Abstract
Background
Excessive alcohol intake can result in the oxidative stress in cells and the genetic variations of alcohol‐metabolizing enzymes are responsible for the different degrees of toxicity of alcohol in several organs, such as the liver and immunological systems. We hypothesized that the alteration of oxidative stress due to some genetic variations of oxidative stress‐related enzymes could result in changes of specific biomarkers, and heavy drinkers could be cautioned about the predictive likelihood to induce drinking‐induced diseases.
Methods
A total of 108 heavy drinkers and 106 nonheavy drinkers were enrolled and the hematological, biochemical, and immunological tests were measured; the genotypes of oxidative stress‐related enzymes, including manganese superoxide dismutase (MnSOD1183T>C), glutathione peroxidase 1 (GPX1Pro198Leu), catalase (CAT‐262C>T), and myeloperoxidase (MPO‐463G>A), were assayed by real‐time polymerase chain reaction (PCR) and PCR‐restriction fragment length polymorphism (PCR‐RFLP).
Results
For the males, the levels of carbohydrate‐deficient transferrin (CDT), malondialdehyde (MDA), CD4+, immunoglobulin G (IgG), immunoglobulin M (IgM), and IL‐6 were significantly different between the two groups. Furthermore, there were higher proportions of CD19+ cells and lower TNF‐α levels in heavy drinkers with the MnSOD C carriers, and there were higher percentages of CD19+ cells and IL‐6 levels in heavy drinkers with the combined genotypes of MnSOD C carriers and MPO A carriers.
Conclusions
Our findings indicate that heavy drinkers may be cautioned predictive likelihood for them to induce drinking‐induced diseases by analyzing their MnSOD genotypes and immunological biomarkers.
Keywords: oxidative stress‐related enzymes, polymorphisms, manganese superoxide dismutase (MnSOD), alcohol, heavy drinkers
INTRODUCTION
Different metabolic pathways affect oxidative stress induced by alcohol intake, as shown in Figure 1. Superoxide dismutase (SOD) can dismutase superoxide anion (O2˙−) to hydrogen peroxide (H2O2) and reduce alcohol‐induced toxicity; therefore, SOD plays a key role in metabolic pathways.
Figure 1.
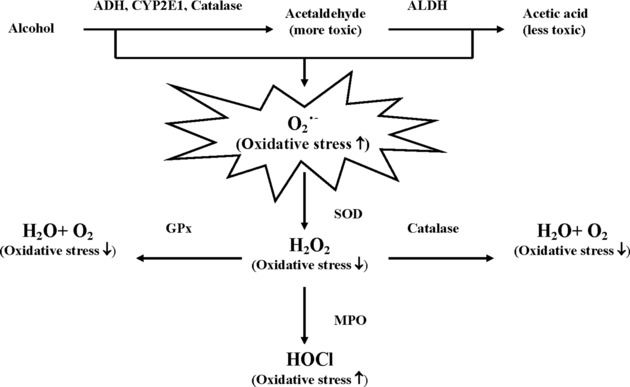
Different metabolic pathways affect oxidative stress induced by alcohol intake. ADH, alcohol dehydrogenase; ALDH, aldehyde dehydrogenase; CYP2E1, cytochrome P450 2E1; O2˙−, superoxide anion; ROS, reactive oxygen species; SOD, superoxide dismutase; H2O2, hydrogen peroxide; GPX, glutathione peroxidase; MPO, myeloperoxidase; HOCl, hypochlorous acid.
Genetic variations leading to alteration of antioxidant activities have been postulated to be a possible mechanism. One of the manganese SOD (MnSOD) polymorphisms leading to the alteration of antioxidant activities, the T to C substitution in nucleotide 1183, results in a change of amino acid from valine to alanine at codon 16, which has been shown to change the structural conformation of the mitochondrial targeting sequence of the enzyme and is predicted to form amphiphilic helix with higher enzyme activity 1, 2. Regarding glutathione peroxidase 1 (GPX1), it has a genetic polymorphism encoded for proline (Pro) or leucine (Leu) at codon 198 of human GPx1 3. Concerning the catalase (CAT) gene, there is a ‐262 C>T substitution on the 5V region of the human CAT gene from the transcription start site and the variant gene displays a lower activity than CAT‐262 C 4. Furthermore, a frequently occurring single nucleotide polymorphism (SNP) in the promoter region of the myeloperoxidase (MPO) gene is a ‐463 G>A substitution, which the MPO (G‐463‐A) A variant allele confers lower transcriptional activation than the G common allele in vitro due to the disruption of the binding site 5, 6. Therefore, the activity of the above‐mentioned enzymes might be affected by their functional polymorphisms in the genes encoding them.
Previous researchers have reported that excessive alcohol intake may lead to oxidative damage of cells and tissues by reactive oxygen species (ROS) and the oxidative damage might be affected by the polymorphisms of alcohol‐metabolizing enzymes and oxidative stress‐related enzymes 7, 8, 9, 10, 11. Our previous studies also showed that the genetic variations of alcohol‐metabolizing enzymes played important roles in trauma patients at an emergency department 12, 13 and the habits of drinking, smoking, and betel chewing, and genetic variations of alcohol‐metabolizing enzymes were associated with immunological biomarkers 10.
Clinically, the alterations of many biomarkers, such as gamma‐glutamyl transferase (GGT), aspartate aminotransferase (AST), malondialdehyde (MDA), and immunological markers have been reported to be associated with excessive alcohol intake 10, 14. Additionally, other previous studies also revealed that excessive alcohol intake was associated with increased iron stores when assessed by serum ferritin concentration 15, 16 and alcohol intake could change the structure of the transferrin (TRF) molecule to become carbohydrate deficient, namely, carbohydrate‐deficient transferrin (CDT), resulting in increased ferritin concentrations in the plasma 17, 18. It is well known that the progression of damage due to chronic alcohol abuse is a multifactorial event that involves some genetic and environmental factors 19.
In this study, we hypothesized that the alteration of oxidative stress due to some genetic variations of oxidative stress‐related enzymes (Fig. 1) could result in changes of specific biomarkers, and heavy drinkers could be cautioned about the predictive likelihood to induce drinking‐induced diseases. Therefore, the genetic polymorphisms of oxidative stress‐related enzymes, including MnSOD, GPX1, CAT, and MPO and clinical biomarkers in heavy drinkers were investigated.
MATERIALS AND METHODS
Study Subjects
A total of 108 heavy drinkers and 106 nonheavy drinkers, excluding those with critical diseases, such as cancer and immune‐related diseases, were enrolled in this study. With informed consent, each participant signed and completed questionnaires, including the data about his/her age, weight, height, lifestyle, and self‐reported alcohol intake status. The participants were classified into two groups according to the modified Alcohol Use Disorders Identification Test (AUDIT) and Cut Down, Annoyed, Guilty, Eye Opener (CAGE) questionnaires in this study as previously described 10. Furthermore, the genetic variations of alcohol‐metabolizing enzymes were apparently different between Taiwanese and Western populations in our previous study 10; thus, the definition of high‐risk or heavy drinkers was different from that established by the World Health Organization 20, and we adopted the suggested definition of heavy drinking in a document from the National Health Research Institute in Taiwan 21. Briefly, heavy drinkers were defined as people with pure alcohol intake of either (a) exceeding 100 g for men and 50 g for women weekly; or (b) more than 40 g a time for men and 20 g for women at least once a week. CAGE is a brief and short evaluation, which includes four questions in order to differentiate individuals with alcohol use disorders. Point of intersection is recommended as two. The AUDIT is a self‐rated ten‐item questionnaire with each item scored 0–4, giving a total score of 40. Point of intersection is assumed to be 8 or 9 22. This study was approved by the institutional review board of the Kaohsiung Veterans General Hospital and informed consent was obtained from all participants.
