Abstract
We investigated the relationship between the urokinase type plasminogen activator receptor (uPAR) in sera and tissues of patients with cervical cancer and the clinical and pathological features of the cancer. Immunohistochemistry (SABC method) was used to detect uPAR expression in cervical cancer and normal tissues; ELISA was employed to assay the uPAR levels in cervical cancer and normal tissues and the corresponding sera. The immunohistochemistry results showed that there were 37 cases of uPAR expression in 56 patients of cervical cancer with a positive expression rate of 66%, whereas there was no uPAR expression in normal cervical tissues. The uPAR levels in cancer tissue from patients with cervical cancer (70.92 ± 28.55 ng/100 mg protein) were significantly higher than those of adjacent tissues obtained from the cancer patients (11.01 ± 5.40 ng/100 mg protein) (P < 0.001). Furthermore, the tissue uPAR levels are correlated with the TNM stages, lymph node metastasis, and the degree of differentiation instead of tumor‐infiltrating and vessel thrombosis. Serum uPAR levels of patients (2.38 ± 0.29 ng/ml) were significantly increased compared with health control group (0.50 ± 0.16 ng/ml) (P < 0.001). Single‐factor analysis shows that the serum uPAR levels of preoperative patients are related with clinical grade, lymph node metastasis, vein embolism, and the depth of infiltration instead of tumor differentiation. We conducted multiple regression analysis and found that the factors affecting preoperative serum suPAR include clinical stage (P = 0.000), pelvic lymph node metastasis (P = 0.000), and depth of myometrial invasion (P = 0.001). The serum suPAR levels of patients with cervical cancer after surgery are significantly decreased compared with preoperation (P < 0.001). The uPAR levels of serum and tissue present a positive correlation (r = 0.705, P < 0.001). The soluble uPAR in serum (suPAR) may be a more convenient indicator to reflect the uPAR system activity in vivo. It could be a tumor marker for clinical diagnosis, treatment, and prognosis monitor of cervical cancer.
Keywords: cervical cancer, serum, tissue, urokinase type plasminogen activator receptor (uPAR)
INTRODUCTION
Cervical cancer is the second most common type of cancer in women worldwide, after breast cancer 1. It was reported that half a million women in the world per year developed cancer of the uterine cervix, resulting in approximately 275,000 deaths yearly 2. Overall, cervical cancer is relatively common in Shanxi Province, where patients located in remote areas often have tumor invasion and metastasis when they seek medical treatment. Cervical cancer is a serious threat to women's physical and mental health owing to the ineffective clinical treatment. The diagnostic value of cytology and colposcopy biopsy has been recognized for the early diagnosis of cervical cancer 3. Tumor markers are substances produced by tumor cells or other cells within the body in response to cancer or certain benign conditions. These markers may be detected within exfoliated or distributed cells, or as circulating agents within the peripheral blood or plasma. With the progress of tumor marker studies, markers have become an indispensable clinical auxiliary diagnosis and pathogenesis analysis technique of tumor. Urokinase type plasminogen activator receptor (uPAR) is a recently found, multifunctional, and ubiquitous receptor involved in the control of a variety of cellular processes frequently found altered in cancer cells 4, 5, 6. uPAR is linked to the cell membrane via a glycosylphosphatidylinositol anchor attached to the C‐terminal hydrophobic domain. Both single chain pro‐uPA and two chain uPA bind to the NH2‐terimnal domain of uPAR. When bound by uPA, uPAR plays a major role in local proteolytic processes, thus facilitaing cell migration as may occur during angiogenesis, neointima and atherosclerotic plaque formation, and tumor cell invasion. uPAR may be a multifunctional receptor, not only promoting pericellular proteolysis, but also involved in integrin‐mediated cell adhension and migration. uPAR has also been implicated in intracellular signaling, cellular differentiation, growth, and chemotaxis. The expression of both u‐PA and uPAR is increased in human atherosclerotic plaque and arterial aneurysms. Previous researches show that uPAR is closely related to the malignant extent, occurrence, development, invasion, and metastasis of tumor 7, 8, 9. In this study, we detected uPAR levels in serum and tissues of tumor patients and analyzed the relationship between uPAR and clinicopathological parameters and the factors affecting preoperative serum uPAR levels. In addition, the study elucidated the potential diagnostic value of uPAR for cervical cancer. uPAR could provide useful information for the design of clinical surgery program, postoperative therapy, disease detection, and evaluation of prognosis.
