Abstract
Background
The metabolic syndrome, syndrome X, is a group of metabolic disorders in which insulin resistance plays a pivotal role. The MS is an important risk factor for subsequent development of type 2 diabetes and cardiovascular disease. Fetuin‐A is a liver derived blood protein that acts as effective inhibitor of soft tissue calcification. Cystatin C is a useful marker in measuring glomerular filtration rate. Moreover, recently it has been suggested that cystatin C may be a potential biomarker for detecting microalbuminuria. Microalbuminuria (MA) is a strong indicator of morbidity related to cardiovascular disorders, and is currently considered a novel diagnostic criterion for MS. It has been also demonstrated that the increased serum fetuin‐A levels is associated with several parameters of MS. In this study, we attempted to investigate the relationship between serum fetuin‐A, cystatin‐C levels and microalbuminuria in patients with MS.
Methods
A total of 50 patients with MS and 25 control were included in this study. We defined MS by the NCEP criteria among nondiabetic outpatients. Patients with MS were further divided into two groups based on MA status. Overall 25 of the participants with MS did not have MA (group I), while the remaining 25 had MA (group II). None of the subjects in the healthy control group (group III) had laboratory findings supporting the presence of MA. The serum fetuin‐A and cystatin‐C levels were measured using ELISA.
Results
Age, distributions of sex, BP and LDL cholesterol levels were similar among all groups. BMI, Waist/hip ratio, FBG, HOMA‐IR, total cholesterol, trigliserid, CRP levels were significantly higher in group I and group II compared to control. In group II, the cystatin‐C and fetuin levels were higher than control. While the cystatin‐C levels were higher in group II compared to group I, the fetuin levels did not different. Morever, the fetuin A and cystatin‐C concentrations were positively correlated with microalbuminuria (r = 0.26, p = 0.02; r = 0.50, p = 0.0001, respectively).
Conclusion
In our study, we found that MS patients with microalbuminuria had high levels of fetuin‐A and cystatin‐C. In conclusion, we suggest that determination of fetuin‐A and cystatin C levels could be useful marker as an early indicator of renal injury in patients with MS.
Keywords: metabolic syndrome, microalbuminuria, fetuin‐A, cystatin‐C
INTRODUCTION
The metabolic syndrome (MS) is a disorder in which insulin resistance plays a pivotal role, and is considered to pose an added metabolic risk for the development of atherosclerotic diseases. The most widely accepted diagnostic criteria for MS are: abdominal obesity (waist circumference of >94 cm in men and >80 cm in women), hypertriglyceridemia (≥150 mg/dl), low high‐density lipoprotein cholesterol (HDL‐C) (<40 mg/dl in men and <50 mg/dl in women), high blood glucose (fasting plasma glucose of ≥100 mg/dl), and elevated blood pressure (≥135/80 mmHg). An individual fulfilling any three of the above criteria may be considered to have MS 1, 2. The frequency of MS in individuals aged 20 years or older in the United States has been reported to be around 27%, with a profound increase in the prevalence among women 3.
Microalbuminuria (MA) is a strong indicator of morbidity related to cardiovascular disorders, and is currently considered a novel diagnostic criterion for MS 4. MA has been shown to develop as a result of transcapillary albumin leakage due to endothelial dysfunction 5. The common belief is that MS is associated with the development of macrovascular and microvascular complications. For this reason, new and simple tests are needed to predict the development of MA early enough to allow for timely intervention to prevent complications.
Fetuin‐A first came to attention by the end of the 1970s as a negative acute phase reactant during acute inflammation, a characteristic shared by albumin 6. This glycoprotein is a potent inhibitor of calcification 7. It has been shown that inhabitation of tyrosine receptor activity of insulin receptors lead to suppression of insulin sensitivity 7. Numerous studies have demonstrated a positive correlation between serum fetuin‐A levels and several parameters of MS 8, 9.
Cystatin C, a protein encoded by the CST3 gene, is suggested to be a reliable marker of glomerular filtration rate (GFR). Moreover, recently, it has been suggested that cystatin‐C may be a potential biomarker for detecting microalbuminuria.
In this study, we attempted to investigate the relationship between serum fetuin‐A, cystatin‐C levels, and microalbuminuria in patients with metabolic syndrome.
