Abstract
Background
Microalbuminuria tests are used as screening tools for diseases, such as diabetic nephropathy, cardiovascular disease, and hypertension. The purpose of this study was to evaluate the utility of a newly introduced semiquantitative urine dipstick, URiSCAN Super cassette ACR (URiSuper‐ACR [where ACR is albumin/creatinine ratio]; YD Diagnostics Corp., Korea), as a screening tool for microalbuminuria.
Methods
Albumin and creatinine levels in randomly selected spot urine samples of 1,040 patients were semiquantitatively measured using URiSuper‐ACR. Results using URiSuper‐ACR system were compared to measurements obtained by quantitative analyzer. We also calculated diagnostic sensitivity, specificity, precision, linearity, and categorical concordance rates for the diagnosis of microalbumiuria using this system. Furthermore, we performed interference tests using standard controls to evaluate possible influence of various factors.
Results
URiSuper‐ACR test showed 88.8% and 86.3% sensitivity and 90.1% and 93.8% specificity for albumin concentration and ACR, respectively. It also showed 91.1% and 92.6% positive predictive values and 87.6% and 88.5% negative predictive values, respectively. The concordance rate between URiSuper‐ACR and quantitative method for albumin and creatinine concentration within the same category were 78.4% and 67.1%, respectively, and for the ±1 category were 98.8% and 99.5%, respectively. For ACR, an 80.0% concordance rate was seen within the same category. The within‐run coefficients of variation (CVs) were 3.0∼15.4% and 5.2∼23.5% for albumin and creatinine, respectively, showing good linearity. In interference tests, no interference was observed except for cases with high specific gravity.
Conclusions
URiSuper‐ACR showed good diagnostic performance for the detection of microalbuminuria and may be a useful screening test in clinical laboratories.
Keywords: microalbuminuria, albuminuria, screening test, urine dipstick, semiquantitative
Abbreviations
- ACR
albumin/creatinine ratio
- URiSuper‐ACR
URiSCAN Super cassette ACR
- SG
specific gravity
INTRODUCTION
In cases of renal failure, urine microalbuminuria is a very valuable marker for the diagnosis of incipient nephropathy 1. The urine microalbumin test is used as a screening test for the diagnosis of renal complications in diseases, such as diabetic nephropathy, cardiovascular disease, and hypertension 1, 2, 3. While the standard method of microalbumin testing uses 24‐hr urine samples, spot urine or first morning urine samples are frequently used nowadays due to the inconvenience of 24‐hr urine collection 4, 5. Urine albumin is very stable and can be stored for 1 week at room temperature or for several weeks at 4°C 6. However, the albumin concentration measurement can be influenced by the amount of urine 7, and the albumin excretion rate can spontaneously change due to various factors, including water intake, hyperglycemia, exercise, and position. Nonetheless, the excretion of creatinine is maintained at a steady rate when the glomerular function is constant; thus, the albumin concentration influenced by quantitative changes in urine can be calibrated by calculating the ratio of albumin to creatinine (albumin/creatinine ratio [ACR]; 8, 9, 10, 11). Therefore, the diagnosis of microalbuminuria is possible using the ratio of microalbumin to creatinine excretion 9, 10, 12, 13.
Recently, a urine dipstick test (URiSCAN Super cassette ACR [URiSuper‐ACR; where ACR is albumin/creatinine ratio], YD Diagnostics Corp., Korea) that can conveniently and semiquantitatively measure concentrations of urine microalbumin and creatinine as well as ACR was developed. The present study evaluated the diagnostic performance of this new semiquantitative dipstick, the URiSuper‐ACR.
MATERIALS AND METHOD
Specimens
Among the urine specimens requested for ACR tests from July 1, 2011, to August 31, 2011, at the Department of Laboratory Medicine, Asan Medical Center, 1,040 specimens were randomly selected for this study. Tests were performed using random, midstream spot urine samples, and all tests were completed within 2 hr of urine collection.