MATERIALS AND METHODS
Subject Investigated
We collected 68 cervical squamous‐celled carcinoma patients (21–78 years old; average age: 42.23) with complete clinicopathological data admitted by Gynecological Cancer Hospital, Shanxi Province, from January 2005 to December 2008. All these cases of cervical cancer have been pathologically confirmed and are without any treatment before operation. The cancer tissue and normal tissue of cervix uteri were collected. The clinical stages were determined according to FIGO standard 10. The growth pattern of tumor included exogenous and endogenous. There are 6 cases of stage IA, 10 cases of stage IB, 40 of stage IIa, and 12 of stage IIb. The control group consisted of 50 cases of healthy subjects with no cancer aged between 20 and 58 years with the average age of 40.48 years.
Specimen Collection
Preoperative fasting venous blood (2 ml) was collected with the tubes containing separating gel and centrifuged (centrifugal radius: 9.4 cm; 3,000 r/min) for 10 min. The serum was separated and stored at −80°C for further detection. Cancer and normal tissues adjacent to cancer tissues at about 4 cm were extracted, washed with physiological saline, and stored at −80°C.
Reagents and Equipment
The low‐temperature high‐speed centrifuge was from Hermle Z 323 K (Hermle Labortecknik, Germany). KDC‐1042 low‐speed centrifuges, microplate reader, and automatic plate washer were purchased from Tecan Group Ltd., Austria. The uPAR Kit was from Assaypro (Winfield, MO). The AssayMax Human uPAR ELISA kit is designed for detection of uPAR in human serum, tissue extracts, cell culture supernatants, and urine. A Murine monoclonal antibody specific for uPAR has been precoated onto amicroplate. uPAR in standards and samples is sandwiched by the immobilized antibody and a biotinylated polyclonal antibody specific for uPAR, which is recognized by a streptavidin‐peroxidase conjugate.
Immunohistochemistry
Fresh cervical tissue samples were removed and immediately fixed with 10% glutaraldehyde solution for more than a 24 hr. The specimens were marked and dehydrated with gradient tertiary‑butyl alcohol, followed by xylene treatment and paraffin embedding. The 3 μm thick consecutive paraffin sections were made and attached to the glass slide followed by baking, staining, and observation under the microscope. Evaluation of conventional histology signals was performed on serial sections by two pathologists independently. These pathologists were blinded for clinical information. The immunohistochemical expression was evaluated as the percentage of the cells that had membranous staining for UPAR with any intensity. The staining for UPAR was scored as follows: 0, no staining of membranous staining in <10% of the cells; 1+, faint incomplete staining in 10–50% of the cells; 2+, weak‐to‐moderate complete staining in >50% of the cells; and 3+, strong complete staining in >50% of the cells.
ELISA
The frozen cervical carcinoma and adjacent tissues were treated with 0.1 M phosphate‐buffered saline (pH 7.4) containing 1% Triton X‐100, ground, and centrifuged at 14,000 g at 4°C for 20 min. The supernatant was collected. Protein content was determined with ultraviolet spectro‐photometer at 280 nm. The tissue extract was diluted 1:5 into MIX Diluent for uPAR assay. The undiluted samples were stored at −20°C. The experimental procedures were operated in strict accordance with the uPAR assay kit manual. Results are considered as positive when suPAR levels in serum are greater than 1.0 ng/ml. The cutoff (1.0 ng/ml) was evaluated from the mean ± 2SEM of survey of healthy people.
Statistical Analysis
The statistical analysis was performed using SPSS12.0. Intergroup differences of the immunohistochemical staining results were analyzed using X2 test (Fisher exact test) and the ELISA results were analyzed by oneway ANOVA. Correlation analysis was performed using multiple linear regression and Spearman correlation analysis. All the values are expressed as mean ± SEM. P‐values less than 0.05 were considered as significant.