Analysis of Biomarkers
To evaluate the oxidative stress‐related biomarkers, three categories of biomarkers, including hematological, biochemical, and immunological tests were assayed. The hematological parameters (counts of neutrophils, lymphocytes, and monocytes and the values of hemoglobin) were measured with an automated hematology analyzer (XE 2100, Sysmex Co., Kobe, Japan). The biochemical tests, including AST, alanine aminotransferase (ALT), GGT, and iron (Fe) were assayed by a biochemical autoanalyzer (Vitros Fusion 5.1, Ortho Clinical Diagnostics, Johnson & Johnson Co.). The concentration of ferritin, TRF, and CDT was measured with two chemical autoanalyzers (Access 2, Beckman Coulter and BN II, Siemens, Germany, respectively). The level of MDA, a biomarker of oxidative damage, was measured as previously described 13. The immunological function tests, including the levels of immunoglobulin G and M (IgG and IgM), the distributions of lymphocyte subpopulations (CD3+, CD4+, CD8+, and CD19+ cells), and the cytokine levels (IL‐2R, IL‐6, IL‐8, IL‐10, and tumor necrosis factor‐α [TNF‐α]), were performed by a biochemical autoanalyzer (Vitros Fusion 5.1, Ortho Clinical Diagnostics, Johnson & Johnson Co.), a flow cytometric analyzer (Coulter Epics XL, Beckman Coulter), and an immunological autoanalyzer (Immulite, DPC/Siemens, Germany), respectively.
Genotyping of Cxidative Stress‐Related Enzymes
The polymorphisms of MnSOD1183T>C (rs1799725) and GPX1Pro198Leu (rs1050450) were analyzed by a real‐time thermo cycler (MJ Research PTC‐200 Peltier Thermal Cycler, Bio‐Rad Co.). For the MnSOD1183T>C polymorphism, primers and probe (Sigma‐Proligo Singapore Pty Ltd) are as follows: primers forward 5′‐AGCCTGCGTAGACGGTCC‐3′ and reverse 5′‐TCGGGGAGGCTGTGCTTC‐3′, allele specific probes: forward 5′‐6‐FAM‐AGCCCAGATACCCCAA AACCGGAGCC‐TAMRA‐3′ and reverse 5′‐HEX‐AGCCCAGATACCCCAAAGCCGGAGCC‐TAMRA‐3′. Thermal cycling was initiated with a first denaturation step of 3 min at 95°C, and then by 40 cycles of 15 sec at 95°C and 60 sec at 55.1°C. As to the GPX1Pro198Leu polymorphism, primers and probes are as follows: primers forward 5′‐CCCCTACGCAGGTACAGC‐3′ and reverse 5′‐ACACCCTCATAGATGAAAACCC‐3′, allele specific probes: forward 5′‐HEX‐CGCGATCGTCTCAA GGGCCCAGCTGTGCCTGATCGCG (BHQ1)‐3′ and reverse 5′‐6‐FAM‐CGCGATCGTCTCAAGGGCTCAG CTGTGCCTGATCGCG (BHQ1)‐3′. Thermal cycling was initiated with a first denaturation step of 3 min at 95°C, and then by 40 cycles of 15 sec at 95°C and 60 sec at 55.1°C. Genotyping for the CAT‐262C>T (rs1001179; 14) and MPO‐463G>A (rs2333227; 23) was analyzed by the polymerase chain reaction‐restriction fragment length polymorphism (PCR‐RFLP) analysis. Primers for the CAT‐262C>T genotyping assay are as follows: forward 5′‐TAAGAGCTGAGAAAGCATAGCT‐3′, reverse 5′‐AGAGCCTCGCCCCGCCGGACCG‐3′. PCR amplification of the CAT gene promoter region was performed with a touchdown program designed as follows: 92°C for 30 sec, 70°C for 40 sec, −0.5°C per cycle (19 cycles), then 92°C for 30 sec, 60°C for 40 sec, 1 sec/cycle (19 cycles). The final elongation step was 10 min at 72.8°C. Then, the PCR products were digested with Sam I for 2 hr at 25°C and viewed with the aid of ethidium bromide staining. Primers for the MPO‐463G>A genotyping assay are as follows: forward 5′‐CGGTATAGGCACACAATGGTGAG‐3′, reverse 5′‐GCAATGGTTCAAGCGATTCTT C‐3′. PCR conditions were 35 cycles of 30 sec at 94°C, 30 sec at 56°C, and 30 sec at 72°C. The PCR product was digested with AciI at 37°C overnight and viewed with the aid of ethidium bromide staining.
Statistical Analysis
Data were analyzed using SPSS software for Windows (version 17.0, SPSS Inc., Chicago, IL). Differences between the two groups were evaluated by using the chi‐square test for discontinuous variables and Student's t‐test for continuous variables. Data were presented as mean ± SD. Probability differences of P < 0.05 were considered statistically significant.
RESULTS
It is well known that gender differences in many biomarkers are apparent; thus, we divided the participants into male and female groups, respectively, and then compared the general characteristics of the study population between heavy drinkers and nonheavy drinkers as shown in Table 1. Two hundred fourteen participants were enrolled in this study. The scores of CAGE and AUDIT in the heavy drinkers were significantly higher than those of the nonheavy drinkers. For the male participants, the heavy drinkers have higher body mass indices (BMIs) than the nonheavy drinkers.
Table 1.
