Abstract
Background
SPAPLUS™ is a turbidimetric immunoassay analyzer for detection of excess free light chain (FLC) antigens in serum. Here, we evaluated the analytical performance of Freelite™ Human Kappa Free and Lambda Free on a SPAPLUS™ instrument.
Methods
We evaluated the precision, linearity, sample carryover, and drift of the SPAPLUS™ instrument and compared it with Hitachi 7600 and BN™ II instruments. We evaluated the detection of antigen excess for 12 specimens from patients with monoclonal gammopathy.
Results
The coefficients of variations of κFLC and λFLC were below 5.0%. Linearity was shown in the range of 9.68–152.25 mg/l for κFLC and 4.96–171.09 mg/l for λFLC, and no drift was observed. The κFLC sample carryover was statistically significant, but much smaller than the optimum allowable bias. Agreement rates with the two comparative methods were 87.1, 87.1, and 97.1% or higher for κFLC, λFLC, and the κ/λ ratio, respectively. Antigen excess signals were observed for all 12 antigen excess specimens.
Conclusions
The Freelite™ on the SPAPLUS™ exhibited appropriate precision, linearity, and relative comparability to the reagents on the other instruments. It was good at detecting specimens that had previously demonstrated the hook effect due to antigen excess.
Keywords: analytical performance, evaluation, free light chain, immunoassay, turbidimetry
INTRODUCTION
In conditions characterized by plasma cell dyscrasias such as multiple myeloma and amyloidosis, the serum concentration of the light chain increases because the excess monoclonal light chains generated by the abnormal proliferation of plasma cells cannot combine with heavy chains and, therefore, exist as free light chains (FLCs) in the blood. Quantifying serum FLC is useful in the diagnosis of monoclonal gammopathy and monitoring the therapeutic effects of treatment 1, 2, 3. The International Myeloma Working Group recommended the addition of a serum FLC test to serum protein electrophoresis and serum immunofixation electrophoresis 1, 4, 5. A recent study that examined around 2,500 patients with multiple myeloma, who were undergoing treatment, revealed that the prevalence of monoclonal gammopathy of undetermined significance, which can develop into multiple myeloma, has reached 3.3% in Korea 6. Because of this, since 2005, more than 20 clinical laboratories in Korea have performed the serum FLC test in patients with monoclonal gammopathy or kidney disease by means of nephelometric or turbidimetric immunoassays 7, 8, 9. Generally, serum FLC can be quantitatively measured by latex particle immunoassays using monospecific polyclonal anti‐FLC antibodies that uniquely detect unbound, free‐form light chains 10. Immunoassay analyzers are usually designed to enter the measurement step after initial standard dilution of specimens with a specific dilution fold because the range of FLC concentrations encountered in the clinical laboratory is broad. However, nonlinearity or the hook effect can be observed due to antigen excess when monoclonal FLC levels are very high 4, 9, 11, 12, 13, 14, 15, 16, 17, 18, 19.
Here, we evaluated the analytical performance of Freelite™ Human Kappa Free and Freelite™ Human Lambda Free (The Binding Site Ltd., Birmingham, UK) on a SPAPLUS™ instrument (The Binding Site Ltd.), which was developed as a dedicated immunoturbidimetric analyzer in 2007 and has been introduced in Korea recently. The precision, linearity, sample carryover, and drift were evaluated in the current study. Method comparisons were performed by additionally testing the Freelite™ Human Kappa Free and Freelite™ Human Lambda Free on the Hitachi 7600 Modular P (Hitachi High‐Tech Co., Tokyo, Japan) and Siemens BN™ II (Siemens Healthcare Diagnostics Inc., Marburg, Germany) instruments. The rate of detection of FLC antigen excess was also evaluated using specimens known to exhibit the hook effect.