RESULTS
Immunohistochemical Staining (SABC Method) Results of Cervical Cancer Tissues
Using microscopy, we found that the positive expression of uPAR in patients with cervical carcinoma was mainly distributed in the membrane surface and the cytoplasm of cancer cells (Fig. 1). The uPAR‐positive staining cells were mainly tumor cells and vascular endothelial cell staining was lower than that of tumor cells. The extent of uPAR staining on the rim of carcinoma nests with sparse tumor cells and strong tumor angiogenesis was deeper than the intensive areas of tumor cell proliferation. The positive rate for uPAR expression of tumor cells was higher and the tumor vascular endothelial uPAR‐positive staining was also positive in areas with exuberant proliferation of tumor vascular. As indicated in Table 1, the positive expression of uPAR in cancer tissues was significantly higher than normal cervical tissue.
Figure 1.
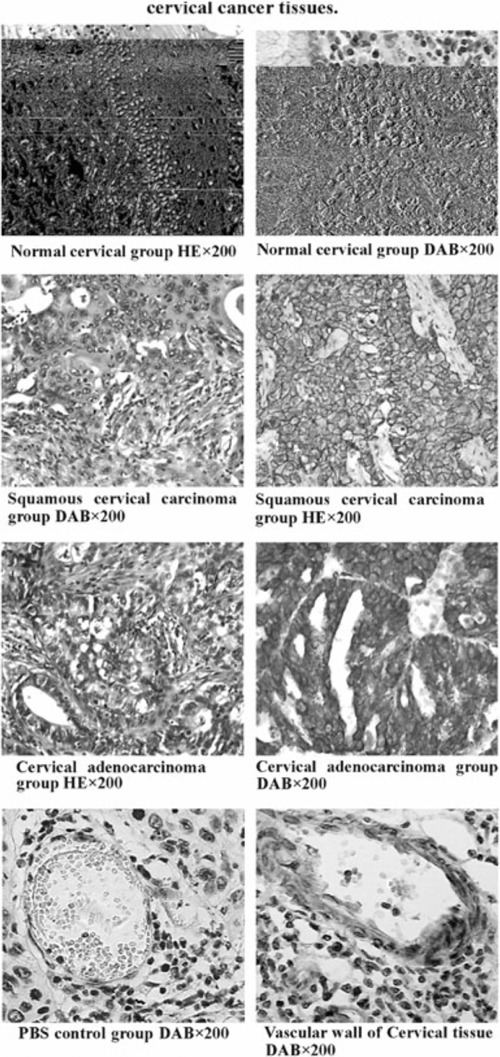
Immunohistochemical staining (SABC method) results of cervical cancer tissues. (1) Normal cervical group HE × 200. (2) Normal cervical group DAB × 200. (3) Squamous cervical carcinoma. (4) Squamous cervical carcinoma group DAB × 200 group HE × 200. (ref SHAPE MERGEFORMAT ref SHAPE MERGEFORMAT 5) Cervical adenocarcinoma. (6) Cervical adenocarcinoma group HE × 200 DAB × 200. (ref SHAPE MERGEFORMAT 7) PBS control group DAB × 200. (8) Vascular wall of Cervical tissue DAB × 200.
Table 1.
Positive Rate of uPAR Expression of Cervical Cancer and Normal Cervical Tissues
| Groups | Cases | uPAR (+) | uPAR(−) | P |
|---|---|---|---|---|
| Cervical cancer tissues | 56 | 37 | 19 | <0.001 |
| Normal cervical tissues | 56 | 0 | 56 |
Comparisons of uPAR Levels in Cervical Cancer and Adjacent Tissues
The uPAR levels in cancer tissue from patients with cervical cancer (70.92 ± 28.55 ng/100 mg protein) were significantly higher than those of adjacent tissues (11.01 ± 5.40 ng/100 mg protein) (P < 0.001).
Relationship Between uPAR Levels in Tumor Tissues and Clinicopathological Features
As shown in Table 2, the uPAR levels of cancer tissues from patients with poorly differentiated cancer are significantly higher than those of the patients with moderately differentiated or well‐differentiated cervical cancer (P < 0.05). The tissue uPAR levels are correlated with the clinical classification and lymph node metastasis (P < 0.05), instead of tumor‐infiltrating and vessel thrombosis (P > 0.05).
Table 2.
