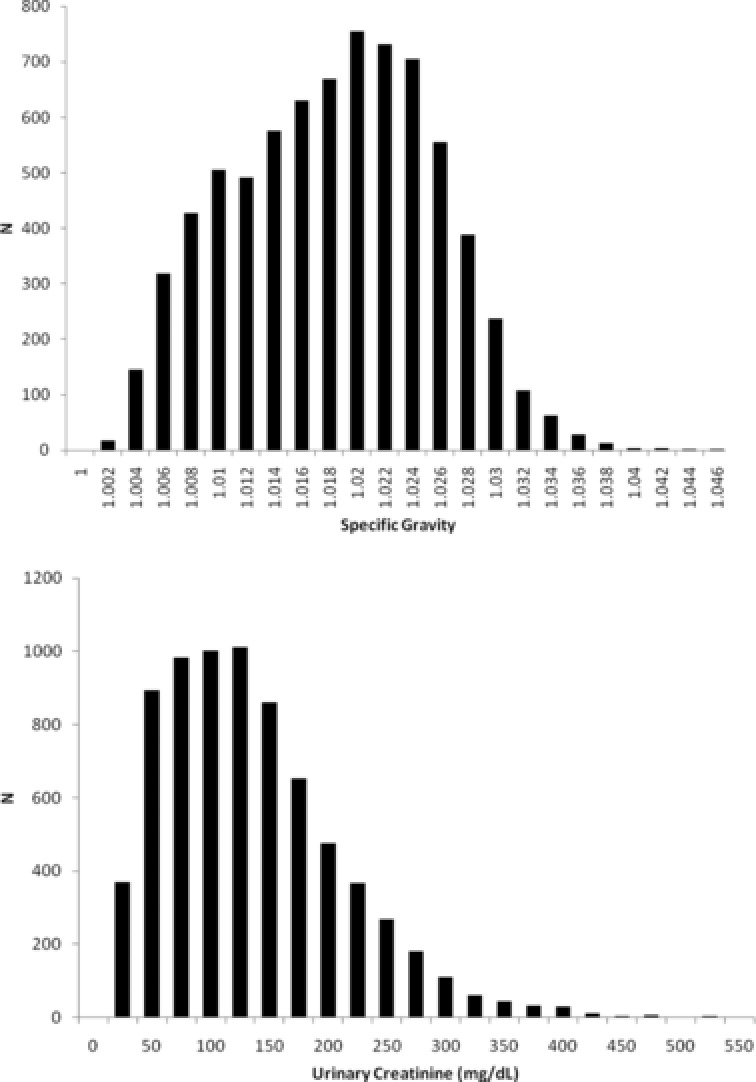
Distribution of creatinine (milligram per deciliter) and specific gravity (SG) among all participants in NHANES 2007–2008.
Figure 2.
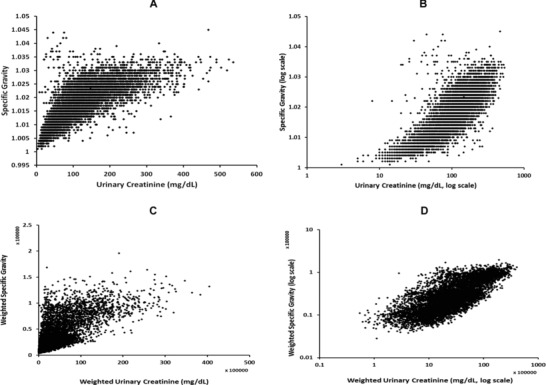
Correlation between creatinine (milligram per deciliter) and specific gravity (SG) among all participants in NHANES 2007–2008. A, unweighted data; B, unweighted data on log scale; C, weighted data; D, weighted data on log scale.
