Abstract
Background
To implement quality screening in a blood service requires the presence of screening strategy with a clear algorithm and supporting standard operating procedures (SOPs), skilled and motivated human resource to perform testing, infrastructure, regular available test kits, and other supplies. In developing countries, smooth supply chain management of critical transfusion transmissible infections (TTIs) screening reagents is a challenge. Therefore, managing the little available kits by knowing the rate of consumption, good forecasting, and monitoring expiry date may be a key in ensuring regular supply.
Method
Test kit monitoring tool (TKMT) for Vironostika HIV Uni‐Form kit/192 1&2 Ag/Ab, Genedia kits for HBsAg and HCV, and RPR for syphilis was developed to track these reagents. This excel tool was developed to assess received reagents, quantity used, quantity remaining, and date of expiration. The tool was evaluated by assessing rerun for each test kits, match tests conducted with blood units tested, adherence to the principle of first in–first out (FIFO), and quantity remaining in the center against the need.
Results
The mean rerun for HIV ELISA Vironistika uniform II Ag/Ab observed over expected was 6.9% (n = 3.8) than 2.4% (n = 1.3), HBsAg was 9.9% (n = 5.7) than 6.7% (3.5) (expected), Genedia for HCV was 1.3% (n = 0.7) than 0.5% (n = 0.3), and RPR test for syphilis 3.3% (n = 1.5) than 0.5%. During implementation, TKMT managed to detect expiring kits in the zonal blood transfusion centers.
Conclusion
A tool‐like TKMT may capture other supplies within blood when expanded. Monitoring of supplies may enable blood service actual accounting and in forecasting supplies and reagents.
Keywords: blood safety supplies, TTI test kits, test kit monitoring tools
LITERATURE REVIEW
Following the adoption of World Health Organization resolution, WHA 28.72, blood safety has become a growing discipline in sub‐Saharan Africa 1. A report by WHO in 2006 showed that 43.2% (n = 19) countries in Africa meets safe blood needs by almost 80–100% via programs of recruitment of Voluntary Non‐Remunerated Blood Donors (VNRBDs). Tanzania is one of countries operating blood service dependent on VNRBD 2. In 2004, in partnership with US government, Tanzania established National Blood Transfusion Service (NBTS) by setting up infrastructures and started blood safety activities with ultimate goal to supply hospitals with processed safe blood and blood products 3.
A critical step in collected blood and blood products processing is to perform a proficient screening to render it free of transfusion transmissible infections (TTIs) in line with WHO recommendation of integrated strategy of provision of safe blood and blood products. The strategy recommends to screen blood to render it free from four TTIs; HIV, HBV (HBsAg), HCV, and syphilis 4.
Quality‐assured screening of blood and blood products requires the presence of four components. These include screening strategy with a clear algorithm supported by standard operating procedures (SOPs); skilled and motivated human resource to perform testing; infrastructure (premises and equipments); and regular available test kits, control materials, and other supplies. In Tanzania, like other developing countries, smooth supply chain management of critical TTI screening reagents is usually a big challenge. Thus, managing of the little available test kits by accounting the quantity received, the rate of consumption and use in line with expiry date are key information to ensure that there is regular supply.
Lack of proper tool to monitor test kits and other supplies has been associated with a loss of millions of dollars through expiration of medical supplies. Following observation of substantial amount of expired drugs in medical stores, Nakayanzi et al. in 2010 reported that expired drugs in Uganda health facilities and National Medical Store remain to be a biggest challenge of the health industry. Similar scenario has been observed in countries such as India, Tanzania, and Botswana. The leading causes of this observation include neglect of stock monitoring and lack of knowledge of basic expiry prevention tools 5.
Blood service in essence is a manufacturing industry as the money is converted to materials (supplies), materials to laboratory results, and the results to money 6. This implies that any expiring supply as a result of poor monitoring is equally the same as wasted money. For example, in 2008 Tanzania NBTS books of accounts showed that test kits and other supplies consumed almost 25% of the annual expenditure budget 7.
