Abstract
Multidrug resistance (MDR) is a multifactorial phenomenon and the role of these proteins in generating the MDR phenotype is controversial. With this in mind, this review compiled the current data on the role of ABCB1, ABCC1, and LRP proteins in the prognosis of hematologic neoplasms and their influence on the choice of therapy. Literature showed that the detection of these proteins, mainly ABCB1, is important in the AL prognosis. However, there is controversy regarding the methodology used for their detection. In summary, the expression and activity profiles of ABCB1, ABCC1, and LRP, proteins capable of promoting the efflux of a variety of chemotherapeutic agents from the cell cytoplasm represent one of the greatest causes of failure in AL treatment. J. Clin. Lab. Anal. 27:62–71, 2013. © 2012 Wiley Periodicals, Inc.
Keywords: multidrug resistance, ABCB1, ABCC1, LRP, acute leukemia
INTRODUCTION
Acute leukemias (ALs) constitute a heterogeneous group of malignant hematologic diseases characterized by the clonal expansion of bone marrow hematopoietic cells with increased blasts and aggressive clinical course. ALs may be of lymphoid or myeloid lineage, and vary in differentiation 1.
The goal of leukemia treatments is to eradicate the leukemic clone and, thus, reestablish normal hematopoiesis. Several therapy methods have been employed in the treatment of leukemia, such as radiotherapy, chemotherapy, immunotherapy, and bone marrow transplant. Although bone marrow transplant is considered an important therapeutic weapon to achieve complete leukemia remission, this procedure has clinical and socioeconomic restrictions. For this reason, chemotherapy is the most common antileukemic therapy employed today, even with the high morbidity associated to it 2.
In spite of progress in chemotherapy, antitumor drug efficacy is still limited by three main factors: (i) by the drug's pharmacokinetic characteristics in terms of reaching the target cell in adequate amounts; (ii) by the adverse events they cause in normal tissues and cells; and (iii) by the resistance of tumors to the cytotoxic agents administered. The latter remains a primary problem in treating AL.
Multidrug resistance (MDR) might be an inherent phenomenon seen prior to medication therapy or acquired after an initially successful therapy begins 3. The MDR phenotype is characterized by the simultaneous resistance against different drugs that have no structural similarity and act on different molecular targets. It is a multifactorial phenomenon with biochemical resistance mechanisms in common, which can include the reduction of intracellular drug concentrations by changes in its influx/efflux 4.
The extracellular efflux of chemotherapy agents fundamentally involves mechanisms mediated by a superfamily of transport proteins called ATP‐binding Cassette, including ABCB1 (P‐Glycoprotein), ABCC1 (MDR‐associated protein [MRP] 1), and a protein involved in the nucleus‐cytoplasm molecule transport called LRP (lung resistance protein; 4, 5).
Protein ABCB1 (P‐Glycoprotein/PgP/MDR1)
ABCB1 (P‐Glycoprotein or MDR1) is a glycosylated protein encoded by the gene abcb1, which is located in chromosome 7q21.12 6, 7. This protein acts as an ATP‐dependant drug efflux pump and belongs to the ABC‐transporter superfamily, which includes proteins responsible for the transport of a broad range of substrates, such as sugars, amino acids, peptides, organic ions, and several hydrophobic and metabolic compounds 8.
ABCB1 was first described by Juliano and Ling (1976) who, by investigating MDR profiles in Chinese hamster ovary cell strains, identified a 170 kDa glycoprotein involved in the change in permeability to drugs; hence it was named P‐Glycoprotein 9. Later, Fojo and colleagues (1985) characterized the presence of a DNA sequence commonly amplified in cells with MDR phenotype, called mdr1, which was responsible for coding a 170 kDa peptide 10. Chen and colleagues (1986) sequenced this mdr1 gene and confirmed that it coded a 1,280‐amino acid protein consistent with the estimated size for P‐Glycoprotein or ABCB1, proving the mdr1 gene was responsible for coding protein ABCB1 11.
ABCB1 is structured in two homologous and symmetric halves, each composed of a transmembrane region made up by an N‐terminal domain, a cytosolic C‐terminal domain and two ATP‐binding sites (Fig. 1; 12. ATP hydrolysis by ABCB1 provides the energy needed for translocating substrates through the cell membrane 13.
Figure 1.
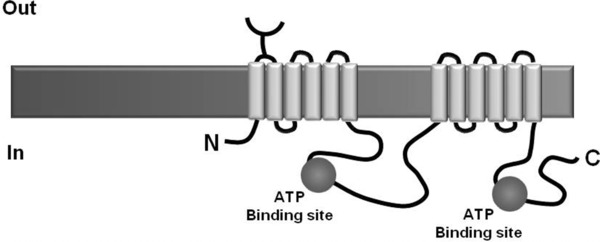
Schematics of protein ABCB1 (Adapted from 14).