MATERIALS AND METHODS
Analytical Systems
Kappa FLC (κFLC) and lambda FLC (λFLC) in the serum were measured using Freelite™ Human Kappa Free and Freelite™ Human Lambda Free on the SPAPLUS™ analyzer (the test method). Method comparison was performed by testing the same reagents, that is, Freelite™ Human Kappa Free and Freelite™ Human Lambda Free on different analyzers, on the Hitachi 7600 P‐module (for immunoturbidimetry) and BN™ II (for immunonephelometry). The initial fold value for the standard dilutions for each of analyzers was set according to the manufacturer's instructions at 1:10 for both κFLC and λFLC for SPAPLUS™ and at 1:100 for those of BN™ II. In the case of the Hitachi 7600 P‐module, the ratios were 1:5 for κFLC and 1:8 for λFLC.
Analytical Performance Evaluation
Precision
κFLC and λFLC were measured in duplicate in each run, and two runs per day for 5 days were run for two concentrations of control materials included in the Freelite™ reagent kits. The calculated total coefficients of variations (CVs) were compared with manufacturer's claims about precision in the reagent package insert sheets and the minimum specifications for imprecision derived from biologic variations (3.3% for κFLC and 5.25% for λFLC, calculated on the basis of desirable specifications reported by Hansen et al. 20). The manufacturer's claim for three concentration levels was presented, and they differed from the averages yielded by this study. Thus, using the linear regression equation derived from the means and SDs among the three concentrations specified on the package insert sheet, we calculated the manufacturer's claimed imprecision corresponding to the mean obtained from this study.
Linearity
Available specimens from patients that had concentrations as close as possible to the upper and lower limits of the measuring interval claimed by the manufacturer were selected. Specimens having the lowest and highest concentrations were mixed at ratios of 4:0, 3:1, 2:2, 1:3, and 0:4 into five concentration levels and were measured in quadruplicate. The minimum specifications for bias derived from biologic variations of 7.95% for κFLC and 11.40% for λFLC, which were calculated as described above, were used as allowable nonlinearities in data analysis. The nonlinearity limit in the absolute concentration unit for the specimen with the second lowest concentration among the five concentration levels examined was also applied to that with lowest concentration.
Sample carryover and drift
Clinical and Laboratory Standards Institute (CLSI) EP10 guidelines were referred to in the evaluation of sample carryover and drift 21. With specimens from patients having concentrations as close as possible to the upper and low limits of the measuring interval claimed by the manufacturer, low and high concentration pools were prepared, and a middle concentration pool was made by mixing the two pools in equal volumes. Aliquots of the pools for these three concentrations were measured in the sequence specified in the CLSI document for five days. We evaluated the existence of carryover and drift through statistical analysis to determine the significance of the regression coefficients.
Method comparison
We analyzed the 80 specimens accepted for κFLC and λFLC tests by the Department of Laboratory Medicine at Seoul St. Mary's Hospital (Seoul, Korea) over a period of 6 months, beginning in May 2011. All the specimens were measured twice by the test method, SPAPLUS™. The upper limits of the reference intervals presented by the manufacturers, that is, 19.40 mg/l for κFLC and 26.30 mg/l for λFLC, were considered to represent the medical decision level (MDL). Estimated differences with 95% confidence intervals (CIs) were derived from a regression analysis of the test and comparative methods and compared with the allowable differences for each analyte. The minimum specifications for total errors derived from biologic variations, that is, 13.35% for κFLC and 20.10% for λFLC, which were calculated from the desirable specification 20, were used as allowable differences. In addition, we calculated the agreement rates when subject specimens were classified on the basis of the references intervals of κFLC, λFLC, and the κ/λ ratio (3.30–19.40, 5.71–26.30, and 0.26–1.65 mg/dl, respectively).
Antigen excess detection capabilities and comparison of the measured values
Eleven specimens from two patients with κFLC monoclonal gammopathy and one specimen from a patient with λFLC monoclonal gammopathy were used. All specimens, following analyses with a Hitachi 7600, had been found to have hook effects due to antigen excess and had been stored at −70°C from March 2009 to May 2011. Before analysis, specimens were thawed at room temperature and then measured once more with a Hitachi 7600 and twice with SPAPLUS™. Spearman's correlation analysis and Passing–Bablok regression were performed for κFLC estimation in specimens from patients with a monoclonal gammopathy.