The fact that reagent and other supplies used to process blood and blood products are always ordered from abroad where they are not readily available and are too expensive. This implies that forecasting and controlling supplies is necessary to ensure that blood and blood products are screened in the agreed testing algorithm, hence meeting clients’ expectations on safety and controlling the use of limited resources. This was a driving force for Tanzania NBTS to design a tool that will be used to monitor TTI test kits. Hence, Test Kit Monitoring Tool (TKMT) was designed to enable blood transfusion center (BTC) staff to manage supplies, Headquarter to perform evidence‐based forecasting and monitoring of the use of supplied test kits.
METHODOLOGY
Design
This was a description of TKMT used to monitor test kits used to screen blood free from TTIs. The data described in this study were collected from BTCs laboratories that perform all screening of all blood units collected by NBTS. The BTC managers, quality officers, and laboratory in‐charges were technically accountable for all screening and data collection in accordance to NBTS quality framework document.
Inclusion Criteria
All BTCs except Dar es Salaam and Zanzibar BTCs were included in the final analysis of TKMT results. Dar es salaam BTC receives direct influence of the headquarter management framework hence the data in this zone may not be representative. On the other hand, management of supplies at Zanzibar BTC is under control of the Ministry of Health, Tanzania Zanzibar implying that NBTS headquarter in Tanzania Mainland was not involved in management.
Test Kit Monitoring Tool (TKMT)
Vironostika HIV Uni‐Form II Ag/Ab ELISA kit/192 Genedia HBsAg ELISA 3.0 kits for HBsAg, Genedia HCV ELISA 3.0 for hepatitis C, and RPR for syphilis and other special supplies to test blood are critical resources that are supposed to be available at all time in a BTC. In other words, these are some of recurrent capitals that determine the quality of blood service given to clients.
TKMT, which is an excel spread sheet tool, was developed to assist Tanzania NBTS to monitor test kits used to test all blood units collected within NBTS networking. Development of this tool emanated following a recurrence of the centers running out of kits unnoticed and making an emergency requests from headquarter. Sometimes, NBTS was caught in unpleasant circumstances to manage emergency requests of test kits, use expensive courier to ship the test kits, and at the same time addressing demands from consumers (hospitals) for safe blood as untested blood was under quarantine and cannot be released under any circumstance from the BTCs.
The designed TKMT had four portions of monitoring, the date of receiving a particular batch of test kits, and quantity received. The tool shows how the kits were used in conducting test through a primary set of the test and rerun in case of positive sample or indeterminate. Finally, the tool automatically calculates the remaining amount of a particular test kit (Box 1). Then, the user has to summarize at the end of month the remaining stock of each test kits (Vironostika HIV Un‐Form Ag/Ab, Genedia HBsAg, and HCV and RPR) by expiry dates. The tool has a section of laboratory and supplies officers to sign to agree with the content. The error in entry of data has been eliminated by inbuilt formulae that require no use of calculator by the user.
Box 1:
Contents of TKMT
Date of receiving test kits and quantity received.
The use of test kits in the laboratory comprised of primary set of the kit and rerun.
Quantity of test kits remaining in stock after use.
Expiry date.
Supervisors section for accountability.
Tool Designing
TKMT was designed using testing expertise narration of experienced laboratory in‐charge from Dar es salaam BTC. This center shares the same building with headquarter. The laboratory language in setting up, running the test, and performing rerun for positive and indeterminate samples using ELISA tests Vironostika HIV Uni‐form kit/192 1&2 Ag/Ab, Genedia kits to test HBsAg, and HCV and RPR test for syphilis was transformed into arithmetic formulae coded in the excel spread sheet. This formula was based on the fact that ELISA plates used to test collected blood units had a total of 96 wells built in 12 strips, each containing eight wells. By calculation, this implies that one strip accounts for approximately 0.085 of the ELISA plate. This formula was practical resolution from the laboratory when blood collection or rerun mandated the testing of few samples leading to the use of only a fractional of ELISA plate (strips). This enabled quantification of rerun tests for positive and indeterminate samples that are cumulatively tested together (Fig. 1).