MATERIAL AND METHODS
Study Design and Subjects
Adult patients (18–65 years) who were diagnosed with MS based on criteria put forth by the physician were screened for eligibility. They gave informed consent, and this study was approved by the institutional ethical committee. Those receiving glucocorticoid medication, with a known liver and/or kidney disorder, abnormal thyroid function tests, a diagnosed malignant disorder, suspected or confirmed pregnancy, a previous diagnosis of diabetes mellitus, or stage II hypertension were not enrolled. The control group consisted of healthy individuals matched for age and gender, with no known chronic/systemic disorder and not on any medications.
A detailed medical history including demographics was obtained from all participants, followed a careful physical examination during which anthropometric measurements were recorded (weight, height, body mass index, waist circumference, hip circumference, waist‐hip ratio). Body mass indices were calculated using the formula [BMI (kg/m2) = weight (kg)/height2 (m2)]. Blood pressure (BP) measurements were obtained for each participant by 24‐hr ambulatory BP monitoring devices.
Blood Sampling and Assay
Venous blood samples were obtained for all patients from the antecubital region between 8.00 and 9.00 a.m. after a 12–14‐hr overnight fast. The blood samples were immediately sent for the determination of fasting blood glucose as well as serum levels of HDL‐C, LDL‐C, TG, creatinine, fasting insulin, and thyroid stimulating hormone. Approximately 4 ml of the supernatant obtained from each of the blood samples was stored at a temperature of –80°C for the en masse determination of cystatin‐C and fetuin‐A levels at the end of the study period.
Insulin resistance was assessed using the homeostasis model assessment (HOMA‐IR) originally described by Mathew et al. 10. HOMA‐IR was calculated using the following formula:
HOMA‐IR has close correlation with the insulin sensitivity index by the standard euglycemic hyperinsulinemic clamp as shown by Mathew et al. 10. Even though the HOMA‐IR score is not considered a diagnostic criterion for the metabolic syndrome, MS patients with a HOMA score of less than 2.5 were excluded from the final analysis. Similarly patients in the control group who had a HOMA score exceeding 2.5 were also excluded.
All participants were asked to collect 24‐hr urine samples 1 day prior to the allotted date for blood sampling. Total urinary volume as well as urinary creatinine and microalbumin concentration was determined. We calculated eGFR with using the MDRD study equation.
Statistical Analysis
Statistics analyses performed using software SPSS for Windows 15. Descriptive data expressed as mean ± standard deviation or median (minimum – maximum). Differences between the groups analyzed using Student's t test, also Kruskal–Wallis and Mann–Whitney U test when necessary. Categorical variables studied with chi‐square test. P values < 0.05 were accepted as statistically significant. A multivariate analysis based on a backward logistic regression was used to evaluate the association between MA and clinical/laboratory variables.
RESULTS
A total of 50 patients with MS and 25 healthy controls were included in the final analysis. Patients with MS were further divided into two groups based on MA status. Overall 25 of the participants with MS did not have MA (group I), while the remaining 25 had MA (group II). None of the subjects in the healthy control group (group III) had laboratory findings supporting the presence of MA.
Age, distributions of sex, BP, and LDL cholesterol levels were similar among all groups. BMI, waist/hip ratio, FBG, HOMA‐IR, total cholesterol, trigliserid, CRP levels were significantly higher in group I and group II compared to control. The demographic characteristics and laboratory findings of the study population have been summarized in Table 1.
Table 1.
