Abstract
Background
For the assessment of inflammatory status, we have developed a simple, reliable radioimmunoassay (RIA) of prostaglandin E‐major urinary metabolite (PGE‐MUM), which remains stable in urine after it is metabolized. Using this method, we conducted a screening study to compare standard values of PGE‐MUM to serum C‐reactive protein (CRP) levels in health check volunteers.
Methods
PGE‐MUM (micrograms per gram creatinine) was measured in normal urine samples obtained from 797 samples in volunteers for health check, using a newly developed RIA, and analyzed in relation to age, gender, smoking, and drinking habits. Results were compared to serum CRP.
Results
PGE‐MUM was significantly higher in males than in females. It was significantly higher in smoking males, compared to males who had never smoked (nonsmokers), particularly in those above 40 years of age. In nonsmokers, PGE‐MUM declined in males with advancing age, while it rose in females. Although PGE‐MUM reflected current smoking status, independent of smoking index (SI), serum CRP indicated both current and former smoking condition, rather dependent upon SI.
Conclusions
PGE‐MUM increases in smokers, as suggested by possible inflammatory injury of pulmonary tissue. This RIA method for PGE‐MUM may be thus a sensitive and reliable biomarker for current inflammation, different from serum CRP.
Keywords: prostaglandin E‐major urinary metabolite, radioimmunoassay, smoking, CRP, age and gender
INTRODUCTION
Prostaglandin E (PGE) is a critical inflammatory mediator and a major cyclooxygenase (COX) product that plays a vital role in human physiology and tumor development and progression 1, 2. It is difficult to measure PGE values directly in local tissue and blood due to its rapid metabolism. Major urinary metabolite (MUM), 7α‐hydroxy‐5, 11‐diketotetranorprosta‐1, 16‐dioic acid derived from PGE1 and PGE2 can be transformed to bicyclic PGE‐MUM by alkali treatment 3. PGE‐MUM is considered to be stable and can be used to assess inflammatory status in the living body. It is important to use a noninvasive method to assess inflammatory status, and PGE‐MUM is a possible inflammation marker candidate. Our previous work focused on the development of a simplified immunoassay (radioimmunoassay [RIA] or enzyme immunoassay [EIA]) in human urine using a specific rabbit antibody obtained by immunization with chemically synthesized 19‐carboxy‐11‐deoxy‐13,14‐dihydro‐15‐dehydro‐2,3,4,5,20‐pentanor‐11ß‐16ε‐cycloprostaglandin E1 (the stable compound, bicyclic PGE‐MUM; 4‐(1‐(2‐carboxyethyl)‐2,5‐dioxooctahydro‐1H‐inden‐4‐yl)butanoic acid) as antigen 3. The benefit of this novel marker is that it allows to assess the activity of chronic inflammatory lesions such as ulcerative colitis (UC) and interstitial pneumonia. In our previous report, we showed that PGE‐MUM is reliable and sensitive to both active and inactive phases of UC, with a marker of activity of UC 4.
However, there is currently no detailed basic analysis data on PGE‐MUM, especially concerning baseline values and differences in age and gender. Although recent reports using isotope dilution techniques employing liquid chromatography/mass spectrometry (LC/MS) have shown an increased PGE‐MUM in smokers, no detailed large‐scale study has been carried out to assess differences in age and gender, as well as smoking effect on PGE‐MUM 5, 6, 7. In the present study, we modified the previous method (3) and developed a new, reliable RIA of PGE‐MUM to determine the basal lines of PGE‐MUM using urine samples from a large pool of health check volunteer subjects. Besides age and gender, the effect of smoking on PGE‐MUM was analyzed and compared to serum C‐reactive protein (CRP), indicating a different possible marker of inflammation by PGE‐MUM from serum CRP.
MATERIALS AND METHODS
Samples and Data Collection
Void urinary and blood serum samples were obtained from 2,500 volunteers who visited the Health Check Department of the Japanese Red Cross Medical Center in 2008 (January to December) for general health checkups. After analysis of routine blood chemistry, hematology, urinalysis, chest X‐ray, and gastrointestinal endoscopy, subjects with neither occult blood in feces nor critical clinical findings were selected to take part in the present study (Tables 1 and 2). It was confirmed that they had normal renal function (blood urea nitrogen [BUN] < 25 mg/dl, creatinine < 1.1 mg/dl) without proteinuria, glucosuria, and urinary tract infection. Patients with high BMI (>25) or hypertension (>140/90), or high serum low‐density lipoprotein [LDL] (>180 mg/dl), or high blood sugar (>126 mg/dl), or HbA1c (>6.0%) were excluded in the present study. After preliminary tests using urine samples collected from 28 volunteers at 7:00 am, 1:00 pm, and 7:00 pm, all urine samples were obtained in the morning (7:00 am) before any examination or treatment. After centrifugation at 1,000 × g for 10 min, the supernatants of all urine samples were stored at −20°C until analysis.
Table 1.