Assay Principle
Detection of albumin and creatinine in the URiSuper‐ACR were performed using the dye‐binding and metal complex methods, respectively. The color change made by the reaction was measured by a reflectance meter (URiSCAN Super Plus, YD Electronics Co., Ltd., Korea). Each specimen was tested twice to measure the percent R (the change of reflectance rate [change%R]), and based on the value, the sample was assigned a category. The URiSuper‐ACR system classifies albumin concentration measurements into four categories (0, 30, 80, and 150 mg/l), and creatinine concentration measurements into five categories (10, 50, 100, 200, and 300 mg/dl), which allows for semiquantitative estimation. It also estimates the ACR using three categories (<30, 30–300, and >300 mg/g).
Diagnostic Sensitivity and Specificity
Using the equipment for quantitative measurement (Cobas Integra 800, Roche Diagnostics, Rotkreuz, Switzerland) as the “gold standard,” the sensitivity and specificity of the URiSuper‐ACR were calculated. The range of microalbuminuria was defined as albumin 20–150 mg/l and ACR 30–300 mg/g, abiding to the ranges reported by other studies 14, 15.
Comparison of the URiSuper‐ACR and the Quantitative Method
The categorical concordance rates of the URiSuper‐ACR results were calculated by comparing the results of the quantitative analyzer (Cobas integra 800, Roche Diagnostics), which use immunoturbidometric and the Jaffe‐kinetic method. Quantitative albumin concentration measurements of <20, 20–60, 61–110, and >110 mg/l were assigned categories defined by 0, 30, 80, and 150 mg/l, respectively. Quantitative creatinine concentration measurements of <24, 24–74, 75–150, 151–250, and >250 mg/dl were matched to categories defined by 10, 50, 100, 200, and 300 mg/dl, respectively. Quantitative ACR measurements of <30, 30–300, and >300 mg/g were regarded as normal, microalbuminuria, and proteinuria, respectively. For albumin and creatinine, a positive concordance rate between the URiSuper‐ACR and quantitative results was calculated when both results were observed in the same category (main diagonal) or in ±1 category (main + first lateral diagonal). For the ACR, concordance was reached when the results were in the same category (main diagonal). In addition, the correlation of the change%R value, which is the mean reflectance rate measured by the reflectance meter by the color change of the URiSuper‐ACR, was compared with quantitative microalbumin and creatinine levels as measured by the Cobas Integra. SPSS version 13.0 (SPSS, Inc., Chicago, IL) was used to compare correlations.
Precision and Linearity of the URiSuper‐ACR
For the evaluation of precision, standard solutions of albumin (10, 30, 80, and 150 mg/l) and creatinine (10, 50, 100, 200, and 300 mg/dl) were prepared and repeatedly measured ten times using the URiSuper‐ACR. Within‐run coefficients of variation (CVs) were calculated using the concentration of standard solution and change%R. For the evaluation of linearity, standard solutions of microalbumin and creatinine were prepared by diluting a 0.2 g/l stock solution of microalbumin and a 10 g/l stock solution of creatinine mixed with 9% NaCl solution. Microalbumin standard solutions with concentrations of 10, 30, 80, and 150 mg/dl and creatinine standard solutions with concentrations of 10, 50, 100, 200, and 300 mg/dl were repeatedly tested ten times. Excel 2007 (Microsoft, Redmond, WA) was used for the calculation of precision and linearity.
Interferences
Interference tests were performed on a variety of conditions that can influence the results of the URiSuper‐ACR. For each standard solution with known concentration, we added 250 erythrocytes/μl, adjusted the pH level to 5 or 9, increased the specific gravity (SG) to 1.030, or added 50 mg/dl ascorbic acid. NaCl was added in normal urine to make high SG urine sample. We repeatedly tested each standard solution ten times and compared the change%R before and after the modifications of the conditions. Interference was considered to have an effect if there was a significant change of the test results for at least one of the ten tests.
RESULTS
Diagnostic Sensitivity and Specificity
For albumin and ACR, the URiSuper‐ACR test showed sensitivities of 88.8% and 86.3%, specificities of 90.1% and 93.8%, positive predictabilities of 91.1% and 92.6%, and negative predictabilities of 87.6% and 88.5%, respectively.
