Abstract
Chronic obstructive pulmonary disease (COPD) is associated with systemic effects, and T‐cell‐mediated immunity was involved in the COPD. COPD Assessment Test (CAT) could provide a valid, reliable, and standardized measure of COPD health status. The objective of this study was determination of lymphocyte subpopulation in patients with stable COPD (n = 52) and to ascertain if a relationship existed between T‐lymphocyte subpopulation and CAT performance. The stable COPD patients were assessed with CAT, and divided into four groups with score >30 (n = 8), 20< score ≤30 (n = 16), 10< score ≤20 (n = 20), and score ≤10 (n = 8). Spearman's rank correlation was used to determine the relationship between proportion of T lymphocyte and CAT score. We found an elevated proportion of CD8+ cells in COPD patients of the group with score >30 compared to other groups. Proportion of CD4+ cells was significantly lower in the groups with score >30 and 20< score ≤30 when compared to groups with 10< score ≤20 and score ≤10. The CD4+:CD8+ ratio was also significantly lower in the groups with score >30 and 20< score ≤30. Of note are the correlations of proportion of CD8+ cells and CD4+:CD8+ ratio with CAT performance when score >20. No correlations existed between proportion of CD4+, CD8+ cells, CD4+:CD8+ ratio, and CAT performance when score ≤20. Our results show that the determinants of T‐lymphocyte subpopulation in COPD patients were value to assess physical conditions. We considered CD4+ and CD8+ T lymphocytes to be a representative and stable parameter in grading of health status in COPD patients.
Keywords: COPD, T lymphocyte, assessment, correlation, health status
INTRODUCTION
Chronic obstructive pulmonary disease (COPD) is a progressive disorder, characterized by poorly reversible airway obstruction and persistent inflammation in the lung tissue. COPD is also a debilitating disorder with impaired exercise capacity, aggravated dyspnea, and impaired quality of life. Increasingly, it has been recognized that health status, especially health‐related quality of life, was an important outcome of medical care 1, 2. For example, as respiratory disease specific questionnaires, the St George's Respiratory Questionnaire (SGRQ) and the Chronic Respiratory Disease Questionnaire (CRQ) include questions designed to assess the effect of dyspnea on everyday activities. These instruments are more likely to be responsive to change in clinical status with treatments that target respiratory symptoms. Among patients with severe COPD, the SGRQ demonstrated a floor effect in 26% of patients; however, both the CRQ and the SGRQ were discriminative in distinguishing patients with different levels of disease severity 3. The COPD Assessment Test (CAT) is a short, simple questionnaire for assessing and monitoring COPD. It has good measurement properties, is sensitive to differences in state, and could provide a valid, reliable, and standardized measure of COPD health status with worldwide relevance 4.
Recently, many data suggest that COPD is a systemic disorder accompanied by chronic inflammation 5. COPD patients presenting with decreased CD4+/CD8+ cell ratio on exacerbation or stable status could represent an interesting phenotype related to accelerated airway inflammation and disease deterioration 6. A few studies have been published on the correlation of the population of circulating T cells in COPD with health‐related quality of life. The aim of our study was to evaluate the proportion of lymphocyte subpopulations and analyze its relationship with CAT scores.
METHODS
Study Participants
In this observational study, 52 patients with stable COPD were included. The diagnosis of COPD was established according to the ATS/ERS standards 7. The group of COPD patients consisted of eight women and 44 men. From all 52 patients based on forced expiratory volume in 1 second (FEV1), 13 patients were classified as moderate, 14 patients were classified as severe, and 25 patients were grouped in extremely severe. Patients with any chronic disease other than COPD and with malignant disease were excluded from this investigation. The study was approved by the Ethics Committee of our Institute of our hospital.
CAT Assessment
Identified items included dyspnea, cough, sputum production, and wheeze, as well as systemic symptoms of fatigue and sleep disturbance. Additional indicators included limitations in daily activities, social life, emotional health, and feeling in control, along with the use of rescue medication. A draft framework and 21 draft items were developed. Items were formatted as a semantic differential 6‐point scale, defined with contrasting adjectives. The eight questions of the CAT must appear verbatim, in order, and together as they are presented and not divided on separate pages. The CAT score is calculated as the sum of the responses present. If more than two responses are missing, a score cannot be calculated; when one or two items are missing, their scores can be set to the average of the nonmissing item scores. Higher scores represent worse health.