Items of Urine Samples for PGE‐MUM in Healthy Volunteers
| Males | Females | |||||||
|---|---|---|---|---|---|---|---|---|
| Smoking status | Smoking status | |||||||
| Age | Total | Never | Former | Current | Total | Never | Former | Current |
| ‐40 | 81 | 37 | 16 | 28 | 56 | 42 | 7 | 7 |
| 41–60 | 296 | 85 | 140 | 71 | 164 | 129 | 25 | 10 |
| 61‐ | 124 | 50 | 61 | 13 | 76 | 65 | 11 | 0 |
| Total | 501 | 172 | 217 | 112 | 296 | 236 | 43 | 17 |
Table 2.
Items of Urine Samples for Serum CRP in Healthy Volunteers
| Males | Females | |||||||
|---|---|---|---|---|---|---|---|---|
| Smoking status | Smoking status | |||||||
| Age | Total | Never | Former | Current | Total | Never | Former | Current |
| ‐40 | 63 | 25 | 15 | 23 | 46 | 34 | 6 | 6 |
| 41–60 | 251 | 71 | 116 | 64 | 128 | 98 | 23 | 7 |
| 61‐ | 95 | 38 | 47 | 11 | 53 | 46 | 7 | 0 |
| Total | 409 | 134 | 178 | 98 | 227 | 178 | 36 | 13 |
Former smokers were divided into two groups according to whether they had quit smoking less than 5 years before this study (<5 years) or 5 years or more (≥5 years). Nonsmokers were defined as those who had smoked fewer than 100 cigarettes in their lifetime. Smoking index (SI, Brinkmann index) was calculated by numbers of cigarette (per day) × accumulated smoking years.
Drinkers were defined as those who drank two days or more per week. Chance drinkers were defined as those who drank one day or less per week. Weekly drinking units were determined by calculating drinking dose (unit/day) × numbers of weekly times, as one drinking unit equaled 80 kcal.
Quantitative Analysis of PGE‐MUM
The identities of the derivatives from the pretreatment of urine samples and the authentic bicyclic PGE‐MUM were compared by high‐performance liquid chromatography‐evaporative light scattering detection system‐liquid chromatography/mas spectrometry (HPLC‐ELSD‐LC/MS) method using HPLC 1100 (Agilent Technologies, Inc., Santa Clara, CA), Evaporative Light Scattering Detector (ELSD Model 300S, Soft A Corp., Westminster, CO), and LCT analyzer (Micromass Ltd., Hertfordshire, UK). Human urine samples pooled from five subjects with a high titer of PGE‐MUM were prepared using the method described later. Both samples of synthetic bicyclic PGE‐MUM and pretreated urine sample aliquot fractionated on HPLC were then simultaneously tested by RIA and the results were compared. Finally, the absolute values of PGE‐MUM in urine were determined. Pooled urine samples were used only for standard quantitative by RIA.
The urine samples were individually subjected to PGE‐MUM assay after being processed using the simplified immunoassay method described by Inagawa et al. 3 with a slight modification. The RIA kit was produced by the Institute of Isotopes Co., Ltd., Budapest, Hungary and distributed by TFB, Inc. Toshima‐ku, Tokyo, Japan.
Briefly, all samples (50 μl) were kept at room temperature for 30 min after the addition of 100 μl of 1N NaOH, and neutralized with 100 μl of 1N HCl. They were then diluted (×25) with 1,000 μl of 50 mM phosphate buffer (pH 7.4) containing 0.1% gelatin and 0.1% sodium azide. The prepared samples were pipetted into assay tubes, 50 μl each. Then, 100 μl of 125I‐bicyclic PGE‐MUM (680 Bq, ∼37 pg) and subsequently 100 μl of rabbit antibicyclic PGE‐MUM were added to each tube. After incubation at room temperature for 2 hr, 250 μl of separating reagent was added to each tube and incubation was further continued for 15 min at room temperature. After centrifugation at 2,000 × g for 10 min, the supernatant was removed by decantation. The radioactivity in the residues was counted with an Auto Well Gamma Counter ARC1000 (ALOKA, Tokyo, Japan).
The performance of the bicyclic PGE‐MUM RIA technique was as follows: intra‐assay variance (CV%, replicate of five) with actual urine samples (10–40 ng/ml) was 3.2–7.2%; minimum measurable concentration of PGE‐MUM was 2.0 ng/ml (or concentration of the minimum standard solution is 0.08 ng/ml). The specificity and cross‐reactivity of antibodies were also checked at the screening stage using corresponding compounds of less than 5%, such as PGE2, 13,14‐dihydro‐15‐keto PGE2, tetranor‐prostaglandin E metabolite (PGEM). PGE‐MUM value was corrected by the concentration of urinary creatinine and expressed as PGE‐MUM (micrograms per gram creatinine) because its concentration depends on urinary volume.
We have also validated the developed RIA for PGE‐MUM in urine sample by the measurement of another type of standard material (Tetranor‐PGEM, Cayman). Thus, the certain quantity of the tetranor‐PGEM gave the theoretical amount by our RIA method. Therefore, the RIA assay gives confident data.
Serum CRP
Serum CRP was measured using the latex agglutination immunoassay with antihuman CRP mouse monoclonal antibody‐coated latex, Nanopia CRP (Sekisui Medical Co., Ltd., Tokyo, Japan) according to the manufacture's guide, using Hitachi model 917 (7170) automated analyzer (Hitachi High‐Technologies, Tokyo, Japan) with serum sample 2.4 μl.