General Characteristics of the Study Populationa
| Males (n = 136) | Females (n = 78) | |||
|---|---|---|---|---|
| Heavy drinkersb | Nonheavy drinkers | Heavy drinkersc | Nonheavy drinkers | |
| (n = 84) | (n = 52) | (n = 24) | (n = 54) | |
| Age (years) | 22—77 | 22–68 | 24–68 | 22–61 |
| CAGE score | 2.1 ± 1.0** | 0.7 ± 1.0 | 1.0 ± 1.2** | 0.2 ± 0.5 |
| AUDIT score | 13.8 ± 5.5** | 2.1 ± 2.4 | 11.2 ± 9.7** | 1.2 ± 0.9 |
| BMI (kg/m2) | 26.1 ± 3.3** | 24.4 ± 2.8 | 21.8 ± 2.7 | 22.1 ± 2.7 |
| Hematological tests | ||||
| Neutrophils (x103/μl) | 3.7 ± 1.4 | 3.7 ± 1.2 | 3.0 ± 1.1 | 3.3 ± 1.1 |
| Mononuclear cells (×103/μl) | 2.7 ± 0.7 | 2.6 ± 0.8 | 2.4 ± 0.4 | 2.5 ± 0.6 |
| Hgb (g/dl) | 15.3 ± 1.2 | 15.3 ± 0.8 | 12.8 ± 0.9 | 12.9 ± 1.1 |
| MCV (fl) | 91.0 ± 6.9* | 88.2 ± 5.7 | 88.1 ± 8.1 | 86.8 ± 9.1 |
| Biochemical tests | ||||
| AST (U/l) | 29 ± 19 | 25 ± 9 | 22 ± 7 | 21 ± 5 |
| ALT (U/l) | 36 ± 20 | 37 ± 23 | 21 ± 8 | 25 ± 9 |
| AST/ALT | 0.8 ± 0.3** | 0.7 ± 0.2 | 1.1 ± 0.4* | 0.9 ± 0.2 |
| GGT (U/l) | 58 ± 37** | 34 ± 17 | 33 ± 22* | 22 ± 9 |
| Fe (μg/dl) | 109 ± 40 | 102 ± 31 | 108 ± 45 | 92 ± 36 |
| Ferritin (ng/ml) | 183 ± 124 | 170 ± 130 | 64 ± 45 | 51 ± 47 |
| Transferrin (mg/l) | 2080 ± 490 | 2180 ± 403 | 1937 ± 516 | 2114 ± 529 |
| CDT% | 1.8 ± 0.7** | 1.3 ± 0.3 | 1.7 ± 0.7 | 1.5 ± 0.4 |
| MDA (μmol/l) | 2.2 ± 2.1* | 1.5 ± 1.1 | 1.2 ± 0.9 | 1.5 ± 0.8 |
| Immunological tests | ||||
| CD3+ cells (%) | 64.9 ± 9.7 | 62.1 ± 9.3 | 69.4 ± 7.0* | 65.3 ± 7.5 |
| CD4+ cells (%) | 35.7 ± 8.5** | 30.0 ± 7.4 | 37.2 ± 11.3 | 34.1 ± 8.3 |
| CD8+ cells (%) | 22.6 ± 6.7 | 25.0 ± 8.0 | 21.8 ± 6.2 | 23.8 ± 4.8 |
| CD19+ cells (%) | 11.2 ± 4.4 | 11.4 ± 4.1 | 11.5 ± 4.0 | 12.6 ± 4.9 |
| IgG (mg/dl) | 1088 ± 216** | 1184 ± 199 | 1265 ± 253 | 1249 ± 193 |
| IgM (mg/dl) | 82 ± 37** | 92 ± 47 | 115 ± 55 | 128 ± 55 |
| IL‐2R (U/l) | 384 ± 127 | 358 ± 110 | 340 ± 166 | 367 ± 174 |
| IL‐6 (ng/ml) | 4.2 ± 2.0** | 3.2 ± 1.9 | 3.3 ± 2.2 | 3.1 ± 1.6 |
| IL‐8 (ng/ml) | 11.2 ± 5.1 | 9.8 ± 6.9 | 7.5 ± 3.0 | 8.6 ± 4.6 |
| IL‐10 (ng/ml) | 1.9 ± 1.0 | 1.9 ± 1.4 | 2.3 ± 1.0* | 1.7 ± 1.0 |
| TNF‐α (pg/ml) | 10.3 ± 8.2 | 10.2 ± 8.5 | 7.5 ± 4.7 | 10.6 ± 9.5 |
Data were expressed as mean ± SD.
b*P < 0.05 and ** P < 0.01 compared with the nonheavy drinkers in the males.
c*P < 0.05 and ** P < 0.01 compared with the non‐heavy drinkers in the females.
CAGE, Cut Down, Annoyed, Guilty, Eye Opener; AUDIT, Alcohol Use Disorders Identification Test; BMI, body mass index; MCV, mean corpuscular volume; AST, aspartate aminotransferase; ALT, alanine aminotransferase; GGT, gamma‐glutamyl transferase; Fe, iron; CDT, carbohydrate‐deficient transferring; MDA, malondialdehyde; CD, cluster differentiation; Ig, immunoglobulin; IL, interleukin; TNF, tumor necrosis factor.
To compare the differences of the biomarkers between the two groups, we measured the hematological, biochemical, and immunological tests in all participants (Table 1). We found that the male heavy drinkers had higher levels of mean corpuscular volume (MCV) than the nonheavy drinkers. Additionally, the levels of AST/ALT, GGT, CDT%, and MDA in the males were higher than those in the nonheavy drinkers; moreover, for female heavy drinkers, the levels of AST/ALT and GGT were higher than in the nonheavy drinkers. However, in practice, the slight difference of AST/ALT ratio might be not significant in clinical interpretation even if there was a statistical difference. For the immunological markers, there were higher percentages of CD4+ cells and IL‐6 levels, but lower IgG and IgM levels in the heavy drinkers than in the nonheavy drinkers for males; however, there were higher IL‐10 levels in the heavy drinkers than in the nonheavy drinkers for females.
As shown in Table 2, there were significant differences of the MnSOD and MPO genotypes between the two groups. In this study, only three heavy drinkers and seven nonheavy drinkers had the MnSOD1183T>C CC genotype; thus, we combined 1 or 2 MnSOD1183T>C C alleles into a single group (as the MnSOD1183T>C C carrier) for the MnSOD1183T>C genotypic classification (Table 2). Because there were very few MPO‐463G>A AA genotype in our subjects, we combined MPO‐463G>AA and MPO‐463G>GA into a single group, which was termed the MPO‐463>A A carrier. In addition, none of the 214 participants was homozygous for the polymorphism of the GPX1Pro198Leu T allele and CAT‐262C>T T allele. Therefore, the participants were classified into two major groups based on the differences of SNP in each enzyme described above.
Table 2.
Frequencies of the Alleles and Genotypes of the Oxidative Stress‐Related Enzymes in the Study Population
| Heavy drinkers | Nonheavy drinkers | ||||
|---|---|---|---|---|---|
| n = 108 | n = 106 | OR | |||
| Genetic polymorphisms | n (%) | n (%) | (95% CI) | P‐value | |
| MnSOD1183T>C | |||||
| Allele | T (lower activity) | 193 (89.4) | 172 (81.1) | 1 | 0.160 |
| C (higher activity) | 23 (10.6) | 40 (18.9) | 0.51 | ||
| (0.28–0.92) | |||||
| Genotype | T/T | 88 (81.5) | 73 (68.9) | 1 | 0.033 |
| T/C + C/C | 20 (18.5) | 33 (31.1) | 0.50 | ||
| (0.27–0.95) | |||||
| GPX1Pro198Leu | |||||
| Allele | C (higher activity) | 204 (94.4) | 197 (92.9) | 1 | 0.518 |
| T (lower activity) | 12 (5.6) | 15 (7.1) | 0.77 | ||
| (0.33–1.80) | |||||
| Genotype | C/C | 96 (88.9) | 91 (85.8) | 1 | 0.503 |
| C/T | 12 (11.1) | 15 (14.2) | 0.76 | ||
| (0.34–1.71) | |||||
| CAT‐262C>T | |||||
| Allele | C (higher activity) | 208 (96.3) | 205 (96.7) | 1 | 0.821 |
| T (lower activity) | 8 (3.7) | 7 (3.3) | 1.13 | ||
| (0.36–3.52) | |||||
| Genotype | C/C | 100 (92.6) | 99 (93.4) | 1 | 0.818 |
| C/T | 8 (7.4) | 7 (6.6) | 1.13 | ||
| (0.40–3.24) | |||||
| MPO‐463G>A | |||||
| Allele | G (higher activity) | 163 (75.5) | 174 (82.1) | 1 | 0.095 |
| A (lower activity) | 53 (24.5) | 38 (17.9) | 1.49 | ||
| (0.91–2.44) | |||||
| Genotype | G/G | 55 (50.9) | 71 (67.0) | 1 | 0.017 |
| G/A + A/A | 53 (49.1) | 35 (33.0) | 1.96 | ||
| (1.12–3.40) | |||||
OR, odd ratios; CI, confidence interval; MnSOD, manganese superoxide dismutase; GPX, glutathione peroxidase; CAT, catalase; MPO, myeloperoxidase.