The weighted urinary creatinine and specific gravity geometric means and their respective upper and lower 95% confidence intervals (CIs) are shown in Table 1. The data are presented by gender, race/ethnicity, and age groups for both nonsmokers and smokers. Males had a significantly higher urinary creatinine geometric mean than females regardless of the smoking status (P < 0.0001) by t‐test. Males also had significantly higher specific gravity than females (1.019 vs. 1.016, P < 0.0001 for the nonsmokers, 1.018 vs. 1.016, P = 0.0008 for smokers). NHB had higher geometric mean creatinine and specific gravity than NHW in both nonsmokers and smokers. Creatinine in children ages 6–11 years is lower than ages 12–19 years (P < 0.0415), and not significantly different from ages 20 and older (P > 0.05), whereas the concentration of urinary creatinine is highest among the 12–19 years age group, progressively declining thereafter among older adults. Specific gravity within the age groups has the same trend as urinary creatinine. The geometric mean of creatinine was higher in smokers than nonsmokers for all (P = 0.01), for NHB (P = 0.002), and for age group of 20–44 years old (P = 0.01), while the geometric mean of specific gravity was higher in smokers than nonsmokers only for the OTH group. t‐Test was used to examine all above tests and all statistical tests were two‐sided with α = 0.05.
Table 1.
Geometric Means of Urinary Creatinine (Milligram per Deciliter) and Specific Gravity (SG) Among All Participants and Tobacco Users* in NHANES 2007–2008
| Nonsmoker geometric mean (95% CI) | Smoker geometric mean (95% CI) | |||||
|---|---|---|---|---|---|---|
| N † | Creatinine | SG | N † | Creatinine | SG | |
| All | 4,993 | 95.11 (91.40–98.97) | 1.017 (1.017–1.018) | 1,391 | 104.34 (97.10–112.13) | 1.017 (1.016–1.018) |
| Male | 2,365 | 115.65 (111.84–119.58) | 1.019 (1.019–1.019) | 853 | 118.29 (107.55–130.10) | 1.018 (1.017–1.019) |
| Female | 2,628 | 80.36 (76.6484.26) | 1.016 (1.015–1.016) | 538 | 85.85 (80.00–94.50) | 1.016 (1.014–1.017) |
| NHW | 1,884 | 90.78 (85.86–95.98) | 1.017 (1.016–1.017) | 707 | 96.52 (89.23–104.41) | 1.016 (1.016–1.017) |
| NHB | 1,041 | 127.19 (121.37–133.29) | 1.019 (1.019–1.020) | 355 | 143.44 (134.04–153.51) | 1.019 (1.018–1.020) |
| MA | 1,192 | 98.70 (92.13–105.73) | 1.019 (1.018–1.019) | 148 | 110.51 (91.62–133.29) | 1.018 (1.016–1.021) |
| OTH race | 876 | 90.53 (84.49–96.99) | 1.017 (1.017–1.018) | 181 | 114.53 (90.87–144.36) | 1.019 (1.017–1.021) |
| Ages 6–11 years | 858 | 77.88 (73.01–82.88) | 1.019 (1.019–1.020) | 4 | 73.14 (N/A) | 1.02 (N/A) |
| Ages 12–19 years | 825 | 126 (118–135) | 1.021 (1.019–1.021) | 120 | 146.55 (122.75–174.97) | 1.021 (1.019–1.023) |
| Ages 20–44 years | 1,262 | 106 (102–111) | 1.018 (1.017–1.018) | 630 | 118.49 (109.49–128.24) | 1.018 (1.017–1.019) |
| Ages 45–64 years | 1,131 | 88 (82–94) | 1.017 (1.016–1.017) | 477 | 86.04 (76.87–96.31) | 1.015 (1.014–1.016) |
| Ages ≥65 years | 917 | 76 (73–80) | 1.015 (1.014–1.015) | 160 | 77.374 (63.93–93.64) | 1.015 (1.014–1.017) |
*Tobacco users/smokers defined as having serum cotinine concentrations >10 ng/ml.
† N = nonmissing and unweighed sample size (excluding unknown smoking status due to missing cotinine measurements).
Upper and lower 95% confidence intervals (CIs) are shown in parenthesis.