The Demographic Characteristic and Laboratory Finding of Study Population
| Group 1 (Mean ± SD) | Group 2 (Mean ± SD) | Control (Mean ± SD) | P value | |
|---|---|---|---|---|
| Demographics | ||||
| Age (years) | 49.2 ± 8.6 | 48.2 ± 7.7 | 46.5 ± 10.5 | ns |
| Sex (female)(%) | 17 (%68) | 17 (%68) | 16 (%64) | ns |
| Measurements | ||||
| BMI (kg/m2) | 34.56 ± 2.63 | 33.68 ± 2.59 | 22.61 ± 1.79a, b | 0.0001 |
| Waist/hip ratio | 0.95 ± 0.06 | 0.99 ± 0.05 | 0.77 ± 0.08a, b | 0.0001 |
| BP systolic (mmHg) | 130.48 ± 22.60 | 128.16 ± 21.68 | 126.28 ± 19.58 | ns |
| BP diastolic (mmHg) | 78.52 ± 16.90 | 82.84 ± 15.13 | 79.40 ± 16.35 | ns |
| FBG (mg/dl) | 115.96 ± 3.62 | 114.28 ± 5.38 | 86.20 ± 9.66a, b | 0.0001 |
| HOMA‐IR | 3.64 ± 1.17 | 3.33 ± 0.51 | 1.01 ± 0.55a, b | 0.0001 |
| Total cholesterol (mg/dl) | 203.53 ± 36.92 | 199.63 ± 39.92 | 180.31±37.71a, b | 0.078 |
| LDL (mg/dl) | 120.48 ± 27.67 | 118.48 ± 40.35 | 105.20 ± 30.76 | 0.220 |
| HDL (mg/dl) | 45.32 ± 8.81 | 43.28 ± 9.94 | 56.40 ± 16.34a, b | 0.001 |
| Trigliserid (mg/dl) | 188.64 ± 75.31 | 189.36 ± 67.70 | 93.56 ± 33.19a, b | 0.0001 |
| CRP (mg/l) | 4.4 ± 3.6 | 3.9 ± 3 | 1.4 ± 2a, b | 0.002 |
| Fetuin‐A (ng/ml) | 65.10 ± 12.38 | 71.37 ± 12.96 | 58.12 ± 8.63a, b | 0.001 |
| Microalbuminuria (mg/gün) | 6.56 ± 5.66 | 146.68 ± 157.24a | 3.3 ± 2.2a, b | 0.0001 |
| Cystatin‐C (ng/ml) | 1,067.61 ± 217.7 | 1,501.28 ± 494.08a | 1,032.00 ± 233.05a | 0.0001 |
| Creatinine clearance ml/min/1.73 m2 | 87.6 ± 15.3 | 79.28 ± 17.7 | 92.8 ± 20.4 | 0,031 |
P < 0,05 as compared with group 1,
P < 0,05 as compared with group 2.
SD, standard deviation; FBG, fasting blood glucose; HOMA‐IR, homeostasis model assessment for insulin resistance; BMI, body mass index; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; TG, triglycerides; BP, blood pressure; MA, microalbuminuria; ns, not significant.
In group II, the cystatin‐C and fetuin levels were higher than control. However, the cystatin‐C levels were not different between group I and control. While the cystatin‐C levels were higher in group II compared to group I, the fetuin levels did not different.
The fetuin‐A and cystatin‐C concentrations were positively correlated with microalbuminuria (r = 0.26, P = 0.02; r = 0.50, P = 0.0001, respectively). The area under the ROC curve was showed that the serum fetuin‐A level greater than 64 ng/ml was associated with sensitivity 72% and specificity 70% for detecting microalbuminuria in patients with metabolic syndrome (Fig. 1).
Figure 1.
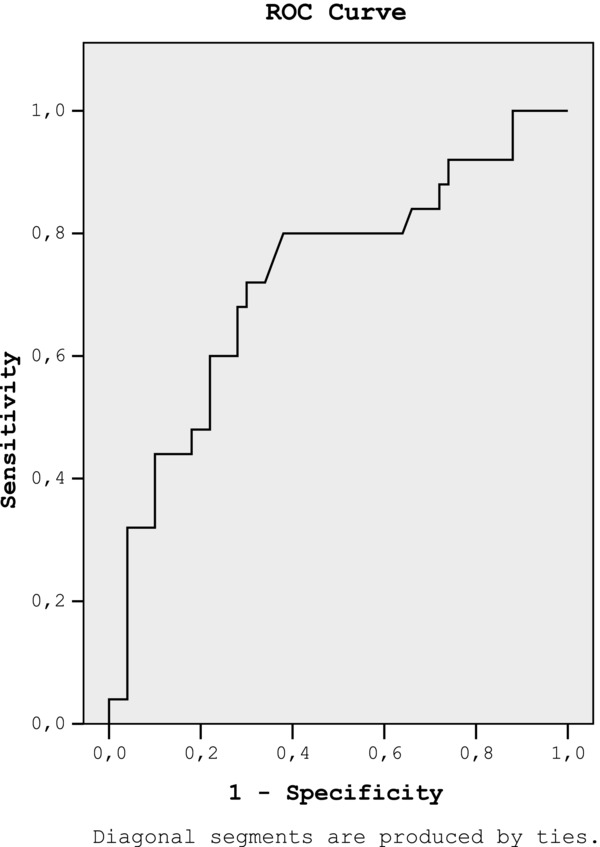
The ROC curve was showed that fetuin‐A can be used to detect microalbuminuria in patients with metabolic syndrome (are under the ROC curve 0.72, 95% CI: 0.59–0.85).