Flow Cytometry Analysis
Ten milliliters of venous blood was drawn in an anticoagulant tube from each subject. The following mixtures of antibodies were used for cell phenotype: anti‐CD3‐FITC and anti‐CD4‐PE and anti‐CD8‐PE. Two‐color analysis of cells was performed. The samples were analyzed by a FACS Calibur flow cytometer. The cells were collected by CELLQuest software (Altra, Beckman Coulter, Miami, FL). Figure 1 presents an example of dot plots showing double staining for CD4/CD3 and CD8/CD3 lymphocytes in patients with COPD.
Figure 1.

(A) Flow cytometry profile of PB lymphocytes (B) lymphocytes CD3+/CD4+ in the upper right quadrant, (C) lymphocytes CD3+/CD8+ in the upper right quadrant.
Statistical Analysis
Values are represented as mean ± SD. Data were analyzed using a statistical package for social sciences (SPSS 10 for windows). Unpaired Student's t‐test with two‐tailed values was performed to compare the differences between the two groups. Spearman's rank correlation coefficient was used to determine the correlation between proportion of T lymphocytes and CAT score.
RESULTS
From all 52 patients based on CAT, eight patients were classified as score >30, 16 patients were classified as 20< score ≤30, 20 patients were grouped in 10< score ≤20, and eight patients were classified as score ≤10.
As shown in the Table 1, the proportion of CD8+ lymphocytes of the group with score >30 was higher than that of other groups, respectively (P < 0.05), but no significant difference of CD8+ lymphocytes was found among the groups with 20< score ≤30, 10< score ≤20, and score ≤10. And CD4+: CD8+ ratio of the group with score >30 was lower than that of other groups, it was also lower in the group with 20< score ≤30 than that in the groups with 10< score ≤20 and score ≤10. For CD4+ lymphocytes, in the group with score >30, it was significantly lower when compared with the groups with 10< score ≤20, score ≤10, but there was no significant difference when compared to the group with 20< score ≤30. No difference was found in the proportion of CD4+, CD8+ lymphocytes, and CD4+: CD8+ ratio between the groups with 10< score ≤20 and score ≤10.
Table 1.
Proportion of CD4+, CD8+, and CD4+: CD8+ Ratio in the PB of COPD Patients with Different CAT Performance
| Group | Mean age | CD4+cells | CD8+cells | CD4/CD8 |
|---|---|---|---|---|
| 30< score | 73.25 ± 1.65 | 21.79 ± 2.35* | 53.14 ± 3.57# | 0.43 ± 0.58§ |
| 20< score ≤30 | 75.81 ± 2.18 | 27.49 ± 2.02** | 27.74 ± 1.94## | 1.09 ± 0.14§§ |
| 10< score ≤20 | 73.44 ± 1.47 | 41.38 ± 2.18*** | 22.98 ± 1.57### | 1.98 ± 0.20§§§ |
| Score ≤10 | 66.41 ± 2.94 | 41.31 ± 2.40 | 21.38 ± 2.57 | 2.19 ± 0.33 |
Data expressed as mean ± SEM.
The age of subjects from the group with score >30, 20< score ≤30, and 10< score ≤20 was comparable.
*No significant difference compared to the group with 20< score ≤30, P > 0.05, significant difference compared to the group with 10< score ≤20 or score ≤10, P < 0.05.
**Significant difference compared to the group with 10< score ≤20 or score ≤10, P < 0.05.
***No significant difference compared to the group with score ≤10, P > 0.05.
#Significant difference between the group with score >30 and other groups, P < 0.05.
##No significant difference compared to the group with 10< score ≤20 or score ≤10, P > 0.05.
###No significant difference compared to the group with score ≤10, P > 0.05.
§Significant difference between the group with score >30 and other groups, P < 0.05.
§§Significant difference compared to the group with 10< score ≤20 or score ≤10, P < 0.05.
§§§No significant difference compared to the group with score ≤10, P > 0.05.