Multiple linear regression models were developed to examine NNAL correction in smokers normalized by creatinine, specific gravity, and both creatinine and specific gravity, respectively. In the models 1, 2, and 3 (NNAL‐CR, NNAL‐SG, and NNAL‐CR‐SG) in Table 2, NNAL corrected by creatinine and specific gravity were the dependent variables. Age, age square, gender, and race/ethnicity were the independent variables. As shown in Table 2, the beta coefficients of age square and race/ethnicity were very similar for NNAL‐CR and NNAL‐SG. However, the beta coefficient of age for creatinine normalization was higher than the beta coefficient of age for specific gravity normalization (beta coefficient of age = 0.0332 and 0.0276, for creatinine and specific gravity, respectively). Also R 2 for creatinine normalization was higher than R 2 for specific gravity normalization (R 2 = 0.1694 and 0.1439, for creatinine and specific gravity, respectively). When NNAL was normalized by both factors (NNAL‐CR‐SG), the R 2 was further improved (R 2 = 0.2068). Age, age‐square term, and race/ethnicity were found to be significant predictors for NNAL‐CR and NNAL‐SG. Gender was not a significant factor in the model for either NNAL‐CR or NNAL‐SG (P‐value >0.05, not shown in Table 2). However, when NNAL was normalized by both creatinine and specific gravity (NNAL‐CR‐SG), gender became significant (P = 0.005).
Table 2.
Beta Coefficients and R 2 from Three Multiple Regression Models for Urinary NNAL (Picogram per Milliliter) Correction Using Normalization Among Tobacco Users* in NHANES 2007–2008
| NNAL correction normalized by | ||||
|---|---|---|---|---|
| Creatinine | Creatinine and SG | |||
| (NNAL‐CR) | SG (NNAL‐SG) | (NNAL‐CR‐SG) | ||
| R 2 | 0.1694 | 0.1439 | 0.2068 | |
| Beta coefficients** | Age | 0.0332 (P < 0.001) | 0.0276 (P < 0.001) | 0.0396 (P < 0.001) |
| Age square | −0.0009 (P < 0.001) | −0.0009 (P < 0.001) | −0.0010 (P < 0.001) | |
| Gender | 0.2446 (P = 0.005) | |||
| Race/ethnicity | −0.2730 (P = 0.0006) | −0.2732 (P = 0.0014) | −0.3512 (P = 0.002) | |
*Tobacco users/smokers defined as having serum cotinine concentrations >10 ng/ml.
**All models were corrected for age, age square, gender, and race/ethinicity.
NNAL correction was also evaluated by fitting creatinine or/and specific gravity as a covariate/covariates in a regression model when NNAL was the dependent variable. As shown in Table 3, model 1 was the base model in which only age, age square, and race/ethnicity were significant factors. In model 2, creatinine was added and appeared as a significant factor, where the R 2 in model 2 was three times as high as in model 1 (0.2226 vs. 0.0716). Additionally, the beta coefficient of age changed by 49% from model 1 (0.0083) to model 2 (0.0124), while the beta coefficient of race/ethnicity also changed by 26% (−0.0896 in model 1 to −0.113 in model 2). Model 3 was developed when specific gravity (log scale) was further added to model 2. In model 3, the beta coefficients of age, age‐square, race/ethnicity, and creatinine remained almost unchanged, compared to model 2. Creatinine remained highly significant upon the addition of specific gravity, and specific gravity was found to be an insignificant factor in model 3 (P = 0.4294). Model 4 was created when specific gravity (log scale) was added to the base model (model 1). In model 4, specific gravity was not only significant, but it also changed the beta coefficients of age and race/ethnicity from model 3 by 36% and 29%, respectively. The last model (model 5) in Table 3 was generated when creatinine was entered into model 4. Compared to model 4, R 2 was slightly improved (from 0.1681 to 0.2275) in model 5, however, the significance of specific gravity was eliminated (from P < 0.0001 to P = 0.4294). Furthermore, the beta coefficient of age was changed by 12% by the addition of creatinine, whereas the beta coefficient of race/ethnicity remained almost unchanged.
DISCUSSION
Our results demonstrate that using multiple regression models to analyze the uncorrected data from population groups is preferable to merely using simple ratios. For urinary NNAL in the NHANES population, creatinine is more influential across age groups, whereas specific gravity is more sensitive to race/ethnicity. Our results also indicate that creatinine is the more influential variable in accounting for urine dilution in tobacco‐specific nitrosamine (TSNA) exposure assessment in the NHANES population.