Relationship Between uPAR Content in Tumor Tissues and Clinicopathological Features
| Feature | Cases | uPAR (ng/100 mg protein) | P |
|---|---|---|---|
| TNM stage | |||
| I | 16 | 50.23 ± 12.34* | <0.05 |
| IIa | 40 | 82.36 ± 29.35 | |
| Lymph node metastasis | |||
| Yes | 24 | 88.56 ± 29.65 | <0.05 |
| No | 32 | 50.23 ± 20.30 | |
| Degree of infiltration | |||
| Myometrial invasion 2/3 | 26 | 68.65 ± 27.68 | >0.05 |
| Myometrial invasion <2/3 | 30 | 80.58 ± 30.16 | |
| Vessel thrombosis | |||
| Yes | 13 | 76.59 ± 30.64 | >0.05 |
| No | 43 | 68.42 ± 21.71 | |
| Pathological classification | |||
| Well differentiated | 12 | 48.69 ± 30.36 | |
| Moderately differentiated | 23 | 48.23 ± 19.68 | |
| Poorly differentiated | 21 | 93.41 ± 24.25** | <0.05 |
*Compared with II phase.
Comparisons of Serum suPAR Levels in Cervical Cancer Patients and Healthy Control and Before and After Surgery
SuPAR levels of patients with cervical cancer (2.38 ± 0.29 ng/ml) was significantly increased compared with healthy group (0.50 ± 0.16 ng/ml) (P < 0.001); the serum suPAR levels of patients with cervical cancer after surgery was significantly decreased compared with preoperation (P < 0.001). (Table 3).
Table 3.
Comparisons of Serum suPAR Levels in Cervical Cancer Patients and Healthy Group
| Cervical cancer and healthy group | Before and after surgery | |||
|---|---|---|---|---|
| Cervical cancer | Healthy group | Preoperation | Postoperation | |
| SuPA(ng/ml) | 2.38 ± 0.29* | 0.50 ± 0.16 | 2.38 ± 0.29** | 0.68 ± 0.16 |
*Compared with healthy group; P‐value < 0.001.
**Compared with postoperation; P‐value < 0.001.
Relationship Between Serum suPAR Levels of Patients With Cervical Cancer and Clinicopathological Features
Single‐factor analysis shows that the serum suPAR levels of patients with cervical cancer are related to clinical grade, lymph node metastasis, vein embolism, and the depth of infiltration (P < 0.05∼P < 0.01) instead of tumor differentiation (P > 0.05). We conducted multiple regression analysis using clinical stage, growth type, histological type, degree of differentiation, lymph node metastasis, and depth of myometrial invasion as variables and serum suPAR levels as dependent variable. Our results indicated that the factors affecting preoperative serum suPAR include clinical stage (P = 0.000), pelvic lymph node metastasis (P = 0.000), and the depth of myometrial invasion (P = 0.001) (Tables 4 and 5).
Table 4.
Relationship Between Serum suPAR Levels of Patients With Cervical Cancer and Clinicopathological Features
| Feature | Cases | suPAR(ng/ml) | P‐value |
|---|---|---|---|
| TNM stage | |||
| I | 16 | 0.95 ± 0.08* | <0.05 |
| IIa | 40 | 2.09 ± 0.34 | |
| IIb | 12 | 2.89 ± 0.28 | |
| Lymph node metastasis | |||
| Yes | 24 | 3.26 ± 0.54 | <0.01 |
| No | 32 | 1.03 ± 0.23 | |
| Depth of infiltration | |||
| Myometrial invasion 2/3 | 26 | 3.02 ± 0.65 | <0.01 |
| Myometrial invasion <2/3 | 40 | 0.98 ± 0.32 | |
| Vessel thrombosis | |||
| Yes | 13 | 3.23 ± 1.23 | <0.01 |
| No | 43 | 1.86 ± 0.56 | |
| Pathological classification | |||
| Well differentiated | 12 | 1.98 ± 0.56 | >0.05 |
| Moderately differentiated | 23 | 2.23 ± 0.68 | |
| Poorly differentiated | 21 | 2.35 ± 0.54 |
*Compared with II‐IIb phase.
Table 5.
Clinicopathological Parameters Affecting Preoperative Serum suPAR Levels
| Parameters | Regression coefficient β | Standardized regression coefficient | T‐value | P‐value |
|---|---|---|---|---|
| Constant term | −0.708 | 0.000 | −4.028 | 0.000 |
| Clinical stages | 0.346 | 0.364 | 3.962 | 0.000 |
| Lymphatic metastasis | 0.525 | 0.341 | 3.868 | 0.000 |
| Myometrial invasion | 0.192 | 0.297 | 3.359 | 0.001 |
Correlation Analysis of uPAR in Serum and Tissue
The uPAR content of serum and tissue presents a positive correlation (r = 0.705, P < 0.001).