Figure 1.
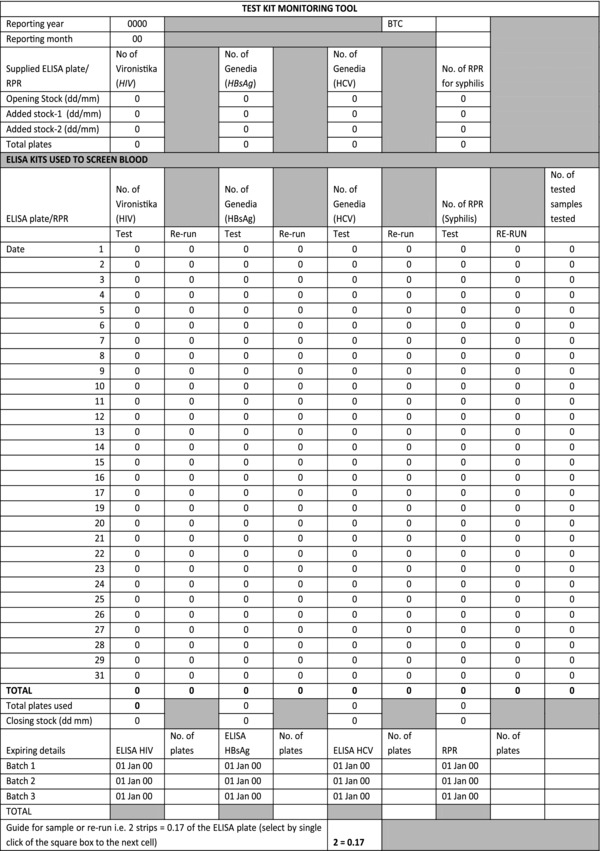
Sample of test kit monitoring tool (TKMT).
To protect TKMT, two levels of passwords were applied; first for authenticity to allow user and second for administrator to protect formula.
Decision for Rerun
Decision for rerun was reached based on the SOPs that dictate that any positive or indeterminate blood sample will undergo rerun using the same test and supplementary test. These blood units with positive and indeterminate results are automatically discarded regardless of the result from rerun or supplementary test.
TKMT Implementation
TKMT was first piloted at a leading Dar es salaam BTC. Feedback from this center enabled designer to sit down and perfect the tool before implementing to other BTCs. One month later, the tool was disseminated to remaining five BTCs. From these BTCs, three members of each center were introduced to the tool. These staffs were quality officer, laboratory in‐charge, and supplies officer. The orientation of the tool was conducted using fixed toll mobile phone conference call, a service that was available from Tanzania mobile phone companies. The designer provided instructions on computer screen from headquarter and the user followed instructions to use the tool on computer screen at BTC. The whole orientation lasted for about 40–60 min.
At the start of the calendar month a new page of TKMT was opened. The number of plates of available test kits received was posted in the tool. Thereafter, everyday a number of plates/or fractional plate used were posted in the tool. Posting of used test kits in TKMT was automatically deducted from the total amount of the available kit in the BTC.
Evaluation of TKMT
Used TKMT was agreed to be returned at the first week of a calendar month. The returning officer (BTC manager) was supposed to convene a meeting with quality officer, laboratory in‐charge, and supplies officer to assess the level of available test kits in the center, use in the previous month, and to come up with a final form to be forwarded to headquarter as soft copy through e‐mail attachment. The headquarter reviewed tool to assess use of test kits and number of rerun, number of test kits remaining and check in practical manner if “first in–first out” (FIFO) principle was followed, and finally to audit if the number of tests tallies with the number of units tested. After reviewing the tool by headquarters, a teleconference was arranged for each zone to give feedback on the key findings of the reported tool. The conference call also gathered the feedback from the centre to know if the tool was helpful to monitor the test kits or rectify some of the fields (Box 2).