Statistical Analysis
Statview software (Abacus Concepts, Berkeley, CA) and SPSS version 13.0J (SPSS Japan, Inc., Shibuya‐Ku, Tokyo, Japan) were used for statistical analyses. Comparisons between two groups were performed using the Mann–Whitney U‐test and among three groups or more using Kruskal–Wallis test. Correlation between PGE‐MUM and other possible markers in all 797 samples was assessed using Spearman's rank correlation coefficients. A P‐value of less than 0.05 was considered of statistical significance.
Ethics
The use of urine and serum samples and laboratory data from the Department of Health Check, Japanese Red Cross Medical Center, with the informed consent of subjects, for the purpose of conducting this study, was approved by our Ethics Committee. The work described in our article has been carried out in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans.
RESULTS
Confirmation of Bicyclic PGE‐MUM in Pretreated Urine Samples and Determination of PGE‐MUM Values by RIA
To determine whether the pretreatment step of urine samples could lead to the desired bicyclic structure exclusively, we checked sample identity by HPLC‐MS method comparing sample behavior to that of an authentic sample on HPLC. Measured HPLC fraction peaks of authentic bicyclic PGE‐MUM were completely consistent with those of human‐pooled urine by RIA (Fig. 1). Fraction 17th urine‐derived sample exhibited a major peak in RIA activity. Mass spectroscopy of this peak confirmed that it was bicyclic PGE‐MUM (MS; minus H2, 309.1350). According to this basic data, real values of PGE‐MUM in urine were determined by RIA. We have estimated the reference value based upon those of healthy volunteer of nonsmoking male (n = 82) and female (n = 105). Then it gave us 28.8 μg/g creatinine for male and 19.9 μg/g creatinine for female.
Figure 1.
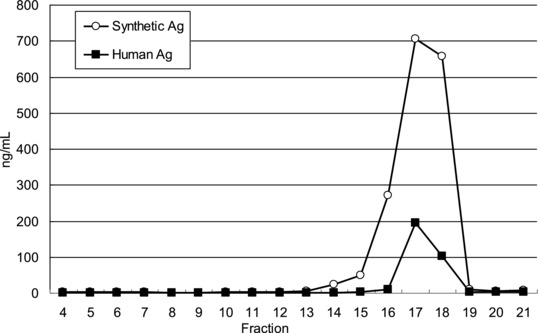
Measured HPLC fractions of synthetic PGE‐MUM and human pooled urine by RIA.
Furthermore, to confirm the quality of RIA, a dilution test was performed. Three representative samples of urine were diluted with ×2, ×3, ×5, ×10, and ×15, and then PGE‐MUM was tested with RIA. Values of PGE‐MUM decreased in proportion (Fig. 2). Next, bicyclic PGE‐MUM of which amount was already determined was added to the urine, and then PGE‐MUM was tested with RIA. Measured PGE‐MUM values subtracted/added PGE‐MUM corresponded to real PGE‐MUM values in urine (data not shown).
Figure 2.
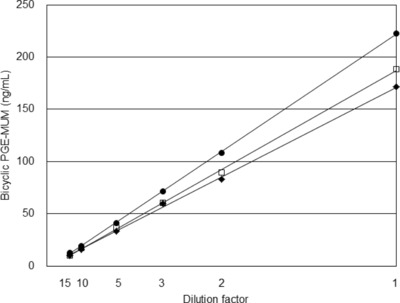
Dilution test using three representative urine samples for confirmation of RIA quality.
PGE‐MUM Comparison Among Morning (7:00 am), Afternoon (1:00 pm), and Evening (7:00 pm) Samples
As a preliminary test, PGE‐MUM (micrograms per gram creatinine) values were compared in urinary samples from 28 subjects (eight males, 49.8 ± 8.8 (mean age ± standard deviation); 20 females, 50.4 ± 5.0) obtained in the morning (7:00 am), afternoon (1:00 pm), and evening (7:00 pm). PGE‐MUM was relatively low and stable in urine obtained in the morning and afternoon compared to urine obtained in the evening (the mean values were 14.0 in the morning, 14.3 in the afternoon, and 14.7 in the evening for males and 13.5, 13.1, and 25.1 for females, respectively.) In females, PGE‐MUM was significantly higher in the evening than at other times (morning and afternoon). Consequently, further examination was carried out on morning urine.
Difference of PGE‐MUM According to Age and Gender
PGE‐MUM was compared in urine samples from males and females of three age groups (≤40, 41–60, and ≥61). A stepwise decrease of PGE‐MUM with age was observed in males (P = 0.001). In contrast, PGE‐MUM increased with advancing age in females (P = 0.003). PGE‐MUM was significantly higher in the male groups aged ≤40 and 40–60 than in the female groups (P < 0.001). There was no difference between males and females aged ≥61 (Fig. 3A).
Figure 3.