DISCUSSION
Cervical screening programs have markedly reduced the incidence of cervical cancer in China, but approximately 12.3 women per 100,000 population with cervical cancer are diagnosed annually; in 2001 alone, there were about 20,000 deaths resulting from the disease 11. Shanxi is one of the provinces with the highest morbidity and mortality of cervical cancer in China. Patients located in remote areas often have tumor invasion and metastasis when they seek medical treatment in Shanxi Province. Cervical cancer is a serious threat to women's physical and mental health owing to the ineffective clinical treatment. Therefore, it is important to identify a sensitive indicator in assessing the occurrence, development, and prognosis of the disease.
UPAR is a recently discovered multifunctional receptor closely related to tumor invasion and metastasis. UPA/uPAR system is a new research hotspot of oncology. A study result from Pei Wang et al. suggested that the serum uPAR of patients with cervical cancer correlated with tumor infiltrating, whereas tissue uPAR was involved in the degree of tumor differentiation 12. Moreover, previous studies substantiated that the over‐expression of uPA and uPAR was found in hepatocellular carcinoma 13, pancreatic cancer 14, nonsmall cell lung cancer 15, gastric cancer 16, nasopharyngeal carcinoma 17, rectal cancer 18, breast cancer 19, especially in the patients with cancer embolus, tumor invasion, and metastasis 13, 18, 19. Intriguingly, Kita et al. found that detection of uPAR mRNA in peripheral blood was a very useful indicator for esophageal cancer recurrence and metastasis 16. However, there are a relatively small number of researches concerning cervical cancer and uPAR. Detection of tissue uPAR levels is also rare in this regard.
Our findings demonstrate that cervical cancer tissues present an abnormally high expression of uPAR relative to normal cervical tissues. The immunohistochemical image reflects the role of uPAR in the invasive process, suggesting that the marginal increase in uPAR levels is necessary for tumor invasion. UPAR may be the pivotal point of initiation, positioning, and development of proteolytic activities in process of the invasion and metastasis. The interaction of uPAR with vitronectin and integrin inhibits tumor cell apoptosis and promotes tumor angiogenesis and tumor occurrence of experimental animals. It can be speculated that uPAR plays an important role in the process of tumor angiogenesis.
In addition, our study indicates that the uPAR levels in tissue are closely related to clinical classification, lymph node metastasis, and tumor differentiation of patients with cervical cancer. But, in serum of the cancer patients, the clinical classification, lymph node metastasis, and degree of the invading played the important role. There is more uPAR in tissue with more late clinical stage. The higher the degree of malignancy of the patients, the higher uPAR content their tissues have. The possible explanation is that uPAR gathers around the surface of tumor tissues and contributes to an increase in the tumor cells load, resulting in abnormal increase in secretion. The multifactor analysis results show that the serum uPAR levels are significantly higher in cervical cancer patients with late clinical stage, lymph node metastasis, and deep infiltration, which may be attributable to tumor uPAR entering the blood flow through lymphatic vessels resulting in an increase in serum uPAR levels. High expression of uPAR prompts the tumor to a higher proliferative activity and a greater ability to invade, implicating a worse prognosis. Therefore, the serum and tissue uPAR detection of patients with cervical cancer will provide a new target for clinical diagnosis, treatment, and prognosis monitor of cervical cancer. It will improve the treatment of patients with cervical cancer, possibly by monitoring disease condition, identifying patients with recurrence or metastasis and providing replacement therapy, lowering relapse rate, and prolonging survival time.
The complexity of tissue sample processing and the many influencing factors limit the clinical applications of the tissue levels of uPAR. In addition, it is not easy to use the tissue levels to detect treatment effects and disease progress owing to the difficulties in extracting tissue samples from postoperative patients. Therefore, the soluble uPAR form in serum (suPAR) may be a more convenient indicator to reflect the uPA system activity in vivo. In future, further studies should be on the significance of suPAR in forecasting the development of cervical cancer.