Statistical data analysis
Microsoft Excel 2007 (Microsoft, Redmond, WA), StatisPro (CLSI, Wayne, PA), and MedCalc statistical software version 12.2.1.0 (Mariakerke, Belgium) were used for data processing and analysis. This study was approved by the Institutional Review Board (IRB) of the Catholic Medical Center at The Catholic University of Korea (XC11DIMI0006H).
RESULTS
Precision
The total CVs for κFLC values were 4.20–4.80%, while those of λFLC were 4.40–4.90%. Although the analyses did not meet the minimum specification for imprecision of 3.3% for κFLC, that for λFLC (5.25%) was met. Moreover, the imprecision was approximately equal to or less than the imprecision claimed by the manufacturer (Table 1).
Table 1.
Precision of the Freelite™ FLC Assay on the SPAPLUS™ Using Two Concentration Levels of Control Material (n = 20)
| Test result | Claim of manufacturer | Allowable imprecisiona | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Within‐run imprecision | Total imprecision | Within‐run imprecision | Total imprecision | |||||||||
| Analyte (mg/l) | SD | 95% CI | %CV | SD | 95% CI | %CV | SD | %CV | SD | %CV | SD | %CV | |
| κFLC | 15.1 | 0.51 | 0.358–0.898 | 3.40 | 0.72 | 0.505–1.275 | 4.80 | 0.319 | 2.11 | 1.584 | 10.49 | 0.50 | 3.30 |
| 29.4 | 0.99 | 0.694–1.742 | 3.40 | 1.23 | 0.922–1.850 | 4.20 | 0.483 | 1.64 | 2.764 | 9.40 | 0.97 | ||
| λFLC | 30.0 | 0.40 | 0.279–0.701 | 1.30 | 1.33 | 0.866–2.823 | 4.40 | 0.754 | 2.51 | 1.495 | 4.98 | 1.58 | 5.25 |
| 60.1 | 1.13 | 0.791–1.988 | 1.90 | 2.96 | 1.872–6.945 | 4.90 | 1.356 | 2.26 | 2.941 | 4.89 | 3.16 | ||
Minimum specifications for imprecision derived from biologic variation of κFLC and λFLC.
Linearity
Linearity was observed in the concentration ranges of 9.68–152.25 mg/l for κFLC and 4.96–171.09 mg/l for λFLC (Table 2).
Table 2.
Linearity of the Freelite™ FLC Assay on the SPAPLUS™ Using Multiple Dilutions of Patient Serum Pools
| Measuring interval | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| claimed by manufacturer | Allowable nonlinearity | Tested range | Linear fit | ||||||
| Lower | Upper | Lower goal for | Upper goal for | ||||||
| Analyte (mg/l) | limit | limit | levels 1 and 2 | levels 3–5 | Slope | y Intercept | SE of fit | ||
| κFLC | 4.0 | 180.0 | <9.7 mg/l | >9.7 mg/l | 9.68 | 152.25 | 1.020 | 1.000 | 3.512 |
| 3.60 mg/l | 7.95% | ||||||||
| λFLC | 4.5 | 165.0 | <5 mg/l | >5 mg/l | 4.96 | 171.09 | 0.996 | −5.120 | 5.833 |
| 5.30 mg/l | 11.4% | ||||||||
Sample Carryover and Drift
In the analysis of the κFLC values, there was no significant drift among the three pools (concentrations of 3.99 ± 0.33, 96.62 ± 2.12, and 162.05 ± 3.20 mg/l). However, a statistically significant sample carryover of 1.2% was observed among these samples. In λFLC analysis, there was no significant sample carryover or drift in specimens with concentrations of 11.89 ± 0.25, 69.28 ± 1.66, and 134.65 ± 3.27 mg/l (Table 3).
Table 3.
Carryover and Drift From Multifactor Regression Showing Components of Measurement Error in Preliminary Evaluation of the Freelite™ FLC Assay on the SPAPLUS™
| Analyte (mg/l) | Components of measurement error | Mean of five runs | Sign statistic | P a |
|---|---|---|---|---|
| κFLC | Carryover | 1.2% | 5 | 0.0625 |
| Drift | −0.018 | 2 | 1.0000 | |
| λFLC | Carryover | 0.0% | 2 | 1.0000 |
| Drift | −0.230 | 1 | 0.3750 |
The sign test was statistically significant at 6.25% significance level.