Comparison of the URiSuper‐ACR and the Quantitative Method
The concordance rates between the URiSuper‐ACR and Cobas Integra on the albumin and creatinine concentration for the same category of microalbumin were 78.4% and 67.1%, respectively, and for ±1 category were 98.8% and 99.5%, respectively. For ACR, the concordance rate was 81.9% for each category (Table 1). The R 2 values for the regression line obtained by the correlation analysis of change%R and the quantitatively measured value were 0.884 for albumin and 0.753 for creatinine, respectively (Fig. 1). The change%R of the URiSuper‐ACR and quantitatively measured values showed strong correlations for both albumin and creatinine.
Table 1.
Comparison of the URiSCAN Super Cassette ACR and the Quantitative Methoda
| (A) Albumin (n = 1,040) | |||||
|---|---|---|---|---|---|
| Quantitative method | |||||
| mg/l | 0 | 30 | 80 | 150 | |
| URiSCAN Super cassette ACR | 0 | 437 | 60 | 2 | |
| 30 | 46 | 79 | 34 | 5 | |
| 80 | 2 | 15 | 28 | 52 | |
| 150 | 3 | 6 | 271 | ||
| Concordance | Main diagonalb 78.4% | ||||
| Main + first lateral diagonalc 98.8% | |||||
| (B) Creatinine (n = 1,040) | ||||||
|---|---|---|---|---|---|---|
| Quantitative method | ||||||
| mg/dl | 10 | 50 | 100 | 200 | 300 | |
| URiSCAN Super cassette ACR | 10 | 17 | 15 | 1 | ||
| 50 | 7 | 200 | 47 | |||
| 100 | 97 | 316 | 39 | |||
| 200 | 1 | 78 | 125 | 14 | ||
| 300 | 3 | 40 | 40 | |||
| Concordance | Main diagonalb 67.1% | |||||
| Main + first lateral diagonalc 99.5% | ||||||
| (C) ACR (n = 1,040) | ||||
|---|---|---|---|---|
| Quantitative method | ||||
| mg/g | <30 | 30–300 | >300 | |
| URiSCAN Super cassette ACR | <30 | 501 | 60 | |
| 30–300 | 49 | 160 | 58 | |
| >300 | 21 | 191 | ||
| Concordance | Main diagonalb 81.9% | |||
Results falling into the main diagonal are bolded, and the results falling into the first diagonal are italicized.
Agreement between the URiSCAN Super cassette ACR and the quantitative method within the same categories.
Agreement between the URiSCAN Super cassette ACR and the quantitative method within ±1 categories.