Protein ABCB1 can be detected in cells from normal tissues involved in physiological absorption and excretion of compounds 14, such as the liver, the kidneys 15, the placenta, the blood–brain barriers, blood–cerebrospinal fluid barriers, and blood–testis barriers 16. ABCB1 is also related to processes involving the regulation of cell differentiation, proliferation 17, and apoptosis, acting in blocking the activation of caspase 8 and caspase 3 18. Moreover, some studies show that protein ABCB1 plays a role in immune responses, being involved in inflammatory processes. This last hypothesis is corroborated by studies showing that some immune system cells express ABCB1 constitutively 19 and that this protein is related to the transport of cytokines, such as interleukin‐2, interleukin‐4, and interferon‐γ 20.
Protein ABCB1 is well characterized as an efflux pump capable of extruding several drugs out of the cells, as doxorubicin, daunorubicin, vinca alkaloids, actinomycin D, paclitaxel, teniposide, and etoposide 6, 21. Several models have been proposed to explain the efflux mechanism promoted by protein ABCB1. One of the most well‐known models is called “hydrophobic vacuum cleaner” 22, where the two subunits of protein ABCB1 form a single transport channel through which the drug is expelled, being it in its neutral or charged forms. An adaptation of this model considers the occurrence of conformational changes in the protein through ATP hydrolysis. These conformational changes lead to the opening of a channel through which the drug is expelled. Higgins (1994) proposed that the action of protein ABCB1 is similar to that of a flippase enzyme, which is present in the cell membrane and aids the transport of phospholipid molecules 23. Currently, the most accepted model is the one that describes the partitioning of the substrate from the lipid bilayer to an inner part of the protein, called “inner leaflet,” where the substrate‐binding site is localized. When ATP binds to the nucleotide‐binding site (NBS), a great conformational change occurs, exposing the substrate‐binding site to the extracellular space, thus allowing for the drug extrusion process to take place 24.
Due to the drug extrusion mechanism, a marked expression of protein ABCB1 has been related to resistance to chemotherapy. Gottesman (2002) highlighted that protein ABCB1 plays an important role in chemotherapy tumor treatment, since (i) the levels of ABCB1 expression in several tumors are high enough to provide significant resistance to drugs; (ii) the acquisition of resistance after chemotherapy is associated with an increase in the levels of this protein; (iii) the expression levels of protein ABCB1 in some tumors can be used to predict the poor response to chemotherapy, when the drugs employed are ABCB1 substrates 25.
Several pieces of evidence indicate that the expression of gene abcb1 contributes to the resistance of leukemia cells against antineoplastic agents 26. In this context, studies have shown that patients with acute myeloid leukemia (AML) and acute lymphoblastic leukemia (ALL) who do not overexpress protein ABCB1 in their cells had a full remission rate after the induction therapy of 89% and 93%, respectively. On the other hand, this rate dropped to 53% and 56% in patients with AML and ALL, respectively, who overexpressed protein ABCB1 27, 28.
Although many studies have shown the relationship between ABCB1 expression and the prognosis of several neoplasms 29, other studies have shown exactly the opposite 30, making the role of protein ABCB1 in MDR controversial. Nevertheless, the expression of protein ABCB1, as well as gene abcb1, is becoming increasingly relevant and recognized in deciding the most appropriate treatment for patients with neoplasms 6. Therefore, many researchers focus their investigations on improving the response to antineoplastic agents in patients who overexpress protein ABCB1, and/or gene abcb1.
Protein ABCC1 (MDR‐Associated Protein 1/MRP1)
ABCC1 (MRP1) is an ATP‐dependant transmembrane protein encoded by gene abcc1, located in chromosome 16p13.12 7. Protein ABCC1 also belongs to the ABC‐transporter superfamily 31 and is involved in the transport of hydrophobic and anionic substances, and also organic anions conjugated with reduced glutathione (GSH), glucuronide, and sulfate 32.
The discovery, characterization, and identification of protein ABCC1 and the gene responsible for its coding follow the same paths of protein ABCB1. Several studies have shown that some tumor cell strains have a MDR phenotype without expressing protein ABCB1, which reinforces the hypotheses of another protein being involved in the efflux of xenobiotics 33, 34, 35. In 1992, Cole and colleagues identified the amplification of a gene sequence as being responsible for the MDR phenotype in ABCB1‐negative tumor cell strains 36. Later, the sequencing of this region identified the coding gene of the MRP, called abcc1. After the discovery of MRP, other five homologous proteins (MRP2–MRP6) were characterized and considered members of the same family. This family was then called ABCC/MRP transporter subfamily, while protein MRP was then called protein ABCC1/MRP1 37. In 1994, Grant and colleagues characterized ABCC1 as a whole‐membrane, glycosylated protein with molecular weight around 190 kDa 38.