Method Comparison
The mean estimated difference (95% CI) between κFLC measurements using SPAPLUS™ and BN™ II at the MDL of 19.40 mg/l was −3.35 mg/l (−8.108 to −1.416), which was not statistically significant considering the allowable difference of 2.59 mg/l. When comparing the SPAPLUS™ results to those from Hitachi 7600, a significant difference over the allowable limit was observed at the MDL of 19.40 mg/l, and the κFLC values obtained by SPAPLUS™ were lower than those obtained by Hitachi 7600 by 5.59 mg/l (2.667–8.505, Table 4). By classifying the specimens on the basis of the reference intervals of κFLC, we achieved a 97.1% agreement between Hitachi 7600 and SPAPLUS™, and a 87.1% agreement between BN™ II and the test method (Table 5). The estimated difference for λFLC measurement at the MDL of 26.30 mg/l, using either Hitachi 7600 or BN™ II as a comparative method, was not significantly different from the allowable difference of 5.29 mg/l (Table 4). Classifying the specimens by the reference intervals of λFLC, we achieved agreement rates of 92.9% between Hitachi 7600 and SPAPLUS™, and 87.1% between BN™ II and the test method (Table 5). The κ/λ ratio of SPAPLUS™ compared with Hitachi 7600 was statistically significantly higher by 0.88 (0.3969–1.3681) at the MDL of 0.26 mg/l; however, there were no significant differences at the MDL of 1.65. The κ/λ ratio of the test method compared with BN™ II was significantly lower, by 0.27 (0.1640–0.3741), at the MDL of 0.26, but was significantly higher, by 0.50 (0.3933–0.6017), at the MDL of 1.65 (Table 4). In the classification of specimens according to the reference intervals, we achieved a 97.1% agreement between Hitachi 7600 and SPAPLUS™, and between BN™ II and SPAPLUS™ (Table 5).
Table 4.
Method Comparison of FLC Measured by the Freelite™ Assay on the SPAPLUS™ Against That on the Hitachi 7600 and BN™ II
| Allowable total errora | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Analyte (unit) | Comparative Method | N | Concentration range from analyzed samples | r | Slope | 95% CI | y Intercept | 95% CI | Sy.x | MDL | Estimated difference | 95% CI | (mg/l) | % |
| κFLC (mg/l) | Hitachi 7600 | 79 | 4.78–1,703.50 | 0.996 | 0.890 | 0.875–0.902 | −3.420 | −6.408 to −0.425 | 18.050 | 19.40 | −5.59 | −8.505 to −2.667 | 2.59 | 13.35 |
| BN II | 80 | 3.43–1,680.00 | 0.990 | 0.990 | 0.968–1.012 | −3.160 | −8.036–1.719 | 29.680 | −3.35 | −8.108–1.416 | ||||
| λFLC (mg/l) | Hitachi 7600 | 78 | 4.27–2,685.50 | 0.996 | 1.030 | 1.014–1.042 | −2.860 | −8.962–3.237 | 36.640 | 26.30 | −2.14 | −8.129–3.860 | 5.29 | 20.10 |
| BN II | 77 | 6.94–2,935.00 | 0.999 | 0.930 | 0.925–0.940 | −2.120 | −5.631–1.401 | 21.110 | −3.90 | −7.358 to −0.432 | ||||
| κ/λ ratio | Hitachi 7600 | 70 | 0.02–478.64 | 0.997 | 0.697 | 0.688–0.705 | 0.961 | 0.4754–1.447 | 2.870 | 0.26 | 0.88 | 0.397–1.368 | ||
| 1.65 | 0.46 | −0.023–0.945 | ||||||||||||
| BN II | 70 | 0.02–214.32 | 1.000 | 1.551 | 1.547–1.556 | −0.410 | −0.5177 to −0.307 | 0.618 | 0.26 | −0.27 | −0.374 to −0.164 | |||
| 1.65 | 0.50 | 0.393–0.602 | ||||||||||||
Minimum specifications for total error derived from biologic variation.
Table 5.