To determine whether microalbuminuria was related to fetuin‐A levels, the patients were separated into two subgroups with respect to fetuin‐A levels: Subgroup I (Fetuin‐A level < 64 ng/ml) n: 42, Subgroup II (Fetuin‐A level > = 64 ng/ml) n: 33. While microalbuminuria and HOMA‐IR were significantly higher in subgroup II, highest fetuin‐A group, remaining parameters (age, BMI, HDL cholesterol, LDL cholesterol, Trigliserid, creatinine clearance and cystatin‐C) were similar among subgroups (Table 2).
Table 2.
The Comparison of Demographic and Laboratory Findings in Patients According to Fetuin‐A Levels
| Lowest fetuin‐A | Highest fetuin‐A | ||
|---|---|---|---|
| Group (mean ± SD) | Group (mean ± SD) | ||
| N:42 | N:33 | P value | |
| Age (years) | 43.61 ± 10.99 | 45.81 ± 10.06 | ns |
| BMI (kg/m2) | 30.92 ± 6.45 | 32.03 ± 4.78 | ns |
| LDL (mg/dl) | 111.33 ± 33.35 | 119.03 ± 33.95 | ns |
| HDL (mg/dl) | 47.69 ± 10.03 | 49.15 ± 16.74 | ns |
| Trigliserid (mg/dl) | 143.83 ± 66.83 | 174.18 ± 83.79 | ns |
| HOMA‐IR | 2.39 ± 1.58 | 3.01 ± 1.12 | 0.019 |
| Microalbuminuria (mg/gün) | 34.20 ± 90.43 | 75.13 ± 132.62 | 0.015 |
| Cystatin‐C (ng/ml) | 1,110.61 ± 352.96 | 1,314.44 ± 426.89 | ns |
| Creatinine clearance ml/min/1.73 m2 | 89.07 ± 19.09 | 82.72 ± 18.74 | ns |
SD, standard deviation; BMI, body mass index; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; TG, triglycerides; HOMA‐IR, homeostasis model assessment for insulin resistance; ns, not significant.
The presence of microalbuminuria was assessed by multiple logistic regression analysis (including age, BMI, HDL cholesterol, LDL cholesterol, Trigliserid, HOMA‐IR, fetuin‐A levels, creatinine clearance, and cystatin‐C). Cystatin‐C was independent predictor for microalbuminuria (Table 3).
Table 3.
Multiple Logistic Regression Analysis for Factors Associated With Microalbuminuria
| Beta | P value | |
|---|---|---|
| Age (years) | 0.14 | 0.9 |
| BMI (kg/m2) | 0.144 | 0.4 |
| LDL (mg/dl) | 0.19 | 0.86 |
| HDL (mg/dl) | –0.122 | 0.32 |
| Trigliserid (mg/dl) | 0.126 | 0.37 |
| HOMA‐IR | –0.269 | 0.17 |
| Fetuin‐A (ng/ml) | 0.163 | 0.15 |
| Cystatin‐C (ng/ml) | 0.42 | 0.03 |
| Creatinine clearance ml/min/1.73 m2 | –0.54 | 0.68 |
SD, standard deviation; BMI, body mass index; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; TG, triglycerides; HOMA‐IR, homeostasis model assessment for insulin resistance; ns, not significant.
DISCUSSION
In our study, we found that MS patients with microalbuminuria had high levels of fetuin‐A and cystatin‐C. However, in multiple logistic regression analysis, we found no association between fetuin‐A and microalbuminuria. On the other hand, the cystatin‐C level was independent predictor for microalbuminuria. We suggested that the cystatin‐C is an important marker for detecting microalbuminuria rather than fetuin‐A in patients with MS.