There were a lot fewer female heavy drinkers than male heavy drinkers in this study; thus, we selected the biomarkers, which were not affected by gender, to investigate whether they were significantly different between the subjects with different genotypes of oxidative stress‐related enzymes as shown in Tables 3, 4, 5.
Table 3.
Comparisons of Biomarkers in the Subjects With Different MnSOD1183T>C Genotypes
| MnSOD1183T>C | ||||
|---|---|---|---|---|
| Heavy drinkersa | Nonheavy drinkersb | |||
| TT | TC + CC | TT | TC + CC | |
| Parameters | (n = 88) | (n = 20) | (n = 73) | (n = 33) |
| Hematological test | ||||
| MCV (fl) | 90.5 ± 7.6 | 90.1 ± 5.5 | 87.4 ± 7.3 | 87.5 ± 8.4 |
| Biochemical tests | ||||
| AST (U/l) | 28 ± 19 | 25 ± 7 | 23 ± 8 | 21 ± 6 |
| ALT (U/l) | 33 ± 19 | 32 ± 16 | 31 ± 20 | 29 ± 14 |
| AST/ALT | 0.9 ± 0.3 | 0.9 ± 0.3 | 0.8 ± 0.2 | 0.8 ± 0.2 |
| GGT (U/l) | 52 ± 36 | 54 ± 33 | 28 ± 13 | 27 ± 18 |
| CDT (%) | 1.7 ± 0.6 | 1.9 ± 1.0 | 1.4 ± 0.3 | 1.4 ± 0.5 |
| MDA (μmol/l) | 1.8 ± 1.5 | 1.6 ± 1.4 | 1.5 ± 1.0 | 1.4 ± 0.9 |
| Immunological tests | ||||
| CD3+ cells (%) | 65.8 ± 9.6 | 66.3 ± 8.5 | 62.8 ± 7.8 | 65.7 ± 9.8 |
| CD4+ cells (%) | 36.5 ± 9.3 | 34.3 ± 8.3 | 32.6 ± 7.9 | 30.4 ± 8.5 |
| CD8+ cells (%) | 22.7 ± 6.8 | 21.2 ± 5.2 | 23.4 ± 5.7 | 26.5 ± 7.8* |
| CD19+ cells (%) | 10.8 ± 4.2 | 13.2 ± 4.5* | 12.4 ± 4.5 | 11.1 ± 4.5 |
| IgG (mg/dl) | 1126 ± 227 | 1132 ± 274 | 1209 ± 272 | 1233 ± 202 |
| IgM (mg/dl) | 90 ± 45 | 88 ± 39 | 100 ± 42 | 132 ± 71 |
| IL‐2R (U/l) | 365 ± 129 | 412 ± 166 | 372 ± 157 | 341 ± 114 |
| IL‐6 (ng/ml) | 3.9 ± 2.1 | 4.6 ± 1.9 | 3.5 ± 2.1 | 2.9 ± 1.5 |
| IL‐8 (ng/ml) | 10.4 ± 4.9 | 10.3 ± 5.2 | 9.8 ± 6.6 | 7.9 ± 3.5 |
| IL‐10 (ng/ml) | 1.9 ± 1.1 | 2.1 ± 0.8 | 1.9 ± 1.3 | 1.5 ± 1.0 |
| TNF‐α (pg/ml) | 10.2 ± 8.4 | 7.5 ± 2.3* | 10.6 ± 9.0 | 10.1 ± 9.0 |
a*P <0.05 compared with the subjects with the TT genotypes in the heavy drinkers.
b*P <0.05 compared with the subjects with the TT genotypes in the nonheavy drinkers.
MCV, mean corpuscular volume; AST, aspartate aminotransferase; ALT, alanine aminotransferase; GGT, gamma‐glutamyl transferase; carbohydrate‐deficient transferring; MDA, malondialdehyde; CD, cluster differentiation; Ig, immunoglobulin; IL, interleukin; TNF, tumor necrosis factor.
Table 4.
Comparisons of the Biomarkers in the Subjects With Different Combined Genotypes of MnSOD1183T>C and MPO‐463G>A
| Heavy drinkers | Nonheavy drinkers | |||||||
|---|---|---|---|---|---|---|---|---|
| MnSOD TT | MnSOD TC + CC | MnSOD TT | MnSOD TC + CC | |||||
| MPO | MPO | MPO | MPO | MPO | MPO | MPO | MPO | |
| GG [A] | GA + AA [B] | GG [C] | GA + AA [D] | GG [A] | GA + AA [B] | GG [C] | GA + AA [D] | |
| Parameters | (n = 45) | (n = 43) | (n = 10) | (n = 10) | (n = 50) | (n = 23) | (n = 21) | (n = 12) |
| Immunological tests | ||||||||
| CD3+ cells (%) | 65.7 ± 11.0 | 65.9 ± 7.9 | 65.3 ± 8.4 | 67.3 ± 8.9 | 63.2 ± 7.3 | 62.3 ± 9.4 | 66.4 ± 9.1 | 64.2 ± 10.9 |
| CD4+ cells (%) | 35.7 ± 9.6 | 37.2 ± 9.0 | 30.3 ± 5.7 | 38.3 ± 8.8 | 32.4 ± 8.1 | 33.0 ± 7.6 | 28.4 ± 7.9 | 33.8 ± 8.7 |
| CD8+ cells (%) | 23.0 ± 7.0 | 22.5 ± 6.8 | 21.7 ± 5.0 | 20.7 ± 5.7 | 23.6 ± 6.0 | 23.1 ± 5.1 | 27.0 ± 8.8 | 25.5 ± 6.0 |
| CD19+ cells (%) | 10.5 ± 3.7 | 11.1 ± 4.6 | 10.5 ± 3.8 | 16.0 ± 3.4a , b , c | 12.2 ± 4.8 | 12.8 ± 3.9 | 11.9 ± 4.6 | 9.8 ± 4.2 |
| IgG (mg/dl) | 1118 ± 200 | 1134 ± 255 | 1195 ± 293 | 1071 ± 253 | 1194 ± 248 | 1243 ± 322 | 1234 ± 213 | 1232 ± 191 |
| IgM (mg/dl) | 82 ± 37 | 98 ± 52 | 92 ± 44 | 84 ± 35 | 95 ± 40 | 111 ± 45 | 156 ± 73a , b , d | 90 ± 45 |
| IL‐2R (U/l) | 359 ± 118 | 371 ± 140 | 382 ± 188 | 443 ± 145 | 361 ± 167 | 392 ± 138 | 338 ± 120 | 357 ± 100 |
| IL‐6 (ng/ml) | 3.9 ± 2.0 | 3.8 ± 2.1 | 3.4 ± 1.1 | 5.8 ± 1.9a , b , c | 3.4 ± 2.2 | 3.6 ± 2.0 | 2.9 ± 1.6 | 2.9 ± 1.4 |
| IL‐8 (ng/ml) | 9.8 ± 4.7 | 11.0 ± 5.1 | 8.6 ± 2.8 | 11.9 ± 6.5 | 9.2 ± 5.2 | 9.7 ± 7.3 | 8.8 ± 7.0 | 8.9 ± 3.3 |
| IL‐10 (ng/ml) | 2.0 ± 1.1 | 1.9 ± 1.0 | 2.3 ± 1.1 | 1.8 ± 0.4 | 1.8 ± 1.3 | 2.1 ± 1.2 | 1.3 ± 0.7 | 1.8 ± 1.4 |
| TNF‐α (pg/ml) | 10.9 ± 9.8 | 9.5 ± 6.6 | 6.8 ± 1.8 | 8.2 ± 2.6 | 10.0 ± 9.1 | 11.7 ± 9.0 | 11.3 ± 11.0 | 8.1 ± 2.8 |
The alphabet in the square bracket indicates the group of the study.