Comparison of Groups Classified by the Reference Intervals of κFLC, λFLC, and the κ/λ FLC Ratio Between SPAPLUS™ and the Other Two Comparative Methods
| Comparative method | SPAPLUS™ | Agreement rate | |||
|---|---|---|---|---|---|
| Hitachi 7600 | κFLC | <3.30 | 3.30–19.40 | >19.40 | |
| <3.30 | 0.0% (0/70) | 0.0% (0/70) | 0.0% (0/70) | 97.1% | |
| 3.30–19.40 | 0.0% (0/70) | 68.6% (48/70) | 0.0% (0/70) | ||
| >19.40 | 0.0% (0/70) | 2.9% (2/70) | 28.6% (20/70) | ||
| λFLC | <5.71 | 5.71–26.30 | >26.30 | ||
| <5.71 | 0.0% (0/70) | 0.0% (0/70) | 0.0% (0/70) | 92.9% | |
| 5.71–26.30 | 2.9% (2/70) | 70.0% (49/70) | 0.0% (0/70) | ||
| >26.30 | 0.0% (0/70) | 4.3% (3/70) | 22.9% (16/70) | ||
| κ/λ FLC ratio | <0.26 | 0.26–1.65 | >1.65 | ||
| <0.26 | 14.3% (10/70) | 1.4% (1/70) | 0.0% (0/70) | 97.1% | |
| 0.26–1.65 | 0.0% (0/70) | 68.6% (48/70) | 1.4% (1/70) | ||
| >1.65 | 0.0% (0/70) | 0.0% (0/70) | 14.3% (10/70) | ||
| BN™ II | κFLC | <3.30 | 3.30–19.40 | >19.40 | |
| <3.30 | 0.0% (0/70) | 0.0% (0/70) | 0.0% (0/70) | 87.1% | |
| 3.30–19.40 | 0.0% (0/70) | 58.6% (41/70) | 0.0% (0/70) | ||
| >19.40 | 0.0% (0/70) | 12.9% (9/70) | 28.6% (20/70) | ||
| λFLC | <5.71 | 5.71–26.30 | >26.30 | ||
| <5.71 | 0.0% (0/70) | 0.0% (0/70) | 0.0% (0/70) | 87.1% | |
| 5.71–26.30 | 2.9% (2/70) | 64.3% (45/70) | 0.0% (0/70) | ||
| >26.30 | 0.0% (0/70) | 10.0% (7/70) | 22.9% (16/70) | ||
| κ/λ FLC ratio | <0.26 | 0.26–1.65 | >1.65 | ||
| <0.26 | 14.3% (10/70) | 1.4% (1/70) | 0.0% (0/70) | 97.1% | |
| 0.26–1.65 | 0.0% (0/70) | 68.6% (48/70) | 1.4% (1/70) | ||
| >1.65 | 0.0% (0/70) | 0.0% (0/70) | 14.3% (10/70) | ||
Capability of Antigen Excess Detection and Comparison of the Measured Values
An antigen excess signal was flagged by SPAPLUS™ for all 11 specimens for κFLC and for a single specimen for λFLC; all of these samples were previously confirmed to contain high levels of the antigen. The pre‐ and postdilutions results from Hitachi 7600 and SPAPLUS™ are shown in Table 6. A significant correlation between the κFLC values measured by Hitachi 7600 (x) and SPAPLUS™ (y) was obtained, with a Spearman's coefficient of 0.922 (0.722–0.980; P = 0.0001), and the regression equation was y = −513.0625 + 1.4392x. The 95% CI of the y intercept was −1887.31–516.83, and that of the slope was 0.746–1.723 (Fig. 1).
Table 6.