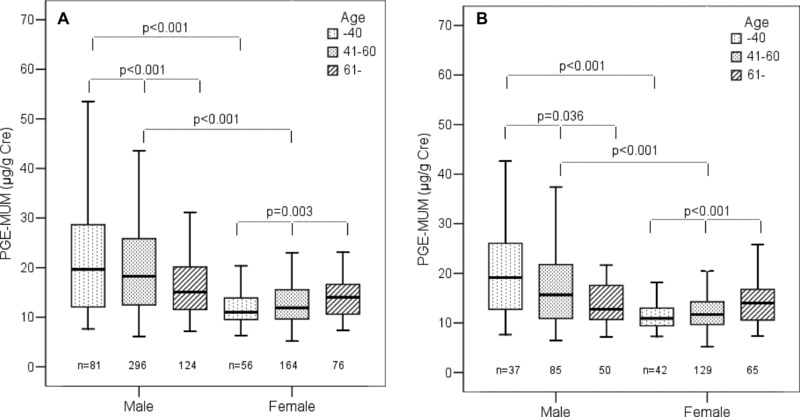
(A) Comparison of PGE‐MUM by age between males and females in total subjects. (Horizontal bar indicates the median value and box shows 25 and 75% percentile.) (B) Comparison of PGE‐MUM by age between males and females in nonsmokers.
In the nonsmoking male groups, a stepwise decrease of PGE‐MUM with age was again confirmed (P = 0.036). In the nonsmoking female groups, a stepwise increase of PGE‐MUM was found (P = 0.001). PGE‐MUM was significantly higher in the nonsmoking male groups aged ≤40 and 41–60 than in the female groups (P < 0.001). No difference was found between males and females aged ≥61 (Fig. 3B).
Difference of PGE‐MUM in Males According to Smoking Status
The PGE‐MUM difference in males was analyzed according to smoking status. A stepwise increase of PGE‐MUM was found in nonsmokers, former smokers (≥5 years and <5 years), and current smokers (P < 0.001). Particularly, PGE‐MUM in former smokers (<5 years) was significantly lower (P = 0.012) than in current smokers (Fig. 4A). When nonsmokers and current smokers were broken down by age group, a nonsignificant difference was seen in the group of current smokers, but a stepwise decrease was seen in the group of nonsmokers (P = 0.036; Fig. 4B).
Figure 4.
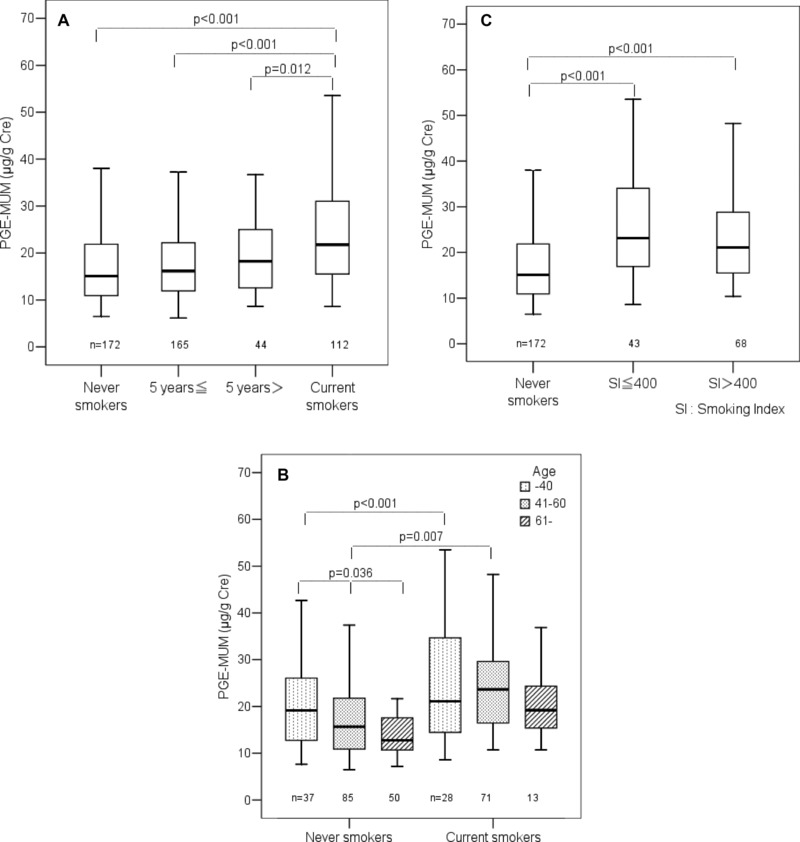
(A) Differences in PGE‐MUM among nonsmokers, former, and current smokers in males (≥5 years: former smokers who quit smoking 5 years or more before the study; <5 years: former smokers who quit smoking less than 5 years before the study). (B) Comparison of PGE‐MUM by age between nonsmokers (never smokers) and current smokers in males. (C) Relation of PGE‐MUM with smoking index in current male smokers.
PGE‐MUM in Relation to SI in Males
PGE‐MUM in males was analyzed in relation to SI (Brinkmann index, number of cigarettes (per day) × accumulated smoking years). Male subjects were divided into three groups: nonsmokers, SI ≤ 400, and SI > 400. There was no significant difference of PGE‐MUM between the groups of SI ≤ 400 and SI > 400, where PGE‐MUM in the two groups was significantly higher (P < 0.001) than that in nonsmokers (Fig. 4C).
Difference of Serum CRP According to Age and Gender
A stepwise increase of serum CRP with advancing age was confirmed in females (P = 0.009), similar to PGE‐MUM. However, a similar stepwise decrease of serum CRP in males with age was not found, as opposed to PGE‐MUM. Serum CRP was significantly higher in males of ≤40, 41–60, and ≥61 than in females (P < 0.001, P < 0.001, and P = 0.016, respectively; Fig. 5A).