P <0.05, compared with Group A in the heavy drinkers or nonheavy drinkers, respectively.
P <0.05, compared with Group B in the heavy drinkers or nonheavy drinkers, respectively.
P <0.05, compared with Group C in the heavy drinkers.
P <0.05, compared with Group D in the nonheavy drinkers.
CD, cluster differentiation; Ig, immunoglobulin; IL, interleukin; TNF, tumor necrosis factor.
Table 5.
Comparisons of the Biomarkers in the Subjects With Different Combined Genotypes of MnSOD1183T>C and PX1Pro198Leu
| Heavy drinkers | Nonheavy drinkers | |||||||
|---|---|---|---|---|---|---|---|---|
| MnSOD TT | MnSOD TC + CC | MnSOD TT | MnSOD TC + CC | |||||
| GPX1 | GPX1 | GPX1 | GPX1 | GPX1 | GPX1 | GPX1 | GPX1 | |
| CC [A] | CT [B] | CC [C] | CT [D] | CC [A] | CT [B] | CC [C] | CT [D] | |
| Parameters | (n = 81) | (n = 7) | (n = 15) | (n = 5) | (n = 66) | (n = 8) | (n = 25) | (n = 7) |
| Immunological tests | ||||||||
| CD3+ cells (%) | 65.6 ± 9.7 | 68.5 ± 7.4 | 65.4 ± 9.6 | 69.0 ± 1.8 | 62.5 ± 7.7 | 65.5 ± 8.0 | 66.4 ± 8.6 | 63.4 ± 15.0 |
| CD4+ cells (%) | 36.2 ± 9.4 | 39.7 ± 7.7 | 32.6 ± 7.9 | 39.3 ± 8.2 | 32.5 ± 7.9 | 32.8 ± 7.6 | 30.6 ± 8.1 | 30.0 ± 11.0 |
| CD8+ cells (%) | 22.8 ± 7.0 | 22.1 ± 4.5 | 21.1 ± 5.1 | 21.4 ± 6.3 | 23.2 ± 5.6 | 23.5 ± 5.4 | 27.3 ± 8.1 | 26.0 ± 7.7 |
| CD19+ cells (%) | 10.8 ± 4.2 | 10.9 ± 4.2 | 12.1 ± 4.1 | 16.8 ± 4.2a | 12.3 ± 4.7 | 12.1 ± 2.3 | 11.7 ± 4.8 | 9.8 ± 3.3 |
| IgG (mg/dl) | 1131 ± 216 | 1020 ± 343 | 1089 ± 228 | 1262 ± 381 | 1234 ± 274 | 1019 ± 106 | 1240 ± 209 | 1194 ± 223 |
| IgM (mg/dl) | 89 ± 44 | 106 ± 53 | 79 ± 30 | 116 ± 53 | 97 ± 41 | 118 ± 54 | 134 ± 57a | 142 ± 105 |
| IL‐2R (U/l) | 365 ± 131 | 361 ± 115 | 383 ± 159 | 500 ± 172 | 380 ± 161 | 337 ± 131 | 334 ± 112 | 332 ± 108 |
| IL‐6 (ng/ml) | 3.9 ± 2.1 | 3.5 ± 1.8 | 4.4 ± 1.9 | 5.3 ± 2.1 | 3.4 ± 2.0 | 4.2 ± 2.8 | 2.7 ± 1.2 | 3.0 ± 2.0 |
| IL‐8 (ng/ml) | 10.5 ± 5.0 | 9.7 ± 3.8 | 10.3 ± 5.9 | 10.2 ± 2.8 | 9.4 ± 5.3 | 9.6 ± 9.4 | 7.6 ± 3.1 | 12.5 ± 11.2 |
| IL‐10 (ng/ml) | 1.9 ± 1.1 | 1.9 ± 0.7 | 2.0 ± 0.7 | 2.1 ± 1.3 | 1.9 ± 1.3 | 1.7 ± 1.1 | 1.6 ± 1.0 | 1.4 ± 1.1 |
| TNF‐α (pg/ml) | 10.4 ± 8.7 | 7.9 ± 2.6 | 6.9 ± 2.1 | 9.3 ± 2.0 | 10.4 ± 9.2 | 10.3 ± 5.8 | 8.4 ± 4.4 | 18.1 ± 16.8 |
The alphabet in the square bracket indicates the group of the study.
P <0.05, compared with Group A in the heavy drinkers or nonheavy drinkers, respectively.
CD, cluster differentiation; Ig, immunoglobulin; IL, interleukin; TNF, tumor necrosis factor.
As shown in Table 3, heavy drinkers with the MnSOD C carrier had higher proportions of CD19+ cells, but lower levels of TNF‐α and nonheavy drinkers with the MnSOD C carrier had higher proportions of CD8+ cells than those with the MnSOD TT genotype. However, there were no significant differences of the biomarkers in the subjects with the different GPX1, CAT, and MPO genotypes (data not shown) mentioned above. Based on these results, we further combined the genotypes of MnSOD with either of the other oxidative stress‐related enzymes to compare their biomarkers among the different genotype combinations. We found that there were significant differences in their biomarkers in the combined genotypes of the MnSOD‐MPO pathway (Table 4) and MnSOD‐GPX1 pathway (Table 5), but not in the combined genotypes of MnSOD and CAT. Table 4 shows that the highest percentage of CD19+ cells and IL‐6 values were in the subjects with the combined genotypes of the MnSOD C carrier (higher activity) and MPO A carrier (lower activity) among the four combined groups in the heavy drinkers. Additionally, the level of IgM was highest in the subjects with the MnSOD C carrier and MPO GG (higher activity) among the four combined groups in the nonheavy drinkers. For the comparisons of the biomarkers in the MnSOD‐GPX1 pathway, Table 5 shows that there were higher percentages of CD19+ cells in the heavy drinkers with the combined genotypes of the MnSOD C carrier and GPX1 CT (lower activity) than those with the combined genotypes of MnSOD TT and GPX1 CC (higher activity). Besides, the level of IgM in the nonheavy drinkers with the combined genotypes of the MnSOD C carrier and GPX1CC was higher than that in the subjects with the combined genotypes of MnSOD TT and GPX1CC.