The metabolic syndrome is a spectrum of disorders associated with an added cardiovascular risk, which develops as a result of several genetic and environmental factors. The basic components of MS are insulin resistance, abdominal obesity, elevated blood pressure, and lipid disorders. Microalbuminuria, which may be defined as mild urinary excretion of albumin, is an early predictor of impending cardiovascular disease and clinical nephropathy. Several studies have reported on a 10–40% prevalence of MA in patients with diabetes mellitus and hypertension 11, 12. It has been postulated that MA is the result of increased vascular permeability that develops as a renal manifestation of generalized vascular endothelial injury, which may make it a valuable early predictor of atherosclerosis and cardiovascular mortality 13. MA is currently considered a novel diagnostic criterion for MS 4. A link between MA and hypertension, central obesity and the other components of the metabolic syndrome have already been demonstrated 14, 15. However, the induction of microalbuminuria as a component of MS remains controversial 16, 17. Early recognition of renal injury in patients with MS is of great importance, since it may allow for the implementation of aggressive therapeutic interventions that may prevent the development or at least slow the progression of end‐organ damage. There is need for a simple, fast, and accurate method of detecting renal injury.
Insulin resistance is the main mechanism underlying the development of MS. The serum fetuin‐A, also referred to as α‐2‐Heremans–Schmid glycoprotein, which is almost exclusively expressed and secreted by the liver, induces insulin resistance, and subclinical inflammation in rodents 18. In animal studies, it has been demonstrated that fetuin‐A leads to insulin resistance via interactions at the level of the insulin receptor 18, 19. Moreover, the fetuin‐A gene is located near the locus that significantly associated with susceptibility to MS 18. Some authors suggested that the serum fetuin‐A levels are strongly associated with metabolic syndrome 9, 20. Joachim et al. reported on a normal distribution of fetuin‐A levels in nondiabetic patients with coronary artery disease. However, in this study, a significant association between highest levels of fetuin‐A and MS was observed 20. Similar to these studies, we found that the serum fetuin‐A was higher in patients with MS than healthy control.
The relationship between serum fetuin‐A levels and several parameters of MS (LDL‐C, TG, BMI, and HOMA‐IR) remains controversial. Roos et al. suggested that only serum triglycerides were found to be associated with fetuin‐A in patients with MS 21. Joachim et al. also concluded that, elevated fetuin‐A levels were found to be strongly associated with MS and an atherogenic lipid profile 20. In our study, there was no association between fetuin‐A and the components of metabolic syndrome including BMI, lipid profile, and blood pressure.
In humans, higher fetuin‐A levels associate with obesity and insulin resistance in patients with CKD and the general population 20, 22. Fetuin‐A and adiponectin, a 30‐kDa protein secreted from adipose tissue, may work in concert to regulate insulin resistance. Genes for both proteins are located at 3q27 in the human genome 23. Recently, Hennige et al. demonstrated that fetuin‐A suppresses mRNA encoding adiponectin in cultured human adipocytes, and treatment of wild‐type mice with fetuin‐A lowered serum adiponectin levels 24. In addition, the association between serum adiponectin and kidney was observed by some authors 25, 26. The pathologic evaluation of adiponectin null mouse demonstrates foot process effacement of podocytes and eventually exhibits proteinuria 26. In conclusion, higher fetuin‐A levels lead to suppression of adiponectin transcription in adipocytes and lower adiponectin levels reduce 5′‐AMP activated protein kinase in podocytes to promote podocyte foot process effacement and albuminuria 27. Reviewing the literature, a possible relationship between fetuin‐A and microalbuminuria has not been reported in patients with MS. There is only one study that was published by Li et al. In this study, they showed that albuminuria was associated with elevated serum fetuin‐A concentrations in middle aged and elderly Chinese patients with normal glucose tolerance 28. Our study is the first study that was shown the possible association between microalbuminuria and fetuin‐A levels in patient with MS.
Cystatin‐C is a useful marker in measuring glomerular filtration rate due to more constant production rate, freely filtration through the glomerulus, and not to be effected by muscle mass and gender. Recent studies indicate that cystatin‐C may be used as an early marker of mild loss in kidney functions 29. Moreover, recently, it has been suggested that cystatin‐C may be a potential biomarker for detecting microalbuminuria 30. In this study, the author was evaluated stage 1 hypertensive patients for a median 3.1 years. They demonstrated that the cystatin‐C was associated with future microalbuminuria. In another study, the serum and urine cystatin‐C levels increased with higher degree of albuminuria in type 2 diabetic patients 31. Similar to this study, we found that cystatin‐C levels were independently predictor for microalbuminuria in patients with MS. We thought that an increased cystatin‐C level associated with an increase in glomerular filtration rate precedes microalbuminuria.
