Abstract
Background
Blood glucose determination is one of the most common clinical diagnostic tests. Often, blood is collected in a field station and analysis is carried out in a remote laboratory. Because blood cells can continue to metabolize glucose, the time of determination of blood glucose after drawing the blood is important.
Method
In order to test the relative suitability of plasma and serum for blood glucose determination, fluoride plasma and Ethylene Diamine Tetra Acetic acid (EDTA) plasma were compared with the serum of the same patient. The analyses were carried out within 10 min of drawing the blood and, thereafter, with a gap of 4 hr and 8 hr.
Results
Serum gave values lower than fluoride plasma by 1.15%. Although this difference was statistically significant, it may not be physiologically relevant. Hence, serum may be used for blood glucose determination with an error of 1.15%. On storing the sample at room temperature for 8 hr, the serum glucose value decreased by 8%. Even fluoride plasma had 4.3% lower glucose.
Conclusion
Hence, blood glucose should be determined within as short a time as possible after drawing the blood. J. Clin. Lab. Anal. 26:317‐320, 2012. © 2012 Wiley Periodicals, Inc.
Keywords: fluoride plasma, EDTA plasma, blood glucose assay
INTRODUCTION
Blood glucose determination is one of the most common clinical diagnostic tests. Accurate and precise measurement of blood glucose is of great importance in the diagnosis and management of diabetes 1. Once the blood is drawn, the concentration of glucose will continue to decrease because of glycolysis, which will occur in erythrocytes, white blood cells (WBCs), and platelets 2, 3, 4, 5, 6. The rate of glycolysis in the whole blood is reported to be 5–7% depending on various factors such as glucose concentration WBC count and hematocrit value 6.
The most practical way to measure blood glucose is in the plasma, which is separated immediately after drawing the blood in the presence of an anticoagulant. Most common anticoagulants used are fluoride, Ethylene diamine Tetra acetic acid (EDTA), and heparin 6.
In some cases, blood needs to be transported from the site where it is drawn to a remote laboratory for analysis. Sometimes, whn automated analysis of blood is done, there may be a waiting period before the analysis is carried out. Also in clinics, immediate measurement of blood glucose is not done for many reasons: often quantity and frequency of blood samples limits the possibility of immediate analysis 7, 8. Under such conditions, it is important to recognize that the blood glucose values can be lower.
In this study, we have investigated the effect of time and the method of processing of blood on the blood glucose levels. We suggested that serum may be a better fluid of choice when there is a delay in the analysis of blood glucose.
MATERIALS AND METHODS
Patients
Blood from 30 different patients who came for routine blood glucose determination were used in the study. Whole blood was divided into three portions. One portion was allowed to clot. To the second portion, sodium fluoride (2mg/ml of blood) was added and to the third portion, EDTA–disodium salt was added. The blood was centrifuged at 2,500 rpm for 10 min and the clear plasma or serum was used in the assay. Blood sugar was estimated using the Beckton Dicknison blood sugar assay Kit (Becton Dickinson India Pvt. Ltd., Bangalore, India). Plasma or serum (10 μl) was mixed with 90 μl of reagent consisting of glucose oxidase–peroxidase‐O‐diamisidine. After 10 min, Optical Density (OD) of the colored complex was measured at 620 nm in an autoanalyzer. Blood sugar in these samples stored at room temperature was measured again after 4 hr and 8 hr.
Statistical Analysis
Fischer's test was used to compare the mean values of the three groups; student's t‐test was used for paired comparisons.
RESULTS
The results of blood glucose measurement of 30 random samples preserved for up to 8 hr are shown in Fig. 1. Fluoride plasma showed the least decrease and EDTA plasma showed the maximum decrease at 8 hr. At 0 time, serum values of blood glucose were 1.15% lower than the corresponding fluoride plasma values and the deference was significant (P < 0.05).
Figure 1.
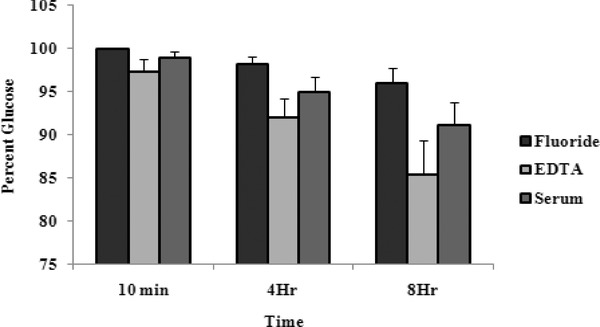
Blood glucose in fluoride plasma, EDTA plasma, and serum assayed immediately after 4 hr and after 8 hr. Values are normalized to fluoride plasma. Results are mean ± SD (n = 30).
The absolute difference in blood glucose followed a normal distribution andwas not related to the absolute blood glucose value (Fig. 2). The blood glucose measured for fluoride plasma correlated linearly with blood glucose in serum (R 2 = 0.999; Fig. 3).
Figure 2.
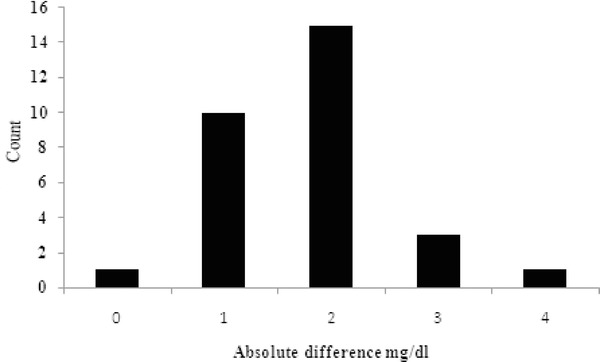
The count of absolute difference between the blood glucose value in fluoride plasma and serum of samples analyzed soon after drawing blood.