Figure 5.
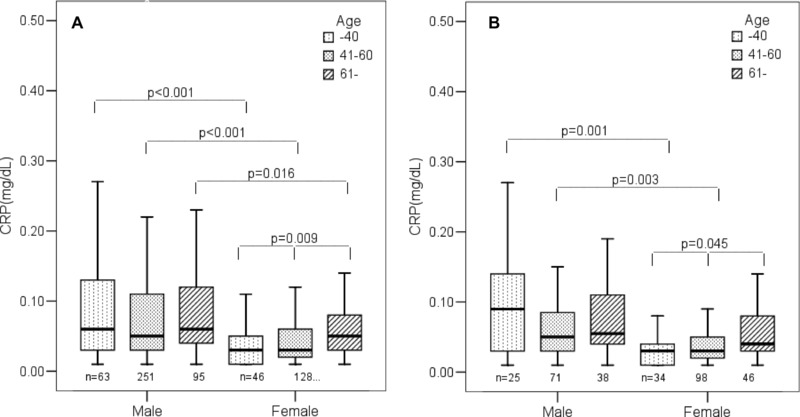
(A) Comparison of serum CRP by age between males and females in total subjects. (B) Comparison of serum CRP by age between males and females in nonsmokers.
This tendency for serum CRP to increase according to age and gender was again confirmed in nonsmokers. A stepwise increase of serum CRP with age was found in females (P = 0.045), but not in males. Serum CRP was significantly higher in males aged ≤40 and 41–60 than in females (P = 0.001 and P = 0.003, respectively; Fig. 5B).
Difference of Serum CRP in Males According to Smoking Status
The serum CRP difference in males was analyzed according to smoking status: nonsmokers, former smokers (≥5 years and <5 years), and current smokers. There was no stepwise increase in serum CRP among nonsmokers, former smokers (≥5 years and <5 years), and current smokers, in contrast with the stepwise increase in PGP‐MEM. No significant difference in serum CRP was found among former smokers (≥5 years and <5 years), and current smokers, although there was a significant difference (P = 0.035) between current smokers and nonsmokers (Fig. 6A).
Figure 6.
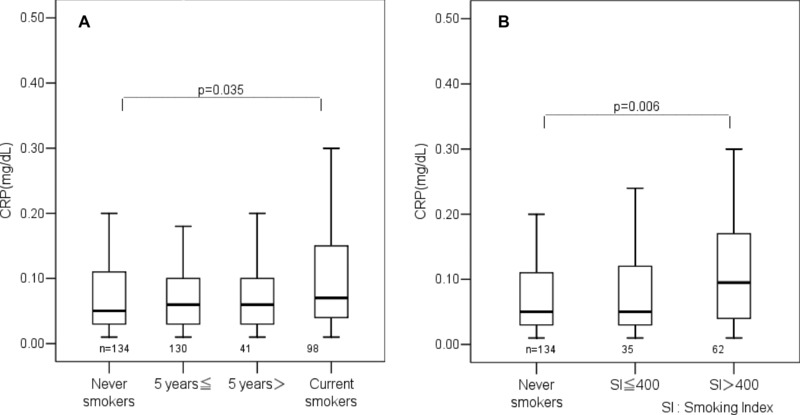
(A) Differences in serum CRP among nonsmokers, former, and current smokers in males (≥5 years: former smokers who quit smoking 5 years or more before the study; <5 years: former smokers who quit smoking less than 5 years before the study). (B) Relation of serum CRP with smoking index in current male smokers.
Serum CRP in Relation With SI in Males
Serum CRP in males was analyzed in relation with SI. Male subjects were divided into three groups of non‐smokers, SI ≤ 400 and SI > 400. There was a tendency for serum CRP to be higher in SI > 400 than SI ≤ 400, but the difference was not significant due to the rather small numbers of subjects. Serum CRP was significantly higher (P = 0.006) in SI > 400 than in nonsmokers (Fig. 6B).
Difference of PGE‐MUM According to Drinking Status and Other Markers
There was no significant difference in PGE‐MUM among the three groups (drinkers, chance drinkers, and nondrinkers) according to drinking status. Furthermore, weekly drinking units had no significant correlation to PGE‐MUM (data not shown). Significant correlation of PGE‐MUM with other markers was not shown using Spearman's rank coefficients, as follows: PGE‐MUM with drinking (r = 0.117, P = 0.0009), BMI (r = 0.014, P = 0.7005), serum CRP (r = 0.029, P = 0.4695), serum LDL (0.011, P = 0.7588), and serum high‐density lipoprotein (HDL) (r = −0.036, P = 0.3128).
DISCUSSION
In the present study, we developed a simple RIA system for PGE‐MUM using urinary samples by making specific rabbit antibody against bicyclic PGE‐MUM (4‐(1‐(2‐carboxyethyl)‐2,5‐dioxooctahydro‐1H‐inden‐4‐yl)butanoic acid). Its specificity was confirmed by inhibition test using bicyclic‐PGE‐MUM (data not shown) similar to the former assay system (3). Furthermore, it was confirmed that the pretreatment of urine samples (PGE metabolites) with 1N NaOH leads to a structure identical to that of the authentic bicyclic PGE‐MUM by HPLC‐ELSD‐LC/MS method. In addition, PGE‐MUM corrected by urinary creatinine as "micrograms per gram creatinine" was also adopted in line with former reports 5, 6, 7. PGE‐MUM values by our RIA were almost consistent with those reported by LC/MS/MS 5, 6, 7. Our RIA method is advantageous in that it can measure lots of samples simultaneously, as opposed to LC/MS. Furthermore, our RIA method is simple and determines tiny amounts of PGE‐MUM, and results can be available for short time, compared to LC/MS.