Box 2:
Elements of evaluation of TKMT
Number of rerun with respect to expected TTI seroprevalence.
Number of tests tallies with number of units.
Verification of principle of FIFO.
Quantity test kits remaining at the center against the need.
Feedback.
Data Presentation
Data analyzed in this presentation were from second quarter covering April to June of 2009. All data were analyzed using simple ratios comparing rerun against calculated TTI seroprevalence as a reference point. Expected numbers of test kits to be used were extrapolated using number of units collected against TTI seroprevalence. Calculated seroprevalence for HIV was 2.8%, HBsAg was 6.6%, HCV was 0.5%, and syphilis was 0.7% (Table 1).
Table 1.
Reference Point; Transfusion Transmissible Infection (TTI) Seroprevalence in Five Blood Transfusion Centers (BTCs) (April–June 2009)
| Kit used | Moshi | Mwanza | Mbeya | Tabora | Mtwara | Overall | ||
|---|---|---|---|---|---|---|---|---|
| TTI | for testing | (N = 4,329) | (N = 6,078) | (N = 3,686) | (N = 5,963) | (N = 3,168) | prevalence | |
| 1 | HIV (%) | ELISA Vironostika HIV Uni‐form II Ag/Ab | 2.0 | 5.1 | 1.5 | 2.3 | 2.5 | 2.8 |
| 2 | HBsAg (%) | ELISA Genedia | 7.1 | 7.5 | 5.1 | 6.0 | 7.7 | 6.6 |
| 3 | HCV (%) | ELISA Genedia | 0.1 | 0.3 | 0.1 | 0.9 | 1.3 | 0.5 |
| 4 | Syphilis (%) | RPR strips | 0.8 | 0.1 | 0.4 | 0.0 | 4.0 | 0.7 |
RESULTS
All five BTC included in this study used the tool to 100%. The analysis has included data from April to June 2009.
During the reported period of TKMT, a total of 23,224 units of blood were tested. All units collected were screened for TTIs using ELISA Vironostika Uni‐form II Ag/Ab (HIV), ELISA Genedia for HBsAg, and HCV and RPR for syphilis (Table 2).
Table 2.
Showing Number of Tests Performed at 1 and 3 Months
| Blood transfusion center (BTC) | Moshi BTC (%) | Mwanza BTC (%) | Mbeya BTC (%) | Tabora BTC (%) | Mtwara BTC (%) | Mean (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of units tested | 4,329 | 6,078 | 3,686 | 5,963 | 3,168 | Overall 23,224 | |||||||
| Observed HIV seroprevalence (%) | 2.0 | 5.1 | 1.5 | 2.3 | 2.5 | Overall 2.8 | |||||||
| Test | 1° + 2° | 2° | 1° + 2° | 2° | 1° + 2° | 2° | 1° + 2° | 2° | 1° + 2° | 2° | 1° + 2° | 2° | |
| ELISA Vironostika | Observed | 50.7 | 3.3 (6.5) | 74.5 | 8 (10.7) | 44.7 | 4.3 (9.6) | 67.0 | 1 (1.5) | 37.8 | 2.4 (6.3) | 54.9 | 3.8 (6.9) |
| HIV Uni‐form II Ag/Ab used | Expected | 49.1 | 1 (2.0) | 70.9 | 3.4 (4.7) | 41.6 | 0.6 (1.4) | 67.8 | 1.5 (2.2) | 35.2 | 0.9 (2.5) | 53.0 | 1.3 (2.4) |
| Observed HBsAg seroprevalence (%) | 7.1 | 7.5 | 5.1 | 6.0 | 7.7 | Overall 6.7 | |||||||