ACR, albumin/creatinine ratio.
Figure 1.
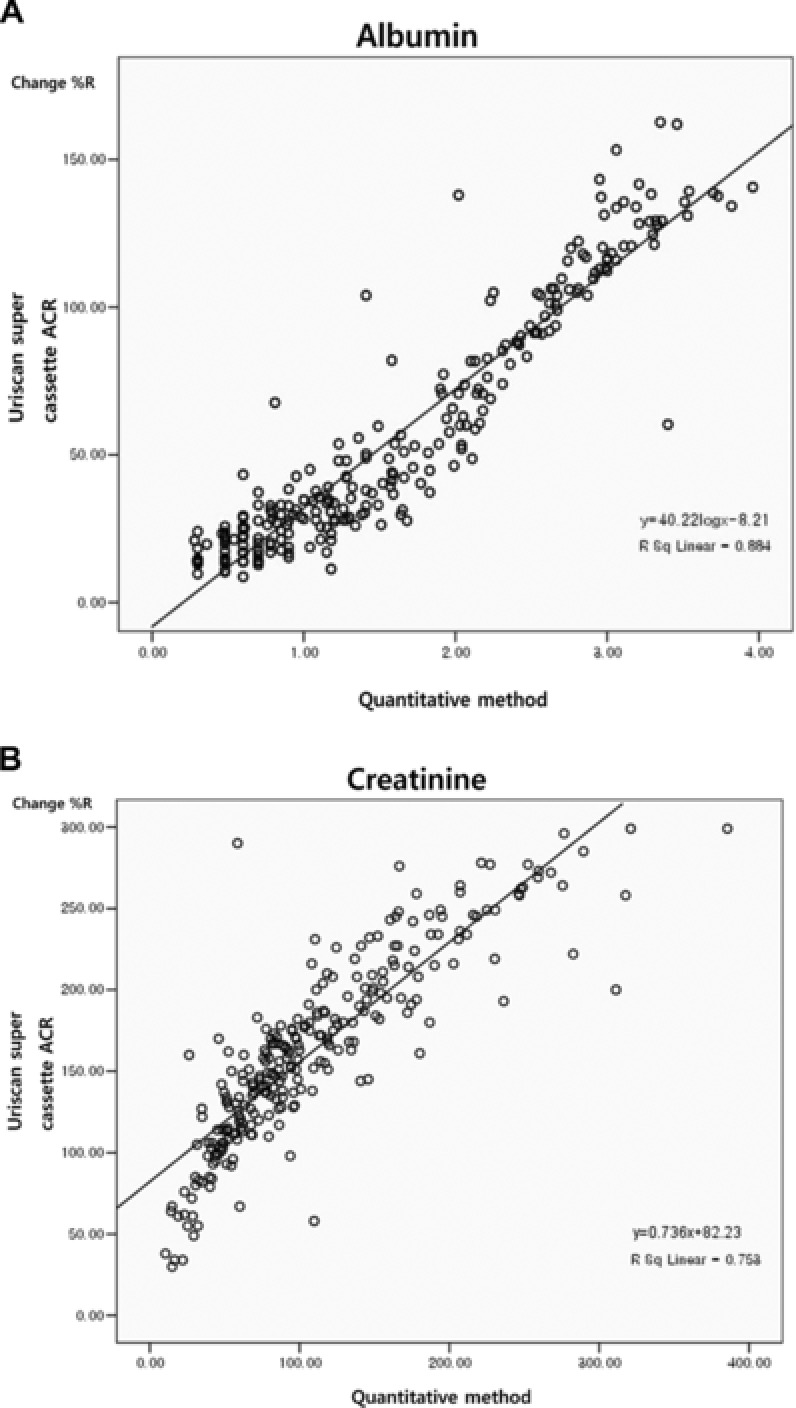
The comparison of the change of reflectance rate (change%R) values from the URiSCAN Super cassette ACR and log transformed quantitative values from the Cobas Integra 800. (A) Albumin (R 2 = 0.884); (B) creatinine (R 2 = 0.753).
Precision and Linearity of the URiSuper‐ACR
The within‐run CV of the URiSuper‐ACR was 3.0∼15.4% for albumin and 5.2∼23.5% for creatinine. Good linearities were shown in the measurement of albumin and creatinine, and the R 2 values were 0.9888 and 0.9927, respectively.
Interferences
Tests were performed in various conditions that can influence the results, including RBC contamination, low and high pH, high SG, and residual ascorbic acid. None of the conditions except for high SG showed interference on the URiSuper‐ACR results for albumin and creatinine (Table 2). Albumin measurements were lower in three (30, 80, and 150 mg/l) of four (0, 30, 80, and 150 mg/l) concentrations tested under high SG. In contrast, creatinine measurements were higher in four (10, 50, 100, and 200 mg/dl) of five (10, 50, 100, 200, and 300 mg/dl) concentrations tested under high SG.
Table 2.
Possible Effects of Interferences on the URiSCAN Super Cassette ACR
| Possible interferences | ||||||
|---|---|---|---|---|---|---|
| Conc. | 250 Ery/μl | pH 5 | pH 9 | High SG (1.030) | Ascorbic acid 50 mg/dl | |
| Albumin (mg/l) | 0 | N | N | N | N | N |
| 30 | N | N | N | Y (10) | N | |
| 80 | N | N | N | Y (10) | N | |
| 150 | N | N | N | Y (80) | N | |
| Creatinine (mg/dl) | 10 | N | N | N | Y (100) | N |
| 50 | N | N | N | Y (200) | N | |
| 100 | N | N | N | Y (200) | N | |
| 200 | N | N | N | Y (300) | N | |
| 300 | N | N | N | N | N | |
“N” indicates that the results were within the same categories with addition of the interferences and “Y” means that the results were within the different categories with addition of the interferences. The numbers in the parentheses represent the concentrations according to the change of reflectance rate (change%R).