Creatinine has been found to be proportional to body weight; it decreases with age in adults; and it is in lower concentration in females than males 12, 17. Our results for NHANES 2007–2008 were consistent with those expectations, as shown in Table 1, such as creatinine was higher in males than females (P < 0.0001), was the highest in 12–19 years age group (P < 0.05), and was highest in NHB (P < 0.05). Specific gravity exhibited the same trends as creatinine when gender, race/ethnicity, and age subgroups were compared, that is, it was higher in males than in females and decreases in adults with increasing age. However, the difference in specific gravity between various subgroups is not statistically as significant as it is for creatinine. For example, among race/ethnicity groups, specific gravity in NHB was significantly higher than NHW (P < 0.0001), but it was not significantly different from MA or OTH. Table 1 also shows creatinine was generally higher (not all significantly though) in smokers than in nonsmokers for each gender, race/ethnicity and age group, but specific gravity was statistically similar in smokers and nonsmokers. Our model calculation indicated creatinine was significantly higher in smokers than in nonsmokers in the NHANES 2007–2008 population (P = 0.01). However, specific gravity was not statistically different between smokers and nonsmokers (P = 0.24).
Although we found that urinary creatinine concentrations were significantly higher among smokers than nonsmokers within the NHANES population (P = 0.01) without adjusted by other covariates, this difference could be accounted for by the demographic differences between smokers and nonsmokers. This was examined through multiple regression analysis in which log creatinine was the dependent variable and smoking status, gender (two subgroups), race (four subgroups), and age (five subgroups) were included as the independent covariates in the model with all possible two‐way interaction terms. In this model, the adjusted smoking status effect was lost (P = 0.80), whereas all the demographic differences remained significant (P = 0.03, P = 0.0076, and P = 0.015 for sex × race, sex × age, and age × race, respectively). Therefore, we conclude that the significance of urinary creatinine differences between smokers and nonsmokers in NHANES is a result of the preponderance of adults, males, and NHB among the smoking population, all of whom tend to have higher creatinine concentrations than children, females, and NHW.
To evaluate methods of correcting NNAL to account for variability in interpersonal urine concentrations in NHANES samples, we included creatinine and specific gravity in the multiple regression analyses in two ways: 1 as correction factors in normalization, and 2 as separate independent variables in the linear regression models. As shown in Table 2, when NNAL was corrected by normalization, the correction by both creatinine and specific gravity (NNAL‐CR‐SG) yielded a somewhat higher R 2 than by creatinine only or by specific gravity only.
In the regression models in which creatinine and specific gravity (log scale) were fitted in the base model separately and then together in sequence, as shown in Table 3, creatinine and specific gravity influence NNAL in population groups differently. The R 2 was improved when creatinine enters the base model (model 1 to model 2). So was the R 2 when specific gravity was added to the base model (model 1 to model 4). Apparently the improvement on R 2 by creatinine is greater than is achieved by specific gravity. Beta coefficient of age was improved more by creatinine than by specific gravity, whereas the improvement of beta coefficient of race/ethnicity was more through specific gravity than through creatinine. This outcome clearly indicated that creatinine is more sensitive than specific gravity when NNAL is compared among different age groups. Conversely, specific gravity is almost as sensitive as creatinine when NNAL is examined across different race/ethnicity groups. This finding can be further examined by the change of beta coefficient of age and race/ethnicity when creatinine and specific gravity entered the model in different orders. When specific gravity followed creatinine in entering the model (model 2 to model 3), specific gravity made almost no change on the beta coefficient of age and improved the beta coefficient of race/ethnicity slightly. In model 5 where creatinine followed specific gravity upon entering the model, creatinine improved the beta coefficient of age, but it did not improve the association of race/ethnicity with the model. It seems age influences creatinine more than race/ethnicity, and specific gravity is more sensitive to the change of race/ethnicity than age. This finding agrees with the conclusion of Suwazono et al. that creatinine‐corrected urinary biomarkers were more affected by age than specific gravity corrected results 10.