κFLC and λFLC Measured by Hitachi 7600 and SPAPLUS™ From Specimens With the Hook Effect Caused by Antigen Excess
| Hitachi 7600 | SPAPLUS™ | ||||||
|---|---|---|---|---|---|---|---|
| Patient ID | Sex/age | Analyte | Specimen ID | Predilution | Postdilution | Predilution | Postdilution |
| A | M/58 | κFLC | A1 | 26.7 | 838.0 | 21.4 | 892.0 |
| A2 | 29.8 | 874.0 | 18.2 | 744.8 | |||
| A3 | 14.4 | 1,094.0 | 14.5 | 1,333.0 | |||
| A4 | 23.0 | 1,167.0 | 19.2 | 1,124.0 | |||
| B | F/66 | κFLC | B1 | 21.7 | 4,547.0 | 24.1 | 6,230.0 |
| B2 | 12.7 | 4,628.0 | 9.9 | 4,031.0 | |||
| B3 | 12.7 | 4,628.0 | 11.8 | 3,285.0 | |||
| B4 | 13.7 | 6,451.0 | 18.6 | 9,114.0 | |||
| B5 | 6.1 | 10,320.0 | 8.0 | 15,890.5 | |||
| B6 | 4.7 | 13,986.0 | <3.8 | 10,951.0 | |||
| B7 | 4.7 | 13,986.0 | 5.5 | 10,541.0 | |||
| C | F/61 | λFLC | C1 | 58.5 | 14,709.0 | 21.5 | 23,400.0 |
Figure 1.
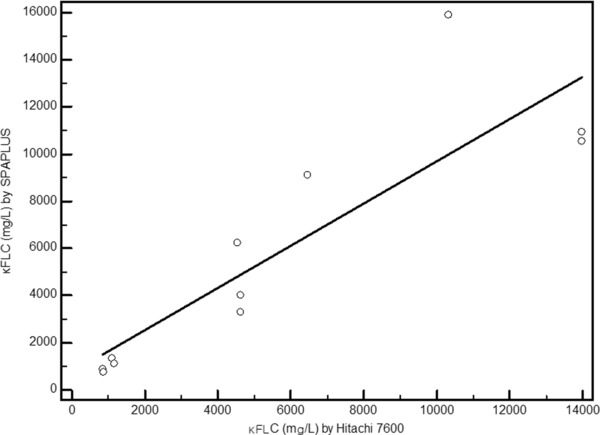
κFLC measured by Hitachi 7600 and SPAPLUS™ from specimens with known hook effects due to antigen excess.
DISCUSSION
To evaluate the analytical performance of Freelite™ Human Kappa Free and Freelite™ Human Lambda Free on SPAPLUS™, we analyzed the precision, linearity, sample carryover, and drift of the samples on the test instrument, and compared the results obtained with those from the Hitachi 7600 Modular P and Siemens BN™ II instruments. Additionally, we examined the capacity to detect antigen excess in specimens known to exhibit the hook effect. Although the number of measurements was relatively small (n = 20), measurement of both κFLC and λFLC by the Freelite™ assay on the SPAPLUS™ instrument met the manufacturer's claims. The observed imprecision of λFLC met the minimum specification for the allowable imprecision, while that of κFLC did not. The desirable specification for imprecision was not fulfilled by either κFLC or λFLC, as described in a previous report testing the Freelite™ FLC assay on the BN™ II instrument 20. Though κFLC showed a statistically significant sample carryover of 1.2% in the concentration range of 3.99–162.05 mg/l, this difference was not thought to be significant when considering the optimum allowable bias of 2.65% for κFLC. Although the estimated difference at the MDL of 19.40 mg/l between the κFLC measured by the test method and Hitachi 7600 was significantly larger than the minimum specification for the allowable total error of 13.35%, the difference from BN™ II was not. For λFLC, the estimated differences at the MDL of 26.30 mg/l between the test method and the two comparative methods were not significantly larger than the minimum specification for total error of 20.10%. Although the Freelite™ Human Kappa and Lambda Free was commonly used on all three analyzers, a certain degree of difference among them was observed. In the comparison of quantitative results, SPAPLUS™, an immunoturbidimetry instrument, seemed to be more comparable with the immunonephelometry instrument, BN™ II, than with another immunoturbidimetry analyzer, Hitachi 7600 Modular P. When comparing the agreement rates in the classification according to the reference intervals, SPAPLUS™ agreed in 92.9% or more of specimens with the Hitachi 7600 analyzer and in 87.1% or more of specimens with the BN™ II analyzer. Here, agreement rates between the two immunoturbidimetry analyzers were higher than those between immunoturbidimetry and immunonephelometry instrument. The agreement rates in the classification of κ/λ ratios for SPAPLUS™ and the Hitachi 7600 or BN™ II analyzers were both 97.1%. The reason for the differences in measured values between analyzers