| Test | 1° + 2° | 2° | 1° + 2° | 2° | 1° + 2° | 2° | 1° + 2° | 2° | 1° + 2° | 2° | 1° + 2° | 2° | |
| ELISA Genedia | Observed | 49.4 | 7.3 (14.8) | 75.0 | 8 (10.8) | 49.8 | 6.5 (13.1) | 72.5 | 3 (4.1) | 40.1 | 3.9 (9.7) | 57.4 | 5.7 (9.9) |
| for HBsAg used | Expected | 51.5 | 3.4 (7.1) | 72.6 | 5.1 (7.5) | 43.1 | 2.1 (5.1) | 70.3 | 4 (6.0) | 37.9 | 2.7 (7.7) | 55.1 | 3.5 (6.7) |
| Observed HCV seroprevalence (%) | 0.1 | 0.3 | 0.1 | 0.9 | 1.3 | Overall 0.5 | |||||||
| Test | 1° + 2° | 2° | 1° + 2° | 2° | 1° + 2° | 2° | 1° + 2° | 2° | 1° + 2° | 2° | 1° + 2° | 2° | |
| ELISA Genedia for | Observed | 47.7 | 0.7 (1.5) | 70.0 | 0.0 | 41.9 | 0.8 (1.9) | 68.5 | 1 (1.5) | 36.7 | 0.8 (2.2) | 53.0 | 0.7 (1.3) |
| HCV used | Expected | 48.1 | 0 (0.1) | 67.7 | 0.2 (0.3) | 41.0 | 0 (0.1) | 66.9 | 0.6 (0.9) | 37.2 | 0.5 (1.3) | 51.9 | 0.3 (0.5) |
| Observed syphilis seroprevalence (%) | 0.8 | 0.1 | 0.4 | 0.0 | 4.0 | Overall 0.7 | |||||||
| Test | 1° + 2° | 2° | 1° + 2° | 2° | 1° + 2° | 2° | 1° + 2° | 2° | 1° + 2° | 2° | 1° + 2° | 2° | |
| RPR test kit | Observed | 42.9 | 5.1 (11.9) | 62 | 0 | 36.7 | 1.5 (4.1) | 63.0 | 0.0 | 37.0 | 3 (8.1) | 46.5 | 1.5 (3.3) |
| Expected | 43.6 | 0.3 (0.8) | 60.9 | 0.1 (0.1) | 37.9 | 1 (0.4) | 59.6 | 0.0 | 36.6 | 1.4 (3.8) | 47.0 | 0.5 (1.1) | |
1°, primary test of all units.
2°, positive and indeterminate units repeat tests (rerun).
During the studied period of TKMT use, four of five BTCs used HIV ELISA Vironostika uniform II Ag/Ab twice as much for rerun than expected. The mean rerun for HIV ELISA Vironostika uniform II Ag/Ab was calculated to be 6.9% (n = 3.8) compared to expected rerun of 2.4% (n = 1.3). In the review of TKMT in testing HBsAg, it was TKMT who observed that two of five BTCs used ELISA Genedia for HBsAg rerun testing was twice than expected. The mean rerun for ELISA Genedia plates in testing HBsAg testing was 9.9% (n = 5.7) compared to 6.7% 3, 5 (expected). On the other hand, although HCV had low seroprevalence among the voluntary blood donors, the proportion of test kit used for rerun using Genedia test kit for HCV was higher with a mean of 1.3% (n = 0.7) than expected 0.5% (n = 0.3). Finally, TKMT observed that the use RPR test kit was not uniform across the BTCs, three of five BTCs showed increased number of RPR strips use with observed mean rerun of 3.3% (n = 1.5) (Table 2).
Neglected Principle of “FIFO”
During this period, TKMT detected kits close to its expiry date piled in two BTCs. These test kits were ELISA Genedia for HBsAg (137), ELISA Genedia for HCV (109), ELISA Vironostika Uniform II Ag/Ab for HIV (30) and RPR (26) (Table 3).