Figure 3.
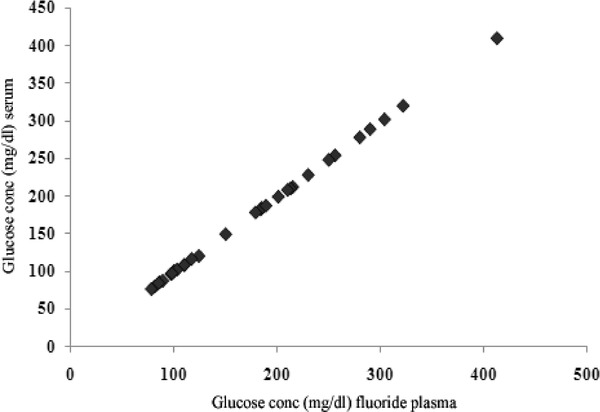
Blood glucose values of fluoride plasma and serum assayed soon after drawing blood was plotted in a scatter diagram (R 2 = 0.999).
DISCUSSION
Several factors have been reported to affect the stability of blood glucose values. Studies have demonstrated that key glycolytic enzyme expression levels is shown to be peak at the onset of circadian night; hence, the early morning sample is shown to be better in stability than the late evening samples 9, 10. All the samples used in this study were drawn at 9.30 am over several days to avoid time of drawing of blood as a variable that could affect the blood glucose measurement. Since same individual was used for three samples, namely the fluoride plasma, EDTA plasma, and serum, the results can be compared between the individuals and within an individual with great accuracy.
The blood glucose values ranged from 76 to 410 covering a wide range of blood glucose values that would be seen in any sample analysis. The absolute amount of glucose that was different between the samples showed a normal distribution andwas independent of the actual blood glucose values, this suggests that the decrease in blood glucose is not entirely proportional to the initial blood glucose level but may have other factors contributing to its overall decrease.
Although plasma is devoid of red blood cells (RBCs) and white blood cells (WBCs), they still contain platelets, and hence, the platelet glucose metabolism can affect the amount of glucose measured with time. On the other hand, serum would be free from any cells and hence this should be suitable for blood glucose measurement particularly when blood glucose is measured after a time delay. Also, Serum is clearer than plasma because of fewer proteins. Proteins are sometimes considered as interfering substances in some tests as they react with the reagent and thereby yield inaccurate results 11.
However, the serum values were significantly lowers (P < 0.05) than plasma values even though the difference was only 1.15%. This small difference can affect diagnosis particularly for patients in the borderline area of impaired glucose tolerance. Since clinical laboratories rely on cutoff values for the classification, even a 1 mg% lower value could miss the potential diabetic patients during diagnosis.
Hence, serum may be a better sample for blood glucose determination particularly when there is a time delay in the measurement. If appropriate correction is applied, it may actually be a better sample than plasma. Moreover, many clinical analysis procedures require serum rather than plasma. Hence, one single drawing of blood may suffice for even blood glucose estimation.
Grant sponsor: Indian Council of Medical Research and Department of Science and Technology.
REFERENCES
- 1. Schrot RJ, Patel KT, Foulis P. Evaluation of inaccuracies in the measurement of glycemia in the laboratory, by glucose meters, and through measurement of hemoglobin A1c. Clin Diabetes 2007;25(2):43–49. [Google Scholar]
- 2. Gambin R. Glucose: A simple molecule that is not simple to quantify. Clin Chem 2007;53(12):2040–2041. [DOI] [PubMed] [Google Scholar]
- 3. Chan AYW, Swaminathan R, Cockram CS. Effectiveness of sodium fluoride as a preservative of glucose in blood. Clin Chem 1989;35(2):315–317. [PubMed] [Google Scholar]
- 4. Steffes MW, Sacks DB. Measurement of circulating glucose concentrations: The time is now for consistency among methods and types of samples. Clin Chem 2005;51(9):1569–1570. [DOI] [PubMed] [Google Scholar]
- 5. Overfield CV, Savory J, Heintges MG. Glycolysis: A re‐evaluation of the effect on blood glucose. Clin Chim Acta 1972;39:35–40. [DOI] [PubMed] [Google Scholar]
- 6. Sacks DB, Goldstein DE, Maclaren NK, McDonald JM, Parrott M. Guidelines and recommendation for laboratory analysis in the diagnosis and management of diabetes mellitus. Diabetes Care 2002;25(4):750–786. [PubMed] [Google Scholar]
- 7. Eliman A, Horal M, Bergstrrom M, Marcus C. Diagnosis of hypoglycemia: Effects of sample handling and evaluation of a glucose photometer in the low glucose range. Acta paediatr 1997;86(5):474–478. [DOI] [PubMed] [Google Scholar]
- 8. Deshpande S, Platt MW. The investigation and management of neonatal hypoglycaemia. Semin Neonatol 2005;10(4):351–361. [DOI] [PubMed] [Google Scholar]
- 9. Daniela E, Acerini CL, Allen JM, et al. Effect of delay on measurement of blood glucose levels in young subjects with type 1 diabetes. Diabetes Res Clin Pract 2009;86:31–33. [DOI] [PubMed] [Google Scholar]
- 10. Reddy AB, Karp NA, Maywood ES, et al. Circadian orchestration of the hepatic proteome. Curr Biol 2006;16(11):1107–1115. [DOI] [PubMed] [Google Scholar]
- 11.ProImmune Limited. Protocols for the Preparation of Blood Plasma and Serum. 2009. Available at http://www.proimmune.com/ecommerce/pdf_files/PR31.pdf. Accessed September 11, 2011.