using the same reagent may be due to the fact that the initial standard sample dilution ratios were different, that is, 1/5, 1/100, and 1/10 in Freelite™ Human Kappa Free, and 1/8, 1/100, and 1/10 in Freelite™ Human Lambda Free for Hitachi 7600, BN™ II, and SPAPLUS™, respectively, according to the package insert sheet. The dilution ratios for the subsequent steps were also different among these three analyzers. Previous studies have commented on the poor linearity of data from these analyzers after sample dilution, especially for the immunonephelometry analyzer 22, 23, 24. However, a thorough evaluation of the dilution effect of Freelite™ assays on SPAPLUS™, for example, comparisons among concentration from more than two dilutions, was not performed in the current study. SPAPLUS™ showed antigen excess signals in all 12 specimens already known to have antigen excess. The κFLC showed a high correlation of 0.922 (Spearman's correlation coefficient) between the postdilution results from Hitachi 7600 and SPAPLUS™ analyzers. Although numerous researchers have reported cases of the hook effect caused by antigen excess in serum FLC measurements 9, 12, 13, 14, 15, 16, the built‐in algorithm in the SPAPLUS™ instrument seemed to detect the hook effect well. Moreover, this algorithm may reduce turnaround time and the chance of missing the need for additional dilution steps because the measuring intervals of SPAPLUS™ (4.0–180 mg/l for κFLC and 4.5–165 mg/l for λFLC) are as wide as those of BN™ II (5.9–190 mg/l for κFLC and 5.0–160 mg/l for λFLC) and wider than those of Hitachi 7600 (3.7–56.2 mg/l for κFLC and 5.6–74.8 mg/l for λFLC).
In conclusion, measurement using the Freelite™ Human Kappa and Lambda Free kit on a SPAPLUS™ analyzer seemed to have appropriate precision and linearity for clinical use and relative comparability with the same reagent on Hitachi 7600 and BN™ II analyzers, which are commonly used in the clinical setting. However, it is necessary to be cautious when determining κFLC and λFLC levels using different analyzers, even if the same reagents are used (i.e., the Freelite™ assay). The SPAPLUS™ instrument also showed excellent performance in detecting specimens exhibiting hook effects due to antigen excess.
REFERENCES
- 1. Dispenzieri A, Kyle R, Merlini G, et al. International Myeloma Working Group guidelines for serum‐free light chain analysis in multiple myeloma and related disorders. Leukemia 2009;23:215–224. [DOI] [PubMed] [Google Scholar]
- 2. Martin W, Abraham R, Shanafelt T, et al. Serum‐free light chain—a new biomarker for patients with B‐cell non‐Hodgkin lymphoma and chronic lymphocytic leukemia. Transl Res 2007;149:231–235. [DOI] [PubMed] [Google Scholar]
- 3. Dispenzieri A, Katzmann JA, Kyle RA, et al. Prevalence and risk of progression of light‐chain monoclonal gammopathy of undetermined significance: A retrospective population‐based cohort study. Lancet 2010;375:1721–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tate J, Bazeley S, Sykes S, Mollee P. Quantitative serum free light chain assay—analytical issues. Clin Biochem Rev 2009;30:131–140. [PMC free article] [PubMed] [Google Scholar]
- 5. Jung S, Kim M, Lim J, et al. Serum free light chains for diagnosis and follow‐up of multiple myeloma. Korean J Lab Med 2008;28:169–173. [DOI] [PubMed] [Google Scholar]
- 6. Park HK, Lee KR, Kim YJ, et al. Prevalence of monoclonal gammopathy of undetermined significance in an elderly urban Korean population. Am J Hematol 2011;86:752–755. [DOI] [PubMed] [Google Scholar]