DISCUSSION
To our knowledge, this is the first report that describes the relationships between the biomarkers and the different combined genotypes of oxidative stress‐related enzymes. In this study, we demonstrated that the effects of MnSOD polymorphisms on the variations of biomarkers might be more crucial than those from the other oxidative stress‐related enzymes in heavy drinkers in Taiwan. In addition, it was shown that the immunological biomarkers might be more suitable for the evaluation of oxidative stress in subjects with excessive alcohol intake than the other biomarkers. In other words, by analyzing their MnSOD genotypes and immunological biomarkers, heavy drinkers may be cautioned about the predictive likelihood for them to induce drinking‐induced diseases.
As shown in Table 1, the scores of CAGE and AUDIT in the males were higher than those in the females. These results showed that the amount of alcohol intake in the males should be more than that in the females, which might easily lead to abnormalities in most of the biomarkers in Taiwan. In addition, the biomarkers, such as CDT%, MDA, CD4+, IgG, IgM, and IL‐6 were significantly different between the heavy drinkers and nonheavy drinkers in the male subjects but not in the female subjects. These results indicated excessive alcohol consumption could increase an individual's oxidative stress and adversely affect many tissues, such as the liver and spleen, as well as the hematopoietic and immunological systems 24.
Furthermore, there was a higher level of ferritin in the males than in the females. These results were consistent with previous studies that reported excessive alcohol intake was associated with increased iron stores as assessed by serum ferritin concentration 15, 16. With the above‐mentioned analyses, the results of many biomarkers, such as Hgb and ferritin, were different between males and females 25; thus, the gender issue must be considered when comparing biomarkers in subjects with excessive alcohol intake. Although studies on biomarkers for the detection of excessive alcohol intake have been performed in many countries 14, the present study uniquely evaluated the differences of the biomarkers in heavy drinkers with different genotype polymorphisms of oxidative stress‐related enzymes.
Many previous studies have indicated that the frequencies of alleles and genotypes of oxidative stress‐related enzymes vary across different populations (Table 6; 3, 23, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38). In this study, the distributions of MnSOD, GPX1, and CAT alleles are similar to those of the Asian population, but not in the distribution of MPO alleles (Table 6). Our previous studies showed that faster elimination of acetaldehyde by higher activity of aldehyde dehydrogenase 2 (ALDH2) *1/*1 led to overdrinking, which made a person become a heavy drinker 10; however, whether the genetic variations of the oxidative stress‐related enzymes are associated with the drinking habit is worth being elucidated. In the present study, there were lower activities of MnSOD in the heavy drinkers; however, most biomarkers were not remarkably affected by the genetic variation of MnSOD except the percentages of CD19+ cells and the TNF‐α level. Therefore, the mechanisms that the alterations of most biomarkers were affected by the toxicity of H2O2 from the MnSOD or accumulation of superoxide anion from the lower active activity of MnSOD need to be further investigated. Furthermore, the subjects with higher activity genotypes of MnSOD (MnSOD C carriers) had a lower level of TNF‐α in the heavy drinkers, and this result was similar to a previous study that indicated that SOD could restore TNF‐α‐induced oxidative stress 39. On the other hand, the biomarkers were not significantly different in the subjects with different genotypes of MPO, CAT, and GPX1 in this study (data not shown).
Table 6.
Frequencies of the Genotypes and Alleles of Oxidative Stress‐Related Enzymes Among Different Ethic Groups
| MnSOD1183T>C | |||||||
|---|---|---|---|---|---|---|---|
| Genotype | Allele | ||||||
| Population | N | TT | TC | CC | T | C | References |
| Chinese | 1197 | 0.739 | 0.242 | 0.019 | 0.860 | 0.140 | 27 |
| Japanese | 291 | 0.728 | 0.251 | 0.015 | 0.856 | 0.143 | 36 |
| Finnish | 482 | 0.317 | 0.479 | 0.203 | 0.557 | 0.443 | 35 |
| German | 1080 | 0.244 | 0.489 | 0.268 | 0.488 | 0.512 | 37 |
| Swedish | 174 | 0.247 | 0.506 | 0.247 | 0.500 | 0.500 | 26 |
| GPX1Pro198Leu | |||||||
| Genotype | Allele | ||||||
| Population | N | CC | CT | TT | C | T | References |
| Chinese | 265 | 0.838 | 0.162 | 0 | 0.919 | 0.081 | 38 |
| Japanese | 2183 | 0.846 | 0.151 | 0.003 | 0.921 | 0.086 | 31 |
| Russian | 250 | 0.668 | 0.308 | 0.024 | 0.822 | 0.178 | 28 |
| Danish | 377 | 0.544 | 0.360 | 0.095 | 0.724 | 0.276 | 3 |
| CAT‐262C>T | |||||||
| Genotype | Allele | ||||||
| Population | N | CC | CT | TT | C | T | References |
| Chinese | 557 | 0.926 | 0.074 | 0 | 0.962 | 0.038 | 33 |
| (Hong Kong) | |||||||
| Korean | 400 | 0.935 | 0.063 | 0.2 | 0.941 | 0.059 | 29 |
| British | 130 | 0.569 | 0.377 | 0.233 | 0.569 | 0.431 | 30 |
| MPO‐463G>A | |||||||
| Genotype | Allele | ||||||
| Population | N | GG | GA | AA | G | A | References |
| Chinese | 320 | 0.709 | 0.272 | 0.019 | 0.845 | 0.155 | 32 |
| Japanese | 163 | 0.706 | 0.251 | 0.043 | 0.831 | 0.169 | 34 |
| Caucasians | 171 | 0.573 | 0.339 | 0.088 | 0.743 | 0.257 | 34 |
| German | 270 | 0.611 | 0.348 | 0.041 | 0.785 | 0.215 | 23 |
Our previous study indicated that there were more abnormal immunological tests in the subjects with combined genotypes of alcohol dehydrogenase 2 (ADH2) *2/*2 (higher activity) and ALDH2 (*1/*2+*2/*2; lower activity; 10). Thus, we suspected that the biomarkers might be affected by the genetic variations of oxidative stress‐related enzymes during alcohol intake. In this study, although MnSOD can reduce oxidative stress, H2O2 derived from superoxide anion by MnSOD needs to be catalyzed to nontoxic molecules by CAT or GPX. The study by Sutton et al. indicated that there were higher risks of hepatocellular carcinoma development in patients with a higher active MnSOD allele than in those with a lower active allele 40. Furthermore, Ambrosone et al. showed that women with high‐activity for MPO, CAT, and MnSOD genotypes associated with higher levels of ROS had better breast cancer survival after treatment than those with alleles that offered better protection from oxidative stress 4. It was indicated in the study of Tohyama et al. 41 that higher oxidative stresses could result in enhanced apoptosis in B lymphocytes; therefore, there were lower percentages of B lymphocytes. In our study, there were higher percentages of CD19+ cells in the genotypes of MnSOD C carriers regardless of the downstream enzyme genotypes. Conclusively, these results indicated that the role of the MnSOD C carrier leading to comparatively higher percentages of CD19+ cells might be more important than those of the downstream enzymes in the oxidative stress‐related metabolic pathways.