The influence of age on proportion of lymphocyte subpopulations cannot be excluded. However, in our study, the age of subjects from the groups with score >30, 20< score ≤30, 10< score ≤20 was comparable.
In some subgroups, we found a significant correlation of the proportion of CD4+, CD8+ lymphocytes, and CD4/CD8 ratio with CAT performance (Fig. 2). Here, the subjects were divided into the groups with CAT score ≤20 and score >20. Some of these analyses were confined to patients with high and low CAT scores. We found a positive correlation of the proportion of CD8+ lymphocytes and negative correlations of CD4/CD8 ratio with CAT performance when score >20. There was no significant correlation of CD8+ lymphocytes and CD4/CD8 ratio with CAT performance when score ≤20. For CD4+ lymphocytes, there was no significant correlation with CAT performance in the both subgroups with score >20 and score ≤20 (Figs. 3 and 4).
Figure 2.
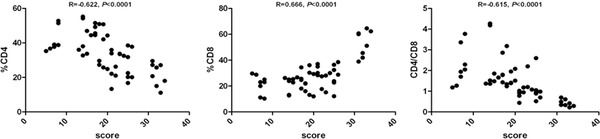
Correlation between proportion of CD4+, CD8+, and CD4+: CD8+ ratio with CAT in the investigated groups.
Figure 3.
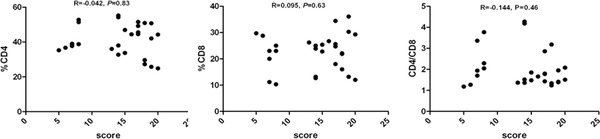
Correlation between proportion of CD4+, CD8+, and CD4+: CD8+ ratio with CAT in the investigated groups with score ≤20.
Figure 4.
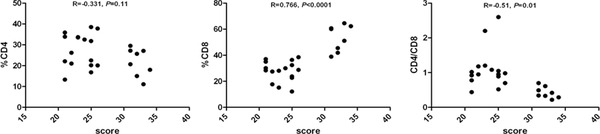
Correlation between proportion of CD4+, CD8+, and CD4+: CD8+ ratio with CAT in the investigated groups with score >20.
Research has shown that CD8+ T cells are overrepresented in the lungs of patients with COPD and that they are inversely related to lung function. Here, we found no significant difference of circulating T‐lymphocyte subpopulation among global initiative for chronic abstractive lung disease (GOLD) grade II, III, IV patients (Table 2).
Table 2.
Proportion of CD4+, CD8+, and CD4+: CD8+ Ratio in the PB of COPD Patients with Different GOLD Stage
| Group | Mean age | CD4+cells | CD8+cells | CD4/CD8 |
|---|---|---|---|---|
| Moderate | 62.35 ± 1.45 | 39.72 ± 2.97* | 28.91 ± 2.69# | 1.84 ± 0.35§ |
| Severe | 69.73 ± 2.18 | 35.88 ± 3.64** | 26.34 ± 1.96 | 1.54 ± 0.29 |
| Extremely severe | 74.52 ± 1.85 | 30.18 ± 1.81 | 26.27 ± 2.18 | 1.42 ± 0.15 |
Data expressed as mean ± SEM.
*No significant difference compared to the severe group, P > 0.05, significant difference compared to the extremely severe group, P < 0.05.
**No significant difference compared to the extremely severe group.
#,§No significant difference existed among the three groups, P > 0.05.
DISCUSSION
COPD is a debilitating disorder characterized by progressive reduction in pulmonary function associated with impaired exercise capacity and quality of life. In addition, COPD is associated with increased risk of comorbid conditions such as skeletal muscle and nutrition abnormalities, immune inhibition 8. Malnutrition produces significant atrophy of lymphoid organs and leads to susceptibility to environmental pathogens, viral reactivation, and development of opportunistic infections 9. Several studies analyzed relationships between nutritional depletion and variables expressing COPD severity 10. In an early study, the prevalence of lean mass depletion was higher among patients with emphysema compared to those with chronic bronchitis. Also, body mass index (BMI) was strongly related to hyperinflation in stable patients with very severe COPD receiving either ventilatory support or long‐term oxygen therapy 11. In contrast, BMI was not related to airway obstruction or hyperinflation in a cohort of stable COPD patients at all stages of the disease 12.