Structurally, protein ABCC1 has two membrane spanning domains (MSD1 and MSD2), each of them with six transmembrane helixes, and a third membrane spanning domain, with approximately 200 amino acids, which is formed by five transmembrane helixes and an amino‐terminal region (Fig. 2; 39). It is believed that the protein binding to its substrates happens by the interaction of these transmembrane helixes 40. ABCC1 is made up of two NBSs, located in the cytoplasmic portion of the protein 41. It must be highlighted that NBS1 has a greater ATP affinity and NBS2 has a greater ATP‐hydrolysis capacity 42.
Figure 2.
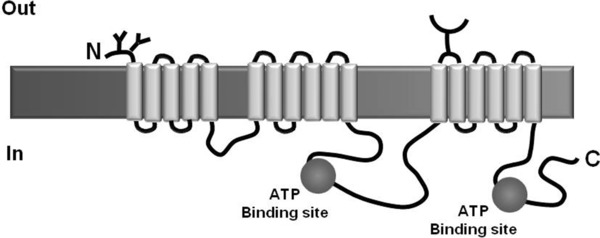
Schematics of protein ABCC1 (Adapted from 99).
The constitutive expression of protein ABCC1 happens in hematopoietic cells of peripheral blood, lung, testicle, placenta, brain, kidneys, adrenal gland, duodenum, heart, colon, and skeletal muscle 7, 37, 43, 44. Protein ABCC1 acts in the transport of physiologic substrates such as leukotriene C4 and oxidized glutathione (GSSG) 45. Moreover, protein ABCC1 protects cells, playing a role in the extrusion of xenobiotic toxic metabolites with the goal of preventing their accumulation 15. In this way, ABCC1 plays an important physiological role in detoxifying cells both from metabolites produced by normal cellular processes and from exogenous toxic agents, such as chemotherapy drugs, which favors the resistance mechanism. Vinca alkaloids, anthracyclines, epipodophyllotoxins, methotrexate, daunorubicin, and doxorubicin are some of the most well‐known antineoplastic agents that are substrates for ABCC1 37.
The likely MDR mechanism induced by ABCC1 is associated with the cotransport of GSH‐conjugated antineoplastic agents, for example, protein ABCC1 acts as a GSH‐dependant drug carrier 46. The importance of this co‐transport mechanism was verified by several studies that show a reduction in the transport of many substrates by protein ABCC1 when GSH was absent or GSH production was inhibited 47. Some studies enabled the confirmation of a direct relationship between the increase in ABCC1 expression and GSH in tumor cells 48, 49. The interaction mechanism between GSH and ABCC1 is complex and not completely understood, but it is believed that these two molecules can interact by means of four different mechanisms. The first mechanism suggests GSH works as a substrate for ABCC1; in the second, GSH plays the role of a co‐transporter for some ABCC1 substrates. Other possible mechanisms show that the enhancement of the ABCC1 transport activity can be mediated by GSH or by other compounds that are not ABCC1 substrates 47.
Several studies in the literature show that the expression of protein ABCC1 is associated with resistance to treatment of different types of cancer 50, 51, 52. Regarding ALs, there is controversy about the role of protein ABCC1 when it is expressed. While some studies claim to find an association of ABCC1 with MDR 53, others show that protein expression seems to have no influence on resistance to treatments 54. One possible explanation for the difficulty in determining the true clinical meaning of these proteins in ALs is based on the fact that the expression of gene abcc1 takes place in all normal hematopoietic cell strains 55. It must be highlighted that many tumor cells co‐express proteins ABCB1 and ABCC1. Considering this, it might be possible that leukemia cells express multiple membrane transporters and that multiple proteins participate in generating the MDR phenotype 56.
Protein LRP (Lung Resistance Protein)
Protein LRP was isolated in 1996 as a vesicular protein of molecular weight of 110 kDa, overexpressed in lung cancer cell strains that had the MDR phenotype. Its identification allowed for the classification of LRP as the main protein of a group of cell organelles discovered in the end of the 1980s called vaults. These proteins include a family of ribonuclear particles present in all eukaryotic cells, explaining why LRP is also called human major vault protein (MVP; 57).