One of the limitations of this study is the small sample size of the patients with MS. However, we believe that this shortcoming did not affect our results and reliability of the study.
In conclusion, our study findings suggest that determination of fetuin‐A and cystatin‐C levels could be useful marker as an early indicator of renal injury in patients with MS. Further studies with larger population are needed to evaluate possible association between fetuin‐A and microalbuminuria in patient with MS.
Authors declare no conflict of interest.
REFERENCES
- 1. Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 2005;112:2735–2752. [DOI] [PubMed] [Google Scholar]
- 2. Sanders BH, Lubsch LM, West DS. Prevalence and treatment of metabolic syndrome in adolescents with type 2 diabetes. Ann Pharmacother 2006;40:1517–1521. [DOI] [PubMed] [Google Scholar]
- 3. Earl S, Ford ES, Giles WH, Mokdad AH. Increasing prevalence of the metabolic syndrome among U.S. adults. Diabetes Care 2004;27:2444–2449. [DOI] [PubMed] [Google Scholar]
- 4. Bendriss L, Lebbaq A, Jallal H, Mrani S, Khatouri A. [Usefulness of microalbuminuria in the metabolic syndrome as a predictor of cardiovascular disease. Prospective study about 78 cases.]. Ann Cardiol Angeiol (Paris) 2012;61:15–19. [DOI] [PubMed] [Google Scholar]
- 5. Michael H Davidson, MD . Management of dyslipidemia in patients with complicated metabolic synsdrome. Am J Cardiol 2005;96:22–25. [DOI] [PubMed] [Google Scholar]
- 6. Lebreton JP, Joisel F, Raoult JP, Lannuzel B, Rogez JP, Humbert G. Serum concentration of human alpha 2 HS gylcoprotein during the inflammatory process: Evidence that alpha 2 HS2glycoprotein is a negative acute‐phase reactant. J Clin Invest 1979;64:1118–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Westenfeld R, Jahnen‐Dechentt W, Ketteler M. Vascular calcification and fetuin‐A deficiency in chronic kidney disease. Trends Cardiovasc Med 2007;17:124–128. [DOI] [PubMed] [Google Scholar]
- 8. Ishibashi A, Ikeda Y, Ohguro T, et al. Serum fetuin‐A is an independent marker of insulin resistance in Japanese men. J Atheroscler Thromb 2010;17:925–933. [DOI] [PubMed] [Google Scholar]
- 9. Xu Y, Xu M, Bi Y, et al. Serum fetuin‐A is correlated with metabolic syndrome in middle‐aged and elderly Chinese. Atherosclerosis 2011;216:180–186. [DOI] [PubMed] [Google Scholar]
- 10. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: Insulin resistance and beta‐cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–419. [DOI] [PubMed] [Google Scholar]
- 11. Bakris GL. Microalbuminuria: Prognostic implications. Curr Opin Nephrol Hypertens 1996;5:219–223. [DOI] [PubMed] [Google Scholar]
- 12. Varghese A, Deepa R, Rema M, Mohan V. Prevalence of microalbuminuria in type‐II diabetes mellitus at a diabetes center in southern India. Postgrad Med J 2001;77:399–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Deckert T, Feldt‐Rasmussen B, Borch‐Johnsen K, Jensen T, Kofoed‐Enevoldsen A. Albuminuria reflects widespread vascular damage. The Steno hypothesis. Diabetologia 1989;32:219–226. [DOI] [PubMed] [Google Scholar]
- 14. Choi HS, Sung KC, Lee KB. The prevalence and risk factors of microalbuminuria in normoglycemic, normotensive adults. Clin Nephrol 2006;65:256–261. [DOI] [PubMed] [Google Scholar]
- 15. Scaglione R, Ganguzza A, Corrao S, et al. Central obesity and hypertension: Pathophysiologic role of renal haemodynamics and function. Int J Obes Relat Metab Disord 1995;19:403–409. [PubMed] [Google Scholar]
- 16. Mykkanen L, Zaccaro DJ, Wagenknecht LE, Robbins DC, Gabriel M, Haffner SM. Microalbuminuria is associated with insulin resistance in nondiabetic subjects: The insulin resistance atherosclerosis study. Diabetes 1998;47:793–800. [DOI] [PubMed] [Google Scholar]