REFERENCES
- 1. Canavan TP, Doshi NR. Cervical cancer. Am Fam Physician 2000;61:1369–1376. [PubMed] [Google Scholar]
- 2. Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin 2005;55:74–108. [DOI] [PubMed] [Google Scholar]
- 3. Tamiolakis D, Kalloniatou M, Lambropoulou M, et al. Contribution of combined colposcopy and cytology in cervical pathology. Arch Gynecol Obstet 2005;273:39–42. [DOI] [PubMed] [Google Scholar]
- 4. Jo M, Thomas KS, O'Donnell DM, Gonias SL. Epidermal growth factor receptor‐dependent and ‐independent cell‐signaling pathways originating from the urokinase receptor. J Biol Chem 2003;278:1642–1646. [DOI] [PubMed] [Google Scholar]
- 5. Huang S, Armstrong EA, Benavente S, Chinnaiyan P, Harari PM. Dual‐agent molecular targeting of the epidermal growth factor receptor (EGFR): Combining anti‐EGFR antibody with tyrosine kinase inhibitor. Cancer Res 2004;64:5355–5362. [DOI] [PubMed] [Google Scholar]
- 6. Guerrero J, Santibanez JF, Gonzalez A, Martinez J. EGF receptor transactivation by urokinase receptor stimulus through a mechanism involving Src and matrix metalloproteinases. Exp Cell Res 2004;292:201–208. [DOI] [PubMed] [Google Scholar]
- 7. Van Buren G 2nd, Gray MJ, Dallas NA, et al. Targeting the urokinase plasminogen activator receptor with a monoclonal antibody impairs the growth of human colorectal cancer in the liver. Cancer 2009;115:3360–3368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liu Y, Pixley R, Fusaro M, et al. Cleaved high‐molecular‐weight kininogen and its domain 5 inhibit migration and invasion of human prostate cancer cells through the epidermal growth factor receptor pathway. Oncogene 2009;28:2756–2765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hildenbrand R, Schaaf A, Dorn‐Beineke A, et al. Tumor stroma is the predominant uPA‐, uPAR‐, PAI‐1‐expressing tissue in human breast cancer: prognostic impact. Histol Histopathol 2009;24:869–877. [DOI] [PubMed] [Google Scholar]
- 10. Barillot I, Horiot JC, Maingon P, et al. Impact on treatment outcome and late effects of customized treatment planning in cervix carcinomas: Baseline results to compare new strategies. Int J Radiat Oncol Biol Phys 2000;48:189–200. [DOI] [PubMed] [Google Scholar]
- 11. Cao ZY. Changes and thinking in the treatment of cervical cancer. Chin J Obstet Gynecol 2004;39:212–213. [Google Scholar]
- 12. Wang P, Zheng SM, Jing JX. The serum and tissue plasminogen activator receptor protein of patients with cervical cancer and its clinical significance. Cancer Res Clin 2008;20:474–475. [Google Scholar]
- 13. Zheng Q, Tang ZY, Xue Q, Shi DR, Song HY, Tang HB. Invasion and metastasis of hepatocellular carcinoma in relation to urokinase‐type plasminogen activator, its receptor and inhibitor. J Cancer Res Clin Oncol 2000;126:641–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Keleg S, Buchler P, Ludwig R, Buchler MW, Friess H. Invasion and metastasis in pancreatic cancer. Mol Cancer 2003;2:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lakka SS, Rajagopal R, Rajan MK, et al. Adenovirus‐mediated antisense urokinase‐type plasminogen activator receptor gene transfer reduces tumor cell invasion and metastasis in non‐small cell lung cancer cell lines. Clin Cancer Res 2001;7:1087–1093. [PubMed] [Google Scholar]
- 16. Kita Y, Fukagawa T, Mimori K, et al. Expression of uPAR mRNA in peripheral blood is a favourite marker for metastasis in gastric cancer cases. Br J Cancer 2009;100:153–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Huang B, Xu YY. Effect of uPAR on invasion and metastasis of nasopharynal carcinoma (NPC). Pract J Cancer 2004;19:58–60. [Google Scholar]
- 18. Illemann M, Bird N, Majeed A, et al. Two distinct expression patterns of urokinase, urokinase receptor and plasminogen activator inhibitor‐1 in colon cancer liver metastases. Int J Cancer 2009;124:1860–1870. [DOI] [PubMed] [Google Scholar]
- 19. Hildenbrand R, Schaaf A. The urokinase‐system in tumor tissue stroma of the breast and breast cancer cell invasion. Int J Oncol 2009;34:15–23. [PubMed] [Google Scholar]