The results from our regression models clearly demonstrate that the order of adding covariates into the model can be used to examine how the entering covariate influences the association between other significant factors and the model. Furthermore, adding a new variable can also change the significance of the existing factor in opposite directions to be more or less significant. When specific gravity was added to model 2 where creatinine already existed, specific gravity strengthened the association of creatinine to the model by 5% (beta coefficient from 0.726 to 0.7637), even though it was not significant in model 3. On the other hand, when creatinine was added to model 4, where specific gravity already existed, creatinine not only weakened the association of specific gravity to the model dramatically (beta coefficient from 57.4876 to −6.2809), but it also eliminated the significance of specific gravity completely (P = 0.4294). Nylander‐French et al. reported the same observation when specific gravity decreased in significance when creatinine and specific gravity were fitted together in the model 39.
Regardless of the opposite order of creatinine and specific gravity's introduction into the model, models 3 and 5 produced essentially the same statistical results: when specific gravity or creatinine was included as a covariate in separate models (models 2 and 4), they, each, were highly significant factors; however, when both correcting variables were included in the model simultaneously as covariates, creatinine remained highly significant, whereas the significance of specific gravity was lost, even though R 2 was somewhat improved. The agreement of model 3 and model 5 enabled us to find the best approach to identifying the correction variable: that approach was to include creatinine and specific gravity in the multiple regression analysis as covariates. This approach allows NNAL concentration to be established, as appropriately corrected by creatinine, specific gravity or both, while other statistical significant variables (age, gender, and race/ethnicity) remain independent of effects of creatinine and specific gravity.
Our study has several strengths and some limitations. An important advantage is the use of a large national population sample. Thus, our results provided a comprehensive estimate on how creatinine and specific gravity affect the correction of certain tobacco biomarkers. Our measurements of NNAL, creatinine, and specific gravity are accurate and precise, resulting from the use of sensitive instruments and specific analysis methods. However, participants who had kidney disease were not excluded from the analysis. This is a potential limitation of this study because kidney malfunction would affect the excretion of creatinine. We also limited our analyses to participants with age of 6 years and older in these assays and were therefore unable to measure NNAL in younger children.
Another possible concern could be the use of dietary supplements. Creatine is the parent compound of creatinine and it is used as a dietary supplement to enhance sports performance among both children and adults. Although some reports have suggested an increase in urinary creatinine following creatine supplementation, as yet that has not been clearly established. For example, both Poortmans et al. and Ropero‐Miller et al. 40, 41 have found little or no increase in urinary creatinine following short‐term creatine supplementation. Even long‐term supplementation was found to have little effect on urinary creatinine levels 42. A recent study reported creatine supplementation to be rather inefficient in attempts to deliberately modify urine creatinine adjustments in samples used for drug analyses 43. Furthermore, although creatine supplementation data are not available in NHANES, according to the National Health Interview Survey, 24,177 children and adolescents reported the use of creatine as a dietary supplement in the 2007 survey, which is about 0.03% of the national estimate of 73.7 million children 44. Also in 2007, 0.39% of adults (843,385 out of 216,781,365) reported the use of creatine 45. These small fractional population estimates suggest that our results are unlikely to be significantly skewed by the use of creatine supplementation in the US population.
Our results are consistent with prior reports in that using multiple regression models with urinary creatinine as a covariate to analyze the uncorrected data from population groups is the preferable approach rather than merely using simple ratios. Although using both creatinine and specific gravity together gives slightly better R 2 values, the fact that specific gravity loses significance when creatinine is present simultaneously in the model suggests that specific gravity provides little improvement in correcting the results. Furthermore, for urinary NNAL in the NHANES population, creatinine is more influential across age groups, whereas specific gravity is more sensitive to race/ethnicity. Both creatinine and specific gravity are sensitive to gender.
CONCLUSIONS
Although we found significant relationships between NNAL concentrations and both urine creatinine and specific gravity, our results indicate that creatinine is the more influential variable in accounting for urine dilution in TSNA exposure assessment in the NHANES population. We conclude that the use of NNAL concentrations corrected by creatinine through the use of an appropriate multiple regression model is the preferred approach for the analysis of these measurements.
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention. Use of trade names and commercial sources is for identification only and does not constitute endorsement by the U.S. Department of Health and Human Services or the Centers for Disease Control and Prevention.