PGE2 level in blood circulation depends on balance of PGE2 production by COX1/2 and its metabolism by 15‐hydroxyprostaglandin dehydrogenase (15‐PGDH; 8). The lung is a principal site of PGE2 clearance by 15‐PGDH 8, 9, although the kidney has also 15PDGH expression 10. Murphey et al. demonstrated that not only the nonselective COX inhibitor ibuprofen but also the COX2 selective inhibitor rofecoxib suppressed levels of PGE‐MUM in healthy humans 5. Further, Uppal et al. showed a relative correlation of urinary PGE2 (nanogram per millimole creatinine) and PGE‐MUM (nanogram per millimole creatinine) in families with wild and heterozygous 15PGDH mutation 9. We deleted subjects with renal dysfunction and diseases in the present study. PGE2 is quickly metabolized. Accordingly, so far as we know, urinary PGE‐MUM is the best measure of total endogenous PGE2 production, although there is a possibility of influence of renal metabolite as well as endogenous PGE2. The influence of renal metabolite remains to be clarified.
For screening to get standard values of PGE‐MUM, we checked for differences in PGE‐MUM among samples obtained in the morning, afternoon, and evening from identical subjects. Since the PGE‐MUM in samples obtained in the morning (7:00 am) was relatively stable and low, we decided to use urinary samples obtained in the morning, routinely. Differences in PGE‐MUM during the day might be caused by hormonal rhythm or lifestyle, although this remains to be confirmed.
The present study demonstrated for the first time that PGE‐MUM was clearly different between males and females, and according to age. The difference was small but significant. Although a stepwise decrease in PGE‐MUM was observed with advancing age in males, a stepwise increase in PGE‐MUM with age was observed in females. These interesting results were found both in nonsmokers and overall subjects. Although standard deviations were relatively large, a decrease tendency of PGE‐MUM with age was shown in males, suggesting a relation with diminished body activity or decrease of insidious inflammation or reduced androgen effect 11. Since little is known about the possibility of PGE2 production with over exercise, further analysis is warranted on this tendency. The analysis of the large fluctuation of PGE‐MUM in males might be able to find unknown factors to give some influence on PGE2 production.
On the other hand, the increase tendency of PGE‐MUM with age in females might be related to the lower level of estrogen during menopause. In fact, PGE‐MUM was significantly higher in ≤40 and 41–60 years in males than in females. However, no difference of PGE‐MUM in ≥61 was observed between males and females. It has been reported that estrogen inhibits the production of PGE2 12, IL‐1α, and IL‐1β 13 as well as the proinflammatory cytokine production of peripheral blood mononuclear cells in vitro level 14. Further, a few reports have suggested that estrogen has anti‐inflammatory activity in microglia 15 and inhibits experimental arthritis 16. Recent reports showed that estrogen suppresses IL‐6 production by Kupffer cells in hepatocellular carcinoma development in female chronic hepatitis in experimental mice and humans 17, 18. Our data concerning the age‐dependent stepwise increase of PGE‐MUM in females may suggest an intimate relation with the down regulation of female sex hormones, although no direct evidence has been confirmed. However, there are several reports suggesting estrogen‐enhanced PGE2 production by macrophages 19 and gingival cells 20, and synergistic upregulation of PGE synthase expression in breast cancer cells 21. Thus, it is still controversial whether female sex hormones, such as 17‐β estradiol, modulate inflammatory reaction, and PGE2 production. The relation between PGE2 production and menstrual cycle in individual subjects remains to be defined.
A significant increase of PGE‐MUM according to smoking status was confirmed in our detailed large‐scale analysis of age‐matched subjects. Our results were in line with previous reports using smaller numbers of subjects 6. Smoking is the most important risk factor of chronic obstructive pulmonary disease (COPD; 22). In fact, over 90% of COPD patients have a smoking history 23. Further, it is reported that enhanced levels of PGE2 and matrix metalloproteinase‐2 correlate with the severity of airflow limitation in stable COPD 24. The effect of smoking on the transcriptional regulation of lung inflammation was apparent in patients with COPD, as opposed to nonsmokers 25. The effect of cigarette smoke extract on COX2 and PGE2 expression, as well as PGE2 release in neutrophils and alveolar macrophages, was also evident 26. In relation with these reports, our data on the increased PGE‐MUM in smokers indicate that smoking induces inflammatory injury of pulmonary tissue.
PGE‐MUM was significantly higher in current smokers than former smokers (<5 years and ≥5 years). In contrast, SIs classified into two groups by SI = 400 showed no difference in PGE‐MUM. This phenomenon suggests that PGE‐MUM shows current inflammatory injury status with smoking.