Conc., concentration; Ery, erythrocyte; SG, specific gravity; ACR, albumin/creatinine ratio.
DISCUSSION
Chronic renal disease patients around the world are undergoing dialysis, and the number of these patients is increasing 16, 17, 18. Urine microalbumin is the first step in screening for renal diseases. There are two known approaches in confirming microalbuminuria: quantitative and semiquantitative methods. The quantitative method is regarded as the “gold standard” used in clinical laboratories. Semiquantitative methods including the URiSuper‐ACR used in this study, which is often used as the bedside diagnostic screening tool, measure microalbuminuria by using low‐cost urine dipstick reagent strips. Other similar reagent strips were introduced for protein detection; however, problems occurred with high false positive rates and low sensitivity 12, 19. Therefore, there is a need for an easy screening test with high sensitivity, specificity, and less interference by other factors.
The URiSuper‐ACR was developed as a screening tool to detect microalbuminuria in a fast and easy manner, and this study is the first evaluation report on this urine dipstick. The URiSuper‐ACR is a newly designed urine strip type to also measure the ACR by the addition of a creatinine pad, giving an advantage over strip types that only measure albumin concentrations. This test can calibrate for urine dilutions, thereby increasing diagnostic sensitivity and specificity. Thus, this tool enables the use of spot urine samples instead of 24‐hr urine samples 15. In addition, the ACR urine strip was reported to measure urinary protein loss more accurately.
The URiSuper‐ACR showed good sensitivity and specificity in this study, with values of 88.8% and 90.1%, respectively, for albumin and 86.3% and 93.8%, respectively, for the ACR. These results indicate that this instrument has adequate sensitivity and specificity as a screening tool. In a diagnostic performance evaluation study 20 of the Clinitek microalbumin 2 semiquantitative strip (Siemens Healthcare Diagnostics, Inc., Tarrytown, NY; Clinitek strip) on samples from the general population, a sensitivity and specificity of 90% and 91%, respectively, were reported, which are comparable to our results.
Compared to the laboratory quantitative method, the URiSuper‐ACR strip detected microalbuminuria in 160 of the total 1,040 urine samples and yielded a high concordance rate of >80% when the ACR was calculated, which was significantly higher than albumin‐only measurements (albumin concordance rate, 78.4%). This correlates with previous reports 15, 21 stating that when using spot urine for albumin excretion estimation, the ACR is more accurate and is recommended for compensation of urine dilution or concentration effects. Furthermore, in the correlation analysis of quantitative measurements by the quantitative method and change%R by the URiSuper‐ACR, the R 2 values for both albumin and creatinine were >0.7 for uncategorized measurements, showing high correlation between the two methods and confirming the accuracy of the URiSuper‐ACR strip test.
In urine dipstick tests, interferences can occur due to various substances within urine samples. In our interference experiment in this study, no conditions other than high SG showed interference. Urine SG usually reflects urine concentration status that may affect the results of urine dipstick 22. High urine SG is a well‐known interference factor to cause lower result in the urine dipstick for the detection of proteinuria 23, 24. This may be explained by the fact that high SG reduces color development in the protein test. Similar negative interference was also observed in our study. High SG's interference for creatinine measurement in urine dipstick is not clear. Detection of creatinine in the URiSuper‐ACR is performed by metal complex methods, in which the optical measurement of color change is included. Thus, one possible explanation is that high SG may positively affect the optical reaction in the creatinine test. Clinically, urine samples are known to show high SG when there is excess water loss/dehydration, adrenal insufficiency, hepatic disease, or digestive heart failure 25. Thus, when testing urine samples from patients with these conditions, urine SG should be verified, and possible interference must be considered if the SG is high.