Many data have confirmed the role of systemic inflammation in COPD. The augmentation of neutrophils and the neutrophils cytokine network in the circulation of patients with COPD was found 13. Mario et al. report that frequency and absolute number of circulating plasmacytoid dendritic cell (pDC)s were significantly reduced in patients with stable COPD. This finding is associated with a consistent increase in peripheral myeloid dendritic cell (mDC)/pDC ratio, suggesting a perturbation of the balance between functionally different DC types in COPD 14.
Recently, the suppressive effect of COPD on T‐cell mediating‐immune response is most readily observed. Based on their respective cytokine profiles, responses to chemokines, and interactions with other cells, these T‐cell subsets can promote different types of inflammatory responses. The CD4+ cells are necessary in the activation and maturation of CD8+ cells. CD8+ cells are mainly divided into three subpopulations, depending on their cytokine profile. Type‐1 cells (Tc1) produce IFN‐γ, type‐2 cells (Tc2) produce IL‐4, while type‐0 (Tc0) cells produce both cytokines. Which subpopulation of CD8+ lymphocytes is mostly involved in COPD pathogenesis is currently under investigation. Nikolaos et al. 15 show that COPD patients had an increased number of CD8+ cells in sputum as compared with smokers without COPD and control subjects. CD8+‐IL‐4 cells were reduced both in COPD and in smokers without COPD compared to controls, while CD8+‐IFN‐γ cells were significantly reduced only in COPD as compared with controls. Domagała‐Kulawik et al. 16 found that proportion of Fas+ T lymphocytes was significantly higher in patients with COPD when compared with asymptomatic smokers and nonsmokers. The proportion of Fas‐positive CD8+ cells significantly correlated with the degree of airway obstruction and hypoxemia. Their observation of an elevated proportion of circulating lymphocytes bearing Fas receptor may play a role in induction of these cells’ apoptosis and indicate the role of Fas/FasL pathway in the changes in proportion of lymphocyte subpopulations in patients with COPD.
Our observational study demonstrated that during the stable phase of COPD, patients with high CAT scores (>20) suffered from more imbalance of circulating T‐lymphocyte subpopulation. In addition, more severe CD4:CD8 ratio impairment in patients was associated with increased CAT scores (>30), as reflected by circulating higher CD8+ T cell and lower CD4+ T cell in this group, but no impairment of CD4+ and CD8+ cell was found in the group with low CAT scores (≤20). Additionally, the correlation of CD8+ lymphocytes with the CAT performance of COPD was observed by us only in the group of subjects with score >20, not in the group of patients with score ≤20. We did not observe any significant correlation of the proportion of CD4+ cells in subgroups with CAT scores >20 and ≤20. Thus our observation suggests that CD4+ cells may not be the only factor responsible for immune dysfunction and that CD8+ cells also play a role in the pathogenesis in COPD.
The stage of COPD was also included to analyses of correlation to circulating T lymphocyte. Here, we found no significant difference of circulating T‐lymphocyte subpopulation among GOLD grade II, III, IV patients. The data have shown that imbalance of circulating T‐lymphocyte subpopulation in the peripheral blood (PB) of patients with COPD was not related to lung function.
To our knowledge, our data are the first to examine the relationships between imbalance of circulating T‐lymphocyte subpopulation and CAT performance. Importantly, our results reflect systemic immune parameters during stable phase of COPD, which CAT performance correlated with PB lymphocyte subsets, suggesting that cell‐mediated immune dysfunction may participate in the pathogenesis of COPD and accelerate the impairment on health status. We concluded that persistent immune disorder in COPD was a cause to damage to in health status. In conclusion, the results of our study confirmed the participation of CD8+ and CD4+ cells in the systemic inflammation in patients with COPD. Our data also raise a question whether drugs targeting immunity could potentially improve clinical outcomes in a subset of COPD patients with high CAT scores.
ACKNOWLEDGMENTS
This work was supported by the project of National Natural Science Foundation of China (81000919) and Jiangsu Province's Key Laboratory of Medicine (xk201135).