Table 3.
Expired Test Kits due to Failure to Follow first In–First Out (FIFO) Detected by Test Kit Monitoring Tool (TKMT)
| Mbeya blood | ||||
|---|---|---|---|---|
| Expiry | transfusion | Moshi | ||
| Type of kit | date | center (BTC) | BTC | Total |
| ELISA Genedia for HBsAg | May 2009 | 39 | 29 | 68 |
| June 2009 | 69 | 69 | ||
| Total | 137 | |||
| ELISA Genedia for HCV | June 2009 | 56 | 56 | |
| May 2009 | 40 | 13 | 53 | |
| Total | 109 | |||
| ELISA Vironostika Uniform II Ag/Ab for HIV | November 2009 | 2 | 2 | |
| December 2009 | 28 | 28 | ||
| Total | 30 | |||
| RPR | April 2009 | 26 | 26 | |
| Total | 26 | |||
CONCLUSION
To achieve quality in blood safety World Health Organization calls for the management team to participate fully in supportive supervision, play a leading role by identifying specific processes and procedures and their control points 8. On the other hand, ISO 9000 on quality management recommends for leaders to establish unity of purpose and direction of the organization. Leaders in an organization should create and maintain the internal environment in which people can become fully involved in achieving the organization's objectives 9. The design of TKMT was partly translating these roles into action. TKMT was translating the role of leadership in quality management, providing enabling environment, and ensuring that control points in TTI testing in BTC laboratories across the country adhere to the set SOPs.
This study is demonstrating that most of the tests performed for positive or indeterminate TTIs sample of blood units were twice more than recommended by Tanzania NBTS SOP 10. One of the reasons for this might have been motivated by compassionate consideration of the BTC laboratory staff on sero‐positive and indeterminate results. It should be understood that at the time of TKMT implementation, Tanzania NBTS was using adapted nation testing algorithm in HIV and syphilis, however, this was not the case for hepatitis B and C infections as the country had no testing algorithm for these TTIs (HBsAg and HCV). Therefore, testing twice a positive sample would have been considered as confirming the test result as there was no available secondary independent supplementary or complementary test kit or confirming test 11.
Health institutions in developing country are struggling to address a major challenge of poor record keeping. In most of these countries, paper‐based tool are the standard means of record keeping. However, paper‐based record keeping is usually labor intensive, subject to wear and tear, and retrieval is usually tedious. A systematic review by William and Boren in 2008 reported that computer record keeping had superior benefits over the paper‐based record keeping, which is labor intensive and prone to error. The benefits on computer‐based record keeping include easy access and retrieve of record; easy to store, communicate, and consistence in work performance. On the other hand, computer‐based record keeping needs few staffs and provides easy monitoring 12. The implementation of TKMT demonstrated easy tracking of the amount of test kits received and used. It is possible that commercial robust tool to monitor laboratory supplies may be available out there as a “standard tool.” However, the commercial electronic monitoring tools (database) are usually expensive to procure and maintain.
Expiration of supplies may result from poor information on the amount of supplies procured, shipped, and remaining at BTC. The use of a computer‐based tool like TKMT helped our organization to identify the state of each of the four test kits in the five BTCs. As pointed earlier, before the introductions of TKMT, BTCs were sometimes providing short notes of out of stock of TTI test reagents. This observation tally with a 2006 report by McHugh that when laboratory staffs were required to review worksheets on lots received, the exercise was time consuming and was prone to human error. McHugh reports that the situation changed significantly after the introduction of computerized tracking system as it was able to display lot number, date of receipt, and expiration date accurately 8. Similar situation was observed upon the introduction of TKMT as NBTS managed to salvage almost all expiring test kits that would have ended up in a dustbin ready for disposal.