Pan et al. 42 showed that the role of H2O2 was a double‐edged sword in oncogenesis; moreover, Frossi et al. also indicated that H2O2 might upregulate the gene expression of IL‐4 and IL‐6 in mast cells 43. In this study, on the basis of Table 2 and Figure 1, we deduced that there were higher activities of MnSOD in the heavy drinkers with MnSOD C carriers; thus, the superoxide anion derived from alcohol metabolism could be quickly dismutased to hydrogen peroxide (H2O2) by MnSOD in the people with MnSOD C carriers. On the other hand, there were lower activities of MPO in the heavy drinkers with MPO A carriers; thus, the cumulative H2O2 were slowly converted to HOCl by MPO in the people with MPO A carriers. Therefore, there were higher levels of IL‐6 in the heavy drinkers with the combined genotypes of MnSOD C carriers and MPO A carriers who had a higher H2O2 level and a lower HOCl level, which indicated there was more accumulation of H2O2 in the blood stream, leading to higher levels of IL‐6. Which one is the most important on the impact of biomarkers: H2O2 generation, alcohol intake, or both? This is a topic that needs to be further investigated. With regard to the above‐mentioned results, we conclude that there were more remarkable differences in the immunological biomarkers than those of hematology and biochemistry in the different combined genotypes of MnSOD and MPO; thus, immunological biomarkers might be more sensitive than the other biomarkers in the evaluation of oxidative stress. However, in this study, the assessment of combined MnSOD‐MPO genotypes is data driven and basically a post hoc analysis that is not based on prior hypothesis. Furthermore, the sample size for the assessment of gene–environment (alcohol intake) and gene–gene interactions in the polymorphisms of oxidative stress‐related enzymes was insufficient, especially in the cases of the female heavy drinkers. However, it is a known fact that there were always more male drinkers than female ones in Taiwan; thus, there were bound to be more heavy male drinkers than female ones in this study.
Taken together, our studies indicated the impact of the risk factors, such as excessive alcohol consumption, genetic variations of alcohol‐metabolizing enzymes, and oxidative stress‐related enzymes, and alcohol‐induced oxidative stress, could result in abnormal alterations of many biomarkers. Furthermore, the present study also indicated that the effects of the MnSOD polymorphisms on the variations of biomarkers were more crucial than the other oxidative stress‐related enzymes in the heavy drinkers in Taiwan. However, the detailed mechanism regarding the relationships between oxidative stress and the alteration of biomarkers in heavy drinkers needs to be further investigated.
Grant sponsor: Kaohsiung Veterans General Hospital Research Program; Grant number: VGHKS95‐53; Grant sponsor: National Science Council, Taiwan; Grant number: NSC 95‐2314‐B‐037‐028.
REFERENCES
- 1. Ho JC, Mak JC, Ho SP, et al. Manganese superoxide dismutase and catalase genetic polymorphisms, activity levels, and lung cancer risk in Chinese in Hong Kong. J Thorac Oncol 2006;1:648–653. [PubMed] [Google Scholar]
- 2. Shimoda‐Matsubayashi S, Matsumine H, Kobayashi T, Nakagawa‐Hattori Y, Shimizu Y, Mizuno Y. Structural dimorphism in the mitochondrial targeting sequence in the human manganese superoxide dismutase gene. A predictive evidence for conformational change to influence mitochondrial transport and a study of allelic association in Parkinson's disease. Biochem Biophys Res Commun 1996;226:561–565. [DOI] [PubMed] [Google Scholar]
- 3. Ravn‐Haren G, Olsen A, Tjonneland A, et al. Associations between GPX1 Pro198Leu polymorphism, erythrocyte GPX activity, alcohol consumption and breast cancer risk in a prospective cohort study. Carcinogenesis 2006;27:820–825. [DOI] [PubMed] [Google Scholar]
- 4. Ambrosone CB, Ahn J, Singh KK, et al. Polymorphisms in genes related to oxidative stress (MPO, MnSOD, CAT) and survival after treatment for breast cancer. Cancer Res 2005;65:1105–1111. [PubMed] [Google Scholar]
- 5. Piedrafita FJ, Molander RB, Vansant G, Orlova EA, Pfahl M, Reynolds WF. An Alu element in the myeloperoxidase promoter contains a composite SP1‐thyroid hormone‐retinoic acid response element. J Biol Chem 1996;271:14412–14420. [DOI] [PubMed] [Google Scholar]
- 6. Rudolph V, Rudolph TK, Kubala L, et al. A myeloperoxidase promoter polymorphism is independently associated with mortality in patients with impaired left ventricular function. Free Radic Biol Med 2009;47:1584–1590. [DOI] [PubMed] [Google Scholar]
- 7. Hashimoto Y, Nakayama T, Futamura A, Omura M, Nakarai H, Nakahara K. Relationship between genetic polymorphisms of alcohol‐metabolizing enzymes and changes in risk factors for coronary heart disease associated with alcohol consumption. Clin Chem 2002;48:1043–1048. [PubMed] [Google Scholar]
- 8. Kim MS, Lee DH, Kang HS, et al. Genetic polymorphisms of alcohol‐metabolizing enzymes and cytokines in patients with alcohol induced pancreatitis and alcoholic liver cirrhosis. Korean J Gastroenterol 2004;43:355–363. [PubMed] [Google Scholar]
- 9. Mantena SK, King AL, Andringa KK, Landar A, Darley‐Usmar V, Bailey SM. Novel interactions of mitochondria and reactive oxygen/nitrogen species in alcohol mediated liver disease. World J Gastroenterol 2007;13:4967–4973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tseng YM, Tsai SM, Chen SY, et al. Roles of the genetic polymorphisms of alcohol‐metabolizing enzymes on the immunology in high‐risk drinkers. Toxicol Sci 2009;111:267–276. [DOI] [PubMed] [Google Scholar]
- 11. Wu D, Cederbaum AI. Alcohol, oxidative stress, and free radical damage. Alcohol Res Health 2003;27:277–284. [PMC free article] [PubMed] [Google Scholar]
- 12. Tseng YM, Hu BW, Tsai SM, et al.Distribution of alcohol‐metabolizing enzyme genotypes in trauma patients with excessive alcohol consumption in the emergency department. Clin Biochem 2007;40:370–376. [DOI] [PubMed] [Google Scholar]
- 13. Tseng YM, Jin YR, Chen IJ, et al. Roles of the genetic variation of alcohol‐metabolizing enzymes on biomarkers in trauma patients with excessive alcohol intake at emergency department. Clin Chim Acta 2008;389:14–18. [DOI] [PubMed] [Google Scholar]
- 14. Conigrave KM, Degenhardt LJ, Whitfield JB, Saunders JB, Helander A, Tabakoff B. CDT, GGT, and AST as markers of alcohol use: The WHO/ISBRA collaborative project. Alcohol Clin Exp Res 2002;26:332–339. [PubMed] [Google Scholar]
- 15. Gordeuk VR, Diaz SF, Onojobi GO, et al. Ferroportin Q248h, dietary iron, and serum ferritin in community African‐Americans with low to high alcohol consumption. Alcohol Clin Exp Res 2008;32:1947–1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liu JM, Hankinson SE, Stampfer MJ, Rifai N, Willett WC, Ma J. Body iron stores and their determinants in healthy postmenopausal US women. Am J Clin Nutr 2003;78:1160–1167. [DOI] [PubMed] [Google Scholar]
- 17. Mundle G, Munkes J, Ackermann K, Mann K. Sex differences of carbohydrate‐deficient transferrin, gamma‐glutamyltransferase, and mean corpuscular volume in alcohol‐dependent patients. Alcohol Clin Exp Res 2000;24:1400–1405. [PubMed] [Google Scholar]
- 18. Reynaud M, Schellenberg F, Loisequx‐Meunier MN, et al. Objective diagnosis of alcohol abuse: Compared values of carbohydrate‐deficient transferrin (CDT), gamma‐glutamyl transferase (GGT), and mean corpuscular volume (MCV). Alcohol Clin Exp Res 2000;24:1414–1419. [PubMed] [Google Scholar]
- 19. Albano E. Alcohol, oxidative stress and free radical damage. Proc Nutr Soc 2006;65:278–290. [DOI] [PubMed] [Google Scholar]
- 20. WHO International guide for monitoring alcohol consumption and related harm. World Health Organization; 2000. [Google Scholar]
- 21. NHRI (National Health Research Institute), Taiwan . Epidemiology of alcohol‐drinking. 2004;54–55.