Cheng Chen and Yu Shen contributed equally and regarded as co‐first authors.
REFERENCES
- 1. Toru O, Koichi N, Mitsuhiro T, Susumu S, Takashi H. Analysis of the factors related to mortality in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2003;167:544–549. [DOI] [PubMed] [Google Scholar]
- 2. Curtis JR, Patrick DL. The assessment of health status among patients with COPD . Eur Respir J Suppl 2003;41:36–45. [DOI] [PubMed] [Google Scholar]
- 3. Windisch W, Budweiser S, Heinemann F, Pfeifer M, Rzehak P. The Severe Respiratory Insufficiency Questionnaire was valid for COPD patients with severe chronic respiratory failure. J Clin Epidemiology 2008;61:848–853. [DOI] [PubMed] [Google Scholar]
- 4. Jones PW, Harding G, Berry P, Wiklund I, Chen WH, Kline LN. Development and first validation of the COPD Assessment Test. Eur Respir J 2009;34:648–654. [DOI] [PubMed] [Google Scholar]
- 5. Gan WQ, Man SFP, Senthilselvan A, Sin DD. Association between chronic obstructive pulmonary disease and systemic inflammation: A systematic review and ameta‐analysis. Thorax 2004;59:574–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kim WD, Kim WS, Koh Y, Lee SD, Lim CM, Kim DS, Cho YJ. Abnormal peripheral blood Tlymphocyte subsets in a subgroup of patients with COPD . Chest 2002;122:437–444. [DOI] [PubMed] [Google Scholar]
- 7. Celli BR, Mac Nee W. Standards for the diagnosis and treatment of patients with COPD: A summary of the ATS/ERS position paper. Eur Respir J 2004;23:932–946. [DOI] [PubMed] [Google Scholar]
- 8. Landbo C, Prescott E, Lange P, Vestbo J, Almdal TP. Prognostic value of nutritional status in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1999;160:1856–1861. [DOI] [PubMed] [Google Scholar]
- 9. Chandra RK. Nutrition and the immune system. Proc Nutr Soc 1993;52:77–84. [DOI] [PubMed] [Google Scholar]
- 10. Skyba P, Kluchova Z, Joppa P, Petrasova D, Tkacova R. Nutritional status in relation to respiratory impairment and systemic inflammation in patients with acute exacerbations of COPD . Med Sci Monit 2009;1510:528–533. [PubMed] [Google Scholar]
- 11. Celli BR, Cote CG, Marin JM, Casanova C, Montes de Oca M, Mendez RA, Pinto Plata V, Cabral HJ. The body‐mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med 2004;350:1005–1012. [DOI] [PubMed] [Google Scholar]
- 12. Vibhuti A, Arif E, Deepak D, Singh B, Qadar Pasha MA. Correlation of oxidative status with BMI and lung function in COPD . Clin Biochem 2007;40:958–963. [DOI] [PubMed] [Google Scholar]
- 13. Barnes PJ, Shapiro SD, Pauwels RA. Chronic obstructive pulmonary disease: molecular and cellular mechanisms. Eur Respir J 2003;22:672–688. [DOI] [PubMed] [Google Scholar]
- 14. Galgani M, Fabozzi I, Perna F, Bruzzese D, Bellofiore B, Calabrese C, Vatrella A, Galati D, Matarese G, Sanduzzi A, Bocchino M. Imbalance of circulating dendritic cell subsets in chronic obstructive pulmonary disease. Clin Immunol 2010;137:102–110. [DOI] [PubMed] [Google Scholar]
- 15. Tzanakis N, Chrysofakis G, Tsoumakidou M, Kyriakou D, Tsiligianni J, Bouros D, Siafakas NM. Induced sputum CD8+ T‐lymphocyte subpopulations in chronic obstructive pulmonary disease. Res Med 2004;98:57–65. [DOI] [PubMed] [Google Scholar]
- 16. Domagała‐Kulawik J, Hoser G, Dabrowska M, Chazan R. Increased proportion of Fas positive CD8+ cells in peripheral blood of patients with COPD . Res Med 2007;101:1338–1343. [DOI] [PubMed] [Google Scholar]