- 17. Zavaroni I, Bonini L, Gasparini P, et al. Dissociation between urinary albumin excretion and variables associated with insulin resistance in a healthy population. J Intern Med 1996;240:151–156. [DOI] [PubMed] [Google Scholar]
- 18. Auberger P, Falquerho L, Contreres JO, et al. Characterization of a natural inhibitor of the insulin receptor tyrosine kinase: cDNA cloning, purification, and anti‐mitogenic activity. Cell 1989;58: 631–640. [DOI] [PubMed] [Google Scholar]
- 19. Srinivas PR, Wagner AS, Reddy LV, et al. Serum alpha 2‐HS‐glycoprotein is an inhibitor of the human insulin receptor at the tyrosine kinase level. Mol Endocrinol 1993;7:1445–1455. [DOI] [PubMed] [Google Scholar]
- 20. Joachim H, Shlipak MG, Brandenburg VM, Ali S, Ketteler M, Whooley MA. Association between human fetuin‐A and the metabolic syndrome: Data from the Heart and Soul Study. Circulation 2006;113:1760–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Roos M, Von Eynatten M, Heemann U, Rothenbacher D, Brenner H, Breitling LP. Serum fetuin‐A, cardiovascular risk factors, and six‐year follow‐up outcome in patients with coronary heart disease. Am J Cardiol 2010;105:1666–1672. [DOI] [PubMed] [Google Scholar]
- 22. Stefan N, Hennige AM, Staiger H, et al. Alpha2‐Heremans‐Schmid glycoprotein/fetuin‐A is associated with insulin resistance and fat accumulation in the liver in humans. Diabetes Care 2006;29:853–857. [DOI] [PubMed] [Google Scholar]
- 23. Vionnet N, Hani EH, Dupont S, et al. Genomewide search for type 2 diabetes‐susceptibility genes in French whites: Evidence for a novel susceptibility locus for early‐onset diabetes on chromosome 3q27‐qter and independent replication of a type 2‐diabetes locus on chromosome 1q21–q24. Am J Hum Genet 2000;67:1470–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hennige AM, Staiger H, Wicke C, et al. Fetuin‐A induces cytokine expression and suppresses adiponectin production. PLoS One 2008;3:e1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tsioufis C, Dimitriadis K, Chatzis D, et al. Relation of microalbuminuria to adiponectin and augmented C‐reactive protein levels in men with essential hypertension. Am J Cardiol 2005;96:946–951. [DOI] [PubMed] [Google Scholar]
- 26. Sharma K, Ramachandrarao S, Qiu G, et al. Adiponectin regulates albuminuria and podocyte function in mice. J Clin Invest 2008;118:1645–1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ix JH, Sharma K. Mechanisms linking obesity, chronic kidney disease, and fatty liver disease: The roles of fetuin‐A, adiponectin, and AMPK. J Am Soc Nephrol 2010;21:406–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Li M, Xu M, Bi Y, et al. Association between higher serum fetuin‐A concentrations and abnormal albuminuria in middle‐aged and elderly chinese with normal glucose tolerance. Diabetes Care 2010;33:2462–2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Filler G, Bökenkamp A, Hofmann W, Bricon TL, Martinez‐ Bru C, Grubb A. Sistatin C as a marker of GFR—History, indications, and future research. Clin Biochem 2005;38:1–8. [DOI] [PubMed] [Google Scholar]
- 30. Palatini P, Benetti E, Zanier A, et al. Cystatin C as predictor of microalbuminuria in the early stage of hypertension. Nephron Clin Pract 2009;113:309–331. [DOI] [PubMed] [Google Scholar]
- 31. Jeon YK, Kim MR, Huh JE, et al. Cystatin C as an early biomarker of nephropathy in patients with type 2 diabetes. J Korean Med Sci 2011;26:258–263. [DOI] [PMC free article] [PubMed] [Google Scholar]