- 7. Bradwell AR, Carr‐Smith HD, Mead GP, et al. Highly sensitive, automated immunoassay for immunoglobulin free light chains in serum and urine. Clin Chem 2001;47:673–680. [PubMed] [Google Scholar]
- 8. Wakasugi K, Suzuki H, Imai A, Konishi S, Kishioka H. Immunoglobulin free light chain assay using latex agglutination. Int J Clin Lab Res 1995;25:211–215. [DOI] [PubMed] [Google Scholar]
- 9. de Kat Angelino CM, Raymakers R, Teunesen MA, Jacobs JF, Klasen IS. Overestimation of serum kappa free light chain concentration by immunonephelometry. Clin Chem 2010;56:1188–1190. [DOI] [PubMed] [Google Scholar]
- 10. Pratt G. The evolving use of serum free light chain assays in haematology. Br J Haematol 2008;141:413–422. [DOI] [PubMed] [Google Scholar]
- 11. Bosmann M, Kossler J, Stolz H, Walter U, Knop S, Steigerwald U. Detection of serum free light chains: The problem with antigen excess. Clin Chem Lab Med 2010;48:1419–1422. [DOI] [PubMed] [Google Scholar]
- 12. Vercammen M, Meirlaen P, Broodtaerts L, Vande Broek I, Bossuyt X. Effect of sample dilution on serum free light chain concentration by immunonephelometric assay. Clin Chim Acta 2011;412:1798–1804. [DOI] [PubMed] [Google Scholar]
- 13. Levinson SS. Hook effect with lambda free light chain in serum free light chain assay. Clin Chim Acta 2010;411:1834–1836. [DOI] [PubMed] [Google Scholar]
- 14. McCudden CR, Voorhees PM, Hammett Stabler CA. A case of hook effect in the serum free light chain assay using the Olympus AU400e. Clin Biochem 2009;42:121–124. [DOI] [PubMed] [Google Scholar]
- 15. Briand PY, Decaux O, Caillon H, Grosbois B, Le Treut A, Guenet L. Analytical performance of the serum free light chain assay. Clin Chem Lab Med 2010;48:73–79. [DOI] [PubMed] [Google Scholar]
- 16. Levinson SS. Regarding the overestimation of serum κ free light chains. Clin Chem 2011;57:775–777. [DOI] [PubMed] [Google Scholar]
- 17. Daval S, Tridon A, Mazeron N, Ristori JM, Evrard B. Risk of antigen excess in serum free light chain measurements. Clin Chem 2007;53:1985–1986. [DOI] [PubMed] [Google Scholar]
- 18. Tate JR, Gill D, Cobcroft R, Hickman PE. Practical considerations for the measurement of free light chains in serum. Clin Chem 2003;49:1252–1257. [DOI] [PubMed] [Google Scholar]
- 19. Tate JR, Mollee P, Dimeski G, Carter AC, Gill D. Analytical performance of serum free light‐chain assay during monitoring of patients with monoclonal light‐chain diseases. Clin Chim Acta 2007;376:30–36. [DOI] [PubMed] [Google Scholar]
- 20. Hansen CT, Munster AM, Nielsen L, Pedersen P, Abildgaard N. Clinical and preclinical validation of the serum free light chain assay: Identification of the critical difference for optimized clinical use. Eur J Haematol 2012;89:458–468. [DOI] [PubMed] [Google Scholar]
- 21. Clinical and Laboratory Standards Institute . Preliminary Evaluation of Quantitative Clinical Laboratory Measurement Procedures; Approved Guideline – Third Edition, EP10‐A3, Wayne, PA: Clinical and Laboratory Standards Institute; 2006. [Google Scholar]
- 22. Jacobs JF, Hoedemakers RM, Teunissen E, van der Molen RG, te Velthuis H. Effect of sample dilution on two free light chain nephelometric assays. Clin Chim Acta 2012;413:1708–1709. [DOI] [PubMed] [Google Scholar]
- 23. Vercammen M, Meirlaen P, Broodtaerts L, Vande Broek I, Bossuyt X. Effect of sample dilution on serum free light chain concentration by immunonephelometric assay. Clin Chim Acta 2011;412:1798–1804. [DOI] [PubMed] [Google Scholar]
- 24. Briand PY, Decaux O, Caillon H, Grosbois B, Le Treut A, Guenet L. Analytical performance of the serum free light chain assay. Clin Chem Lab Med 2010;48:73–79. [DOI] [PubMed] [Google Scholar]