REFERENCES
- 1. Xia Y, McGuffey JE, Bhattacharyya S, et al. Analysis of the tobacco‐specific nitrosamine 4‐(methyinitrosamino)‐1‐(3‐pyridyl)‐1‐butanol in urine by extraction on a molecularly imprinted polymer column and liquid chromatography/atmospheric pressure ionization tandem mass spectrometry. Anal Chem 2005;77(23):7639–7645. [DOI] [PubMed] [Google Scholar]
- 2. Hecht SS, Carmella SG, Murphy SE, Akerkar S, Brunnemann KD, Hoffmann D. A tobacco‐specific lung carcinogen in the urine of men exposed to cigarette‐smoke. New Engl J Med 1993;329(21):1543–1546. [DOI] [PubMed] [Google Scholar]
- 3. Hecht SS, Trushin N. DNA and hemoglobin alkylation by 4‐(methylnitrosamino)‐1‐(3‐pyridyl)‐1‐butanone and its major metabolite 4‐(methylnitrosamino)‐1‐(3‐pyridyl)‐1‐butanol in F344 rats. Carcinogenesis 1988;9(9):1665–1668. [DOI] [PubMed] [Google Scholar]
- 4. Hecht SS. Biochemistry, biology, and carcinogenicity of tobacco‐specific N‐nitrosamines. Chem Res Toxicol 1998;11(6):559–603. [DOI] [PubMed] [Google Scholar]
- 5. Hoffmann D, Djordjevic MV, Rivenson A, Zang E, Desai D, Amin S. A study of tobacco carcinogenesis. LI. Relative potencies of tobacco‐specific N‐nitrosamines as inducers of lung tumours in A/J mice. Cancer Lett 1993;71(1–3):25–30. [DOI] [PubMed] [Google Scholar]
- 6. Rivenson A, Hoffmann D, Prokopczyk B, Amin S, Hecht SS. Induction of lung and exocrine pancreas tumors in F344 rats by tobacco‐specific and areca‐derived N‐nitrosamines. Cancer Res 1988;48(23):6912–6917. [PubMed] [Google Scholar]
- 7. Moore RR, HirataDulas CA, Kasiske BL. Use of urine specific gravity to improve screening for albuminuria. Kidney Int 1997;52(1):240–243. [DOI] [PubMed] [Google Scholar]
- 8. Barr DB, Wilder LC, Caudill SP, Gonzalez AJ, Needham LL, Pirkle JL. Urinary creatinine concentrations in the US population: Implications for urinary biologic monitoring measurements. Environ Health Persp 2005;113(2):192–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Muscat JE, Liu A, Richie JP. A comparison of creatinine vs. specific gravity to correct for urinary dilution of cotinine. Biomarkers 2011;16(3):206–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Suwazono Y, Akesson A, Alfven T, Jarup L, Vahter M. Creatinine versus specific gravity‐adjusted urinary cadmium concentrations. Biomarkers 2005;10(2–3):117–126. [DOI] [PubMed] [Google Scholar]
- 11. Heavner DL, Morgan WT, Sears SB, Richardson JD, Byrd GD, Ogden MW. Effect of creatinine and specific gravity normalization techniques on xenobiotic biomarkers in smokers’ spot and 24‐h urines. J Pharmaceut Biomed 2006;40(4):928–942. [DOI] [PubMed] [Google Scholar]
- 12. Boeniger MF, Lowry LK, Rosenberg J. Interpretation of urine results used to assess chemical‐exposure with emphasis on creatinine adjustments – A review. Am Ind Hyg Assoc J 1993;54(10):615–627. [DOI] [PubMed] [Google Scholar]
- 13. Laguna TA, Wagner BD, Accurso FJ. The applicability of urinary creatinine as a method of specimen normalization in the cystic fibrosis population. J Cyst Fibros 2010;9(3):212–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Thompson SG, Barlow RD, Wald NJ, Vanvunakis H. How should urinary cotinine concentrations be adjusted for urinary creatinine concentration? Clin Chim Acta 1990;187(3):289–296. [DOI] [PubMed] [Google Scholar]
- 15. Dasgupta PK, Ohira SI, Kirk AB, Dyke JV. Creatinine adjustment of spot urine samples and 24 h excretion of iodine, selenium, perchlorate, and thiocyanate. Environ Sci Technol 2008;42(24):9419–9423. [DOI] [PubMed] [Google Scholar]