Serum CRP, which was simultaneously examined by a relatively highly sensitive method in the present study, showed a similar tendency for serum CRP to be significantly higher in males aged ≤40 and 41–60, as compared to their female counterparts, in line with the previous report 27. However, serum CRP did not decrease with age, as opposed to PGE‐MUM in males. In contrast, a tendency of stepwise increase in serum CRP with age was demonstrated in females, similar to PGE‐MUM. Further, current male smokers showed a significant increase in serum CRP, compared to nonsmokers, in line with PGE‐MUM. However, there was no difference of serum CRP between current smokers and former smokers (<5 years or ≥5 years). There was also a tendency for serum CRP in SI > 400 to be higher than in SI ≤ 400. It can be suggested that serum CRP reflects not only current but also accumulated smoking status, clearly different from PGE‐MUM, which indicates current active smoking injury, independent of SI. In fact, no significant correlation was found between PGE‐MUM and serum CRP in the present study. Serum CRP concentration is reported to be associated with oxidative stress and risk factors of cardiovascular disease and atherothrombosis but not with cancer development 28, 29, 30, 31, while PGE products are known to be risk factors for both cardiovascular disease and cancer development 1, 2, 31. These differences might have some relation with cell or tissue injury types.
According to the present study, our newly developed RIA method can detect precise values of PGE‐MUM, and the results are highly sensitive and reliable, because the values reflect minor injuries such as smoking. This method makes the estimation and screening of inflammation status in the living body easily available, without any injury to the body. Particularly, this method may be useful for screening of chronic inflammation, including UC and interstitial pneumonia. Altogether, PGE‐MUM is higher in males than in females, although age influences PGE‐MUM. PGE‐MUM increases in smokers, suggesting significant and fairly current inflammatory injury of pulmonary tissue, as opposed to serum CRP that shows both current and accumulated smoking injury. This RIA method of PGE‐MUM may be useful for screening and estimation of inflammation status in the living body, in addition to basic clinical examination.
CONFLICT OF INTEREST
The authors have read the journal's policy and have the following conflicts: Dr. Okayasu, Dr. Fujiwara, and Fujirebio Co., Inc. have kept the patent for method for determining the stage of ulcerative colitis or interstitial pneumonitis and reagent kit thereof (Japan #4,914,347; USA #8,053,202). Dr. Ohnishi is an employee of TFB Co., Inc., which is a subsidiary company of Fujirebio Co., Inc. Dr. Azumi is an employee of Fujirebio Co., Inc. Dr. Ito is a consultant for Fujirebio Co., Inc. and receives consultancy fee from Fujirebio Co., Inc.
REFERENCES
- 1. Thun MJ, Namboodiri MM, Heath CW, Jr . Aspirin use and reduced risk of fatal colon cancer. N Engl J Med 1991;325:1593–1596. [DOI] [PubMed] [Google Scholar]
- 2. DuBois RN, Smalley WE. Cyclooxygenase, NSAIDs, and colorectal cancer. J Gastroenterol 1996;31:898–906. [DOI] [PubMed] [Google Scholar]
- 3. Inagawa T, Imaki K, Masuda H, et al. Simplified immunoassays of prostaglandin E main metabolite in human urine. Adv Prostaglandin Thromboxane Leukot Res 1983;11:191–196. [PubMed] [Google Scholar]
- 4. Fujiwara M, Okayasu I, Oritsu M, et al. Significant increase in prostaglandin E‐main urinary metabolite by laxative administration: Comparison with ulcerative colitis. Digestion 2000;61:201–206. [DOI] [PubMed] [Google Scholar]
- 5. Murphey LJ, Williams MK, Sanchez SC, et al. Quantification of the major urinary metabolite of PGE2 by a liquid chromatographic/mass spectrometric assay: Determination of cyclooxygenase‐specific PGE2 synthesis in healthy humans and those with lung cancer. Anal Biochem 2004;334:26–75. [DOI] [PubMed] [Google Scholar]
- 6. Gross ND, Boyle JO, Morrow JD, et al. Levels of prostaglandin E metabolite, the major urinary metabolite of prostaglandin E2, are increased in smokers. Clin Cancer Res 2005;11:6087–6093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Duffield‐Lillico AJ, Boyle JO, Zhou XK, et al. Levels of prostaglandin E metabolite and leukotriene E(4) are increased in the urine of smokers: Evidence that celecoxib shunts arachidonic acid into the 5‐lipoxygenase pathway. Cancer Prev Res (Phila) 2009;2:322–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ferreira SH, Vane JR. Prostaglandins: Their disappearance from and release into the circulation. Nature 1967;216:868–873. [DOI] [PubMed] [Google Scholar]
- 9. Uppal S, Diggle CP, Carr IM, et al. Mutations in 15‐hydroxyprostaglandin dehydrogenase cause primary hypertyrophic osteoartheropathy. Nat Genet 2008;40:789–793. [DOI] [PubMed] [Google Scholar]