In addition to measuring albumin and creatinine, the URiSuper‐ACR also detects ten items (blood, bilirubin, urobilinogen, ketone bodies, protein, nitrite, glucose, pH, leukocyte, and SG) that are already included in conventional clinical laboratory urine strip tests that are currently used in our laboratory. We investigated the effect of the addition of creatinine and albumin test pads by comparing the URiSuper‐ACR results with those of conventional urine strip (URiSCAN Super cassette; YD Diagnostics Corp., Korea) having ten test items. We found concordance rates within each category of 92.5–100%, and within ±1 categories of 99.2–100%, showing the insignificant effect exerted on the other ten test items by the addition of microalbumin and creatinine pads (data not shown).
The limitation of this study was the selection of a heterogeneous patient group rather than a specific patient group, which limits the prediction of test performance in patients with specific diseases, such as hypertensive diseases or diabetes. As an example, in the Clinitek microalbumin/creatinine urinalysis dipstick evaluation study for pregnant cohorts, the sensitivity was 18.5–59.4% and specificity was 45.4–84.2%, and this instrument was reported to be inadequate as a screening tool 12. However, when the same evaluation was performed for the general population, the sensitivity was 90% and specificity was 91%, making it a useful screening tool for the detection of microalbuminuria 20. Therefore, the diagnostic performance can differ according to the patient population, and separate evaluation studies for specific disease groups are needed.
In this evaluation study on a heterogeneous patient group, the URiSuper‐ACR showed good diagnostic performance, such as good sensitivity, specificity, concordance rate with quantitative method, linearity, and precision, and this method could be used as an effective screening tool for the detection of microalbuminuria.
CONFLICT OF INTEREST
All authors have no conflicts to disclose.
REFERENCES
- 1. Parving HH, Oxenboll B, Svendsen PA, Christiansen JS, Andersen AR. Early detection of patients at risk of developing diabetic nephropathy. A longitudinal study of urinary albumin excretion. Acta Endocrinol (Copenh) 1982;100(4):550–555. [DOI] [PubMed] [Google Scholar]
- 2. Pugh JA, Medina RA, Cornell JC, Basu S. NIDDM is the major cause of diabetic end‐stage renal disease. More evidence from a tri‐ethnic community. Diabetes 1995;44(12):1375–1380. [DOI] [PubMed] [Google Scholar]
- 3. Mogensen CE, Damsgaard EM, Frøland A, Nielsen S, de Fine Olivarius N, Schmitz A. Microalbuminuria in non‐insulin‐dependent diabetes. Clin Nephrol 1992;38(Suppl 1):S28–S39. [PubMed] [Google Scholar]
- 4. Watts GF, Hodgson B, Morris RW, Shaw KM, Polak A. Side‐room tests to screen for microalbuminuria in diabetes mellitus. Diabet Med 1988;5(3):298–303. [DOI] [PubMed] [Google Scholar]
- 5. Nathan DM, Rosenbaum C, Protasowicki VD. Single‐void urine samples can be used to estimate quantitative microalbuminuria. Diabetes Care 1987;10(4):414–418. [DOI] [PubMed] [Google Scholar]
- 6. Collins AC, Sethi M, MacDonald FA, Brown D, Viberti GC. Storage temperature and differing methods of sample preparation in the measurement of urinary albumin. Diabetologia 1993;36(10):993–997. [DOI] [PubMed] [Google Scholar]
- 7. Viberti GC, Mogensen CE, Keen H, Jacobsen FK, Jarrett RJ, Christensen CK. Urinary excretion of albumin in normal man: The effect of water loading. Scand J Clin Lab Invest 1982;42(2):147–157. [PubMed] [Google Scholar]
- 8. Al RA, Baykal C, Karacay O, Geyik PO, Altun S, Dolen I. Random urine protein‐creatinine ratio to predict proteinuria in new‐onset mild hypertension in late pregnancy. Obstet Gynecol 2004;104(2):367–371. [DOI] [PubMed] [Google Scholar]