- 22. Gul S, Akvardar Y, Tas G, Tuncel P. The diagnostic validity of screening tests and laboratory markers in alcohol use disorders. Turk Psikiyatri Derg 2005;16:3–12. [PubMed] [Google Scholar]
- 23. Cascorbi I, Henning S, Brockmoller J, et al. Substantially reduced risk of cancer of the aerodigestive tract in subjects with variant–463A of the myeloperoxidase gene. Cancer Res 2000;60:644–649. [PubMed] [Google Scholar]
- 24. Tseng YM, Tsai SM, Lin WS, et al. Effects of whey protein concentrate (WPC) on the distributions of lymphocyte subpopulations in rats with excessive alcohol intake. J Agric Food Chem 2010;58:12729–12734. [DOI] [PubMed] [Google Scholar]
- 25. Burtis CA, Ashwood ER, Bruns DE. Tietz Fundamentals of Clinical Chemistry W.B. Philadelphia, PA: Saunders Company; 2001. [Google Scholar]
- 26. Bergman M, Ahnstrom M, Palmeback Wegman, P , Wingren S. Polymorphism in the manganese superoxide dismutase (MnSOD) gene and risk of breast cancer in young women. J Cancer Res Clin Oncol 2005;131:439–444. [DOI] [PubMed] [Google Scholar]
- 27. Cai Q, Shu XO, Wen W, et al. Genetic polymorphism in the manganese superoxide dismutase gene, antioxidant intake, and breast cancer risk: Results from the Shanghai Breast Cancer Study. Breast Cancer Res 2004;6:R647–R655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chistiakov DA, Zotova EV, Savost'anov KV, et al. The 262T>C promoter polymorphism of the catalase gene is associated with diabetic neuropathy in type 1 diabetic Russian patients. Diabetes Metab 2006;32:63–68. [DOI] [PubMed] [Google Scholar]
- 29. El‐Sohemy A, Cornelis MC, Park YW, Bae SC. Catalase and PPARgamma2 genotype and risk of rheumatoid arthritis in Koreans. Rheumatol Int 2006;26:388–392. [DOI] [PubMed] [Google Scholar]
- 30. Goulasa A, Fidanib I, Kotsisb A, et al. An association study of a functional catalase gene polymorphism, ‐262C>T, and patients with Alzheimer's disease. Neurosci Lett 2002;330:210–212. [DOI] [PubMed] [Google Scholar]
- 31. Kuzuya M, Ando F, Iguchi A, Shimokata H. Glutathione peroxidase 1 Pro198Leu variant contributes to the metabolic syndrome in men in a large Japanese cohort. Am J Clin Nutr 2008;87: 1939–1944. [DOI] [PubMed] [Google Scholar]
- 32. Lu W, Xing D, Qi J, Tan W, Miao X, Lin D. Genetic polymorphism in myeloperoxidase but not GSTM1 is associated with risk of lung squamous cell carcinoma in a Chinese population. Int J Cancer 2002;102:275–279. [DOI] [PubMed] [Google Scholar]
- 33. Mak JC, Leung HC, Ho SP, et al. Polymorphisms in manganese superoxide dismutase and catalase genes: Functional study in Hong Kong Chinese asthma patients. Clin Exp Allergy 2006;36:440–447. [DOI] [PubMed] [Google Scholar]
- 34. Marchand L, Seifried A, Lum A, Wilkens LR. Association of the myeloperoxidase ‐463G–>a polymorphism with lung cancer risk. Cancer Epidemiol Biomarkers Prev 2000;9:181–184. [PubMed] [Google Scholar]
- 35. Mitrunen K, Sillanpaa P, Kataja V, et al. Association between manganese superoxide dismutase (MnSOD) gene polymorphism and breast cancer risk. Carcinogenesis 2001;22:827–829. [DOI] [PubMed] [Google Scholar]
- 36. Nomiyama T, Tanaka Y, Piao L, et al. The polymorphism of manganese superoxide dismutase is associated with diabetic nephropathy in Japanese type 2 diabetic patients. J Hum Genet 2003;48:138–141. [DOI] [PubMed] [Google Scholar]
- 37. Slanger TE, Chang‐Claude J, Wang‐Gohrke S. Manganese superoxide dismutase Ala‐9Val polymorphism, environmental modifiers, and risk of breast cancer in a German population. Cancer Causes Control 2006;17:1025–1031. [DOI] [PubMed] [Google Scholar]
- 38. Tang NP, Wang LS, Yang L, et al. Genetic variant in glutathione peroxidase 1 gene is associated with an increased risk of coronary artery disease in a Chinese population. Clin Chim Acta 2008;395:89–93. [DOI] [PubMed] [Google Scholar]
- 39. Mariappan N, Soorappan RN, Haque M, Sriramula S, Francis J. TNF‐alpha‐induced mitochondrial oxidative stress and cardiac dysfunction: Restoration by superoxide dismutase mimetic Tempol. Am J Physiol Heart Circ Physiol 2007;293:H2726–2737. [DOI] [PubMed] [Google Scholar]
- 40. Sutton A, Nahon P, Pessayre D, et al. Genetic polymorphisms in antioxidant enzymes modulate hepatic iron accumulation and hepatocellular carcinoma development in patients with alcohol‐induced cirrhosis. Cancer Res 2006;66:2844–2852. [DOI] [PubMed] [Google Scholar]
- 41. Tohyama Y, Takano T, Yamamura H. B cell responses to oxidative stress. Curr Pharm Des 2004;10:835–839. [DOI] [PubMed] [Google Scholar]
- 42. Pan JS, Hong MZ, Ren JL. Reactive oxygen species: A double‐edged sword in oncogenesis. World J Gastroenterol 2009;15:1702–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Frossi B, De Carli M, Daniel KC, Rivera J, Pucillo C. Oxidative stress stimulates IL‐4 and IL‐6 production in mast cells by an APE/Ref‐1‐dependent pathway. Eur J Immunol 2003;33:2168–2177. [DOI] [PubMed] [Google Scholar]