- 10. Yao B, Xu J, Harris RC, Zhang M‐Z. Renal localization and regulation of 15‐hydroxyprostaglandin dehydrogenase. Am J Physiol Renal Physiol 2008;294:F433–F439. [DOI] [PubMed] [Google Scholar]
- 11. Razmara A, Krause DN, Duckles SP. Testosterone augments endotoxin‐mediated cerebrovascular inflammation in male rats. Am J Physiol Heart Circ Physiol 2005;289:H1843–H1850. [DOI] [PubMed] [Google Scholar]
- 12. Miyagi M, Morishita M, Iwamoto Y. Effects of sex hormones on production of prostaglandin E2 by human peripheral monocytes. J Periodontol 1993;64:1075–1078. [DOI] [PubMed] [Google Scholar]
- 13. Morishita M, Miyagi M, Iwamoto Y. Effects of sex hormones on production of interleukin‐1 by human peripheral monocytes. J Periodontol 1999;70:757–760. [DOI] [PubMed] [Google Scholar]
- 14. Dienstknecht T, Schwacha MG, Kang SC, et al. Sex steroid‐mediated regulation of macrophage/monocyte function in a two‐hit model of trauma‐hemorrhage and sepsis. Cytokine 2004;25:110–118. [DOI] [PubMed] [Google Scholar]
- 15. Vegeto E, Bonincontro C, Pollio G, et al. Estrogen prevents the lipopolysaccharide‐induced inflammatory response in microglia. J Neurosci 2001;21:1809–1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ganesan K, Balachandran C, Manohar BM, et al. Comparative studies on the interplay of testosterone, estrogen and progesterone in collagen induced arthritis in rats. Bone 2008;43:758–765. [DOI] [PubMed] [Google Scholar]
- 17. Naugler WE, Sakurai T, Kim S, et al. Gender disparity in liver cancer due to sex differences in MyD88‐dependent IL‐6 production. Science 2007;317:121–124. [DOI] [PubMed] [Google Scholar]
- 18. Nakagawa H, Maeda S, Yoshida H, et al. Serum IL‐6 levels and the risk for hepatocarcinogenesis in chronic hepatitis C patients: An analysis based on gender differences. Int J Cancer 2009;125:2264–2269. [DOI] [PubMed] [Google Scholar]
- 19. Lu B, Jiang YJ, Choy PC. 17‐Beta estradiol enhances prostaglandin E2 production in human U937‐derived macrophages. Mol Cell Biochem 2004;262:101–110. [DOI] [PubMed] [Google Scholar]
- 20. ElAttar TM. Prostaglandin E2 in human gingiva in health and disease and its stimulation by female sex steroids. Prostaglandins 1976;11:331–341. [DOI] [PubMed] [Google Scholar]
- 21. Frasor J, Weaver AE, Pradhan M, et al. Synergistic up‐regulation of prostaglandin E synthase expression in breast cancer cells by 17beta‐estradiol and proinflammatory cytokines. Endocrinology 2008;149:6272–6279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Buist AV, Vollmer WM. Smoking and other risk factors In: Murray JE, Nadel JA. (Eds.), Textbook of Respiratory Medicine. WB Saunders, Philadelphia, PA, 1994. p 1259–1287. [Google Scholar]
- 23. Snider GL. Chronic obstructive pulmonary disease: Risk factors, pathophysiology and pathogenesis. Annu Rev Med 1989;40:411–429. [DOI] [PubMed] [Google Scholar]
- 24. Chen Y, Chen P, Hanaoka M, et al. Enhanced levels of prostaglandin E2 and matrix metalloproteinase‐2 correlate with the severity of airflow limitation in stable COPD. Respirology 2008;13:1014–1021. [DOI] [PubMed] [Google Scholar]
- 25. Szulakowski P, Crowther AJ, Jimenez LA, et al. The effect of smoking on the transcriptional regulation of lung inflammation in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2006;174:41–50. [DOI] [PubMed] [Google Scholar]
- 26. Profita M, Sala A, Bonanno A, et al. Chronic obstructive pulmonary disease andneutrophil infiltration: Role of cigarette smoke and cyclooxygenase products. Am J Physiol Lung Cell Mol Physiol 2010;298:L261–L269. [DOI] [PubMed] [Google Scholar]
- 27. Ballou SP, Lozanski FB, Hodder S, et al. Quantitative and qualitative alterations of acute‐phase proteins in healthy elderly persons. Age Ageing 1996;25:224–230. [DOI] [PubMed] [Google Scholar]
- 28. Yamada S, Gotoh T, Nakashima Y, et al. Distribution of serum C‐reactive protein and its association with atherosclerotic risk factors in a Japanese population: Jichi Medical School Cohort Study. Am J Epidemiol 2001;153:1183–1190. [DOI] [PubMed] [Google Scholar]
- 29. Ito Y, Suzuki K, Tamakoshi K, et al. Colorectal cancer and serum C‐reactive protein levels: A case‐control study nested in the JACC Study. J Epidemiol 2005;15(Suppl 2):S185–S189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dohi Y, Takase H, Sato K, et al. Association among C‐reactive protein, oxidative stress, and traditional risk factors in healthy Japanese subjects. Int J Cardiol 2007;115:63–66. [DOI] [PubMed] [Google Scholar]
- 31. Hegener HH, Diehl KA, Kurth T, et al. Polymorphisms of prostaglandin‐endoperoxide synthase 2 gene, and prostaglandin‐E receptor 2 gene, C‐reactive protein concentrations and risk of atherothrombosis: A nested case‐control approach. J Thromb Haemost 2006;4:1718–1722. [DOI] [PubMed] [Google Scholar]