- 9. Waugh J, Bell SC, Kilby MD, et al. Urinary microalbumin/creatinine ratios: Reference range in uncomplicated pregnancy. Clin Sci (Lond) 2003;104(2):103–107. [DOI] [PubMed] [Google Scholar]
- 10. Wallace JF, Pugia MJ, Lott JA, et al. Multisite evaluation of a new dipstick for albumin, protein, and creatinine. J Clin Lab Anal 2001;15(5):231–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Birmingham DJ, Rovin BH, Shidham G, et al. Spot urine protein/creatinine ratios are unreliable estimates of 24 h proteinuria in most systemic lupus erythematosus nephritis flares. Kidney Int 2007;72(7):865–870. [DOI] [PubMed] [Google Scholar]
- 12. Wilde HM, Banks D, Larsen CL, Connors G, Wallace D, Lyon ME. Evaluation of the Bayer microalbumin/creatinine urinalysis dipstick. Clin Chim Acta 2008;393(2):110–113. [DOI] [PubMed] [Google Scholar]
- 13. Croal BL, Mutch WJ, Clark BM, et al. The clinical application of a urine albumin:creatinine ratio point‐of‐care device. Clin Chim Acta 2001;307(1–2):15–21. [DOI] [PubMed] [Google Scholar]
- 14. Molitch ME, DeFronzo RA, Franz MJ, et al. Nephropathy in diabetes. Diabetes Care 2004;27(Suppl 1):S79–S83. [DOI] [PubMed] [Google Scholar]
- 15. Eknoyan G, Levin NW. K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am J Kidney Dis 2002;39(2 Suppl 1):S1–266. [PubMed] [Google Scholar]
- 16. Coresh J, Astor BC, Greene T, Eknoyan G, Levey AS. Prevalence of chronic kidney disease and decreased kidney function in the adult US population: Third National Health and Nutrition Examination Survey. Am J Kidney Dis 2003;41(1):1–12. [DOI] [PubMed] [Google Scholar]
- 17. El Nahas M. The global challenge of chronic kidney disease. Kidney Int 2005;68(6):2918–2929. [DOI] [PubMed] [Google Scholar]
- 18. Kausz AT, Khan SS, Abichandani R, et al. Management of patients with chronic renal insufficiency in the Northeastern United States. J Am Soc Nephrol 2001;12(7):1501–1507. [DOI] [PubMed] [Google Scholar]
- 19. Phelan LK, Brown MA, Davis GK, Mangos G. A prospective study of the impact of automated dipstick urinalysis on the diagnosis of preeclampsia. Hypertens Pregnancy 2004;23(2):135–142. [DOI] [PubMed] [Google Scholar]
- 20. Graziani MS, Gambaro G, Mantovani L, et al. Diagnostic accuracy of a reagent strip for assessing urinary albumin excretion in the general population. Nephrol Dial Transplant 2009;24(5):1490–1494. [DOI] [PubMed] [Google Scholar]
- 21. Kim TY, Kim WB, Kim ES, et al. Serum thyroglobulin levels at the time of 131I remnant ablation just after thyroidectomy are useful for early prediction of clinical recurrence in low‐risk patients with differentiated thyroid carcinoma. J Clin Endocrinol Metab 2005;90(3):1440–1445. [DOI] [PubMed] [Google Scholar]
- 22. Lamb EJ, Price CP. Kidney function tests Burtis CA, Ashwood ER, Bruns DE, et al. (eds.). Tiez Textbook of Clinical Chemistry and Molecular Diagnostics, 5th edition. St. Louis: Elsevier; 2012: 671–673. [Google Scholar]
- 23. Makihara N, Yamasaki M, Morita H, Yamada H. A dipstick test combined with urine specific gravity improved the accuracy of proteinuria determination in pregnancy screening. Kobe J Med Sci 2011;56(4):165–172. [PubMed] [Google Scholar]
- 24. Newman DJ, Pugia MJ, Lott JA, Wallace JF, Hiar AM. Urinary protein and albumin excretion corrected by creatinine and specific gravity. Clin Chim Acta 2000;294(1–2):139–55 [DOI] [PubMed] [Google Scholar]
- 25. Grunwald F, Menzel C, Fimmers R, Zamora PO, Biersack HJ. Prognostic value of thyroglobulin after thyroidectomy before ablative radioiodine therapy in thyroid cancer. J Nucl Med 1996;37(12):1962–1964. [PubMed] [Google Scholar]