- 16. Waikar SS, Sabbisetti VS, Bonventre JV. Normalization of urinary biomarkers to creatinine during changes in glomerular filtration rate. Kidney Int 2010;78(5):486–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Alessio L, Berlin A, Dellorto A, Toffoletto F, Ghezzi I. Reliability of urinary creatinine as a parameter used to adjust values of urinary biological indicators. Int Arch Occup Environ Health 1985;55(2):99–106. [DOI] [PubMed] [Google Scholar]
- 18. Sata F, Araki S. Adjustment of creatinine‐adjusted value to urine flow rate in lead workers. Arch Environ Health 1996;51(4):329–333. [DOI] [PubMed] [Google Scholar]
- 19. Fried PA, Perkins SL, Watkinson B, Mccartney JS. Association between creatinine‐adjusted and unadjusted urine cotinine values in children and the mothers report of exposure to environmental tobacco‐smoke. Clin Biochem 1995;28(4):415–420. [DOI] [PubMed] [Google Scholar]
- 20. Parikh CR, Gyamlani GG, Carvounis CP. Screening for microalbuminuria simplified by urine specific gravity. Am J Nephrol 2002;22(4):315–319. [DOI] [PubMed] [Google Scholar]
- 21. Haddow JE, Knight GJ, Palomaki GE, Neveux LM, Chilmonczyk BA. Replacing creatinine measurements with specific‐gravity values to adjust urine cotinine concentrations. Clin Chem 1994;40(4):562–564. [PubMed] [Google Scholar]
- 22. Newman DJ, Pugia MJ, Lott JA, Wallace JF, Hiar AM. Urinary protein and albumin excretion corrected by creatinine and specific gravity. Clin Chim Acta 2000;294(1–2):139–155. [DOI] [PubMed] [Google Scholar]
- 23. Gaines LGT, Fent KW, Flack SL, et al. Effect of creatinine and specific gravity normalization on urinary biomarker 1,6‐hexamethylene diamine. J Environ Monit 2010;12(3):591–599. [DOI] [PubMed] [Google Scholar]
- 24. Levine L, Fahy JP. Evaluation of urinary lead determinations. J Ind Hyg Toxicol 1945. 27:217–223. [PubMed] [Google Scholar]
- 25. Araki S. Effects of urinary volume on urinary concentrations of lead, delta‐aminolevulinic‐acid, coproporphyrin, creatinine, and total solutes. Br J Ind Med 1980;37(1):50–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vij HS, Howell S. Improving the specific gravity adjustment method for assessing urinary concentrations of toxic substances. Am Ind Hyg Assoc J 1998;59(6):375–380. [DOI] [PubMed] [Google Scholar]
- 27. Vahter M, Nermell B, Lindberg AL, et al. Urinary arsenic concentration adjustment factors and malnutrition. Environ Res 2008;106(2):212–218. [DOI] [PubMed] [Google Scholar]
- 28. Sorahan T, Pang D, Esmen N, Sadhra S. Urinary concentrations of toxic substances: An assessment of alternative approaches to adjusting for specific gravity. J Occup Environ Hyg 2008;5(11):721–723. [DOI] [PubMed] [Google Scholar]
- 29. Bartolucci GB, Carrieri M, Trevisan A. Adjustment to concentration‐dilution of spot urine samples: Correlation between specific gravity and creatinine. Int Arch Occup Environ Health 2001;74(1):63–67. [DOI] [PubMed] [Google Scholar]
- 30. CDC . National Health and Nutrition Examination Survey. Centers for Disease Control and Prevention. Available from: http://www.cdc.gov/nchs/nhanes.htm. Access date September 30, 2013.
- 31. CDC . Analytic and reporting guidelines, the National Health and Nutrition Examination Survey (NHANES). Centers for Disease Control and Prevention. Available from: http://www.cdc.gov/nchs/data/nhanes/nhanes_03_04/nhanes_analytic_guidelines_dec_2005.pdf. Access date September 30, 2013.