A tool, such as TKMT, may be expanded to capture other supplies within blood services that are routinely used such as blood bags, vacutainers, ABO blood groups, Rhesus reagents, etc. Monitoring of these supplies may enable blood service to know exact amounts used per year hence helping forecasting (planning), monitoring, and certainly serve some money that may be wasted due to overpurchase, overconsumption, or unnecessary overtesting.
In order to make a progress in development for any sector, training is necessary and this requires financial resource of which is limited. In this study intervention, convention classroom was not an option instead innovation of mobile phone teleconference was used to save a limited financial resource. This innovation was practically quick to cover all centers, effective, and less costly. The innovation to orient staff took advantage of already existing resources, that is, the same available human resources (as there was no need of hiring of an expert), computers with connected broadband services, and mobile phone airtime special package. In so doing, the program salvaged substantial amount of funds than if conventional training was used. Based from this experience, it is the opinion of this study that a lot more may be done by using the available IT solutions, such as teleconference facilities in mobile phone, skype, etc., in building capacity of staff and solving other challenges of monitoring and evaluation and supportive supervision.
ACKNOWLEDGMENTS
This study would not be possible without the work of all NBTS staff at the Headquarter of BTCs, so, we would like to thank the team for their contribution. Second, we would like to express our sincere contribution of Egid Minja, former quality systems officer at Dar es Salaam, who provided initial technical guidance to design the TKMT. Third, thanks should go to zonal managers Grace Mlingi (Dar es Salaam), Abdul Mahamoud (Mwanza), Lelo Baliyima (Mbeya), Abdul Juma (Tabora), Vincent Mtweve (Mtwara), and Christopher Msoma (Moshi), who in their capacity ensured TKMT is implemented. And finally, we would like to register special thanks to all zonal administrators, quality system officers, and lab managers of the time for their dedicated work to use TKMT.
REFERENCES
- 1. Aide Memoire for national blood programme . World Health Organization 2002. WHO/BCT/02.03. [Google Scholar]
- 2. Tapko JB, Mainuka Paul, Diarra‐Nama AJ. Status of blood safety in the WHO African region. Report of the 2006 survey.
- 3. Screening donated blood for transfusion transmissible infections . World Health Organization 2010. ISBN‐10 924154788X. [PubMed]
- 4. Nakyanzi JK, Kitutu FE, Oriaa H, Fadhiru Kamba PF. Expiry of medicines in supply outlets in Uganda. Bull World Health Organ 2010;88:154–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. de Kieviet W, Frank E, Stekel H. Essentials of clinical laboratory management in developing regions. Committee on Clinical Laboratory Management (C‐CLM), Education and Management Division (EMD). Available at www.ifcc.org/PDF/homepage/ifcc_book.pdf
- 6. Tanzania National Blood Transfusion Service, annual expenditure . 2008. (Budget reports).
- 7. Williams F, Boren SA. The role of electronic medical record in care delivery in developing countries. Inform Prim Care 2008; 16:139–145. [DOI] [PubMed] [Google Scholar]
- 8. McHugh TM. Supply chain management in the clinical laboratory. Clin Leadersh Manag Rev 2006; 20(1: E4. [PubMed] [Google Scholar]
- 9. Nolen LB, Braveman P, Dachs JN, et al. Strengthening health information systems to address health equity challenges. Bull World Health Organ 2005; 83(8):597–603. [PMC free article] [PubMed] [Google Scholar]
- 10. Tanzania National Blood Transfusion Service . Standard Operating Procedure (SOP) 2008. [Google Scholar]
- 11. Makuwani AM, Nkya AE, Minja E, Haule DP. Observed high HBsAg Sero‐prevalence among blood donors—a lead cause of blood discards in Tanzania. African Sanguine 2010; 13(1):9–12. [Google Scholar]
- 12. Williams F, Boren SA. The role of electronic medical record in care delivery in developing countries. Inform Prim Care 2008; 16:139–145. [DOI] [PubMed] [Google Scholar]