- 32. Xia Y, Bernert JT, Jain RB, Ashley DL, Pirkle JL. Tobacco‐specific nitrosamine 4‐(methylnitrosamino)‐1‐(3‐pyridyl)‐1‐butanol (NNAL) in smokers in the United States: NHANES 2007–2008. Biomarkers 2011;16(2):112–119. [DOI] [PubMed] [Google Scholar]
- 33. Bernert JT, Pirkle JL, Xia Y, Jain RB, Ashley DL, Sampson EJ. Urine concentrations of a tobacco‐specific nitrosamine carcinogen in the U.S. population from secondhand smoke exposure. Cancer Epidemiol Biomarkers Prev 2010;19(11):2969–2977. [DOI] [PubMed] [Google Scholar]
- 34. Xia Y, Bernert JT. Stability of the tobacco‐specific nitrosamine 4‐(methylnitrosamino)‐1‐(3‐pyridyl)‐1‐butanol in urine samples stored at various temperatures. J Anal Toxicol 2010;34(7):411–415. [DOI] [PubMed] [Google Scholar]
- 35. Xia Y, Bernert JT. Stability of the tobacco‐specific nitrosamine 4‐(methylnitrosamino)‐1‐(3‐pyridyl)‐1‐butanol in urine samples stored at various temperatures. J Anal Toxicol 2010;34(7):411–415. [DOI] [PubMed] [Google Scholar]
- 36. Pirkle JL, Bernert JT, Caudill SA, Sosnoff CS, Pechacek TF. Trends in the exposure of nonsmokers in the US population to secondhand smoke: 1988–2002. Environ Health Persp 2006;114(6):853–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Caudill SP, Schleicher RL, Pirkle JL. Multi‐rule quality control for the age‐related eye disease study. Stat Med 2008;27(20):4094–4106. [DOI] [PubMed] [Google Scholar]
- 38. Pirkle JL, Flegal KM, Bernert JT, Brody DJ, Etzel RA, Maurer KR. Exposure of the US population to environmental tobacco smoke – The third National Health and Nutrition Examination Survey, 1988 to 1991. J Am Med Assoc 1996;275(16):1233–1240. [PubMed] [Google Scholar]
- 39. Nylander‐French LA, Gaines LGT, Fent KW, et al. Effect of creatinine and specific gravity normalization on urinary biomarker 1,6‐hexamethylene diamine. J Environ Monitor 2010;12(3):591–599. [DOI] [PubMed] [Google Scholar]
- 40. Ropero‐Miller JD, Paget‐Wilkes H, Doering PL, Goldberger BA. Effect of oral creatine supplementation on random urine creatinine, pH, and specific gravity measurements. Clin Chem 2000;46(2):295–297. [PubMed] [Google Scholar]
- 41. Poortmans JR, Auquier H, Renaut V, Durussel A, Saugy M, Brisson GR. Effect of short‐term creatine supplementation on renal responses in men. Eur J Appl Physiol Occup Physiol 1997;76(6):566–567. [DOI] [PubMed] [Google Scholar]
- 42. Robinson TM, Sewell DA, Casey A, Steenge G, Greenhaff PL. Dietary creatine supplementation does not affect some haematological indices, or indices of muscle damage and hepatic and renal function. Br J Sports Med 2000;34(4):284–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Villena VP. Beating the system: A study of a creatinine assay and its efficacy in authenticating human urine specimens. J Anal Toxicol 2010;34(1):39–44. [DOI] [PubMed] [Google Scholar]
- 44. Evans MW, Jr. , Ndetan H, Perko M, Williams R, Walker C. Dietary supplement use by children and adolescents in the United States to enhance sport performance: Results of the National Health Interview Survey. J Prim Prev 2012;33(1):3–12. [DOI] [PubMed] [Google Scholar]
- 45. Wu CH, Wang CC, Kennedy J. Changes in herb and dietary supplement use in the U.S. adult population: A comparison of the 2002 and 2007 National Health Interview Surveys. Clin Ther 2011;33(11):1749–1758. [DOI] [PubMed] [Google Scholar]