Morphologically, vaults are organelles made up by four structures: the MVP itself, adenosine diphosphate ribose, telomerase‐associated protein, and the small untranslated RNA. Despite their complex structure and composition, they are highly conserved among different phylogenetic species and are present in different cell types. It has been suggested that vaults perform basic functions in all cells, especially in mechanisms that involve the protection of the cell nucleus against toxic compounds and in the intracellular transport of ribosomes and steroid receptors, including those related to progesterone, estrogen, and glucocorticoids 4. There are reports of the expression of protein LRP in the bronchial epithelium and in the digestive tract, in keratinocytes, in the adrenal cortex, in macrophages, in the kidney proximal tubules, in the urothelium, in the pancreatic duct, in germinative cells, in fibroblasts, in Purkinje cells, and in the endothelium of different tissues 58.
Immunihostochemical evaluations reinforce the correlation between LRP levels and drug resistance in cell cultures, the aggressiveness of tumors, and the bad prognosis in cancer treatment. The molecular mechanism involved in MDR mediated by LRP has not been fully determined, but the early idea suggests that the protein acts in the transport of molecules from the nucleus to the cytoplasm, hypothesis generated due to the structural similarity and close fitting to the nuclear pores. Besides transporting toxic substances to the cytoplasm, it is believed that the proteic complex of vaults can arrest molecules in vesicles for later taking them out of the cells by exocytosis (Fig. 3; 58–60).
Figure 3.
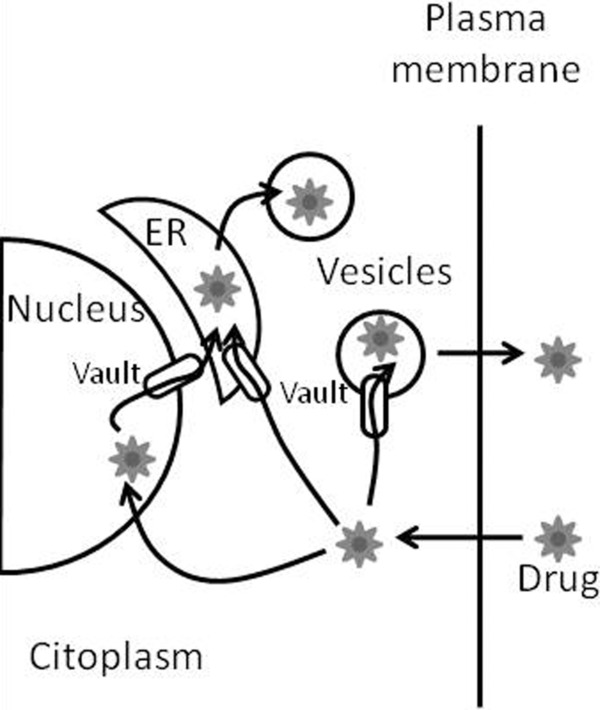
Hypothetical role of LRP in mediating resistance against chemotherapy drugs. LRP/vaults act in the nucleocytoplasmic and vesicular transport of drugs. ER, endoplasmic reticulum (Adapted from 59).
The hypothesis of drug arrest followed by its efflux by using proteic pumps has been explained by investigations involving cell signaling mechanisms and nuclear magnetic resonance spectroscopy. It has been proposed that the vaults act as structured proteins in the signaling pathways of the epidermal growth factor. These proteins can take part in multiple protein–protein interactions, which result in a greater efficiency of the intracellular signaling mechanisms. This model suggests that the role played by LRP involves stimulation of the activation pathways of molecules associated with cell proliferation mechanisms and not necessarily the levels of LRP expressed in the cell 59, 60.
Studies employing ovarian carcinoma cells show that a high LRP expression is required, but not enough, for the development of the MDR phenotype in these cells. Moreover, no relation was seen between the resistance to drugs such as etoposide, daunorubicin, and vincristine and the overexpression of the vaults 61. Van Zon and colleagues (2004) assessed the efflux and the arrest of anthracyclines in vesicles and found these mechanisms to be independent of the increased LRP expression 62. The absence and the reduction of vaults expression were also assessed and were shown not to induce hypersensitivity to cytostatic drugs and not to affect the cells resistance ability 63.
However, other studies suggest the overexpression of LRP can be seen as a phenomenon related to resistance against several chemotherapy agents, such as doxorubicin, vincristine, etoposide, and taxol in patients with solid tumors such as sarcomas, ovary, kidney, bladder, colon, and lung cancer 64. In the case of neoplasms of hematologic origin, LRP expression did not show any significant correlation with risk groups identified by initial white blood cell (WBC) count, gender, and age, while the presence of the MDR phenotype seems to be significantly influenced by them 65. The expression of LRP in AML, multiple myeloma, and diffuse large B‐cell lymphomas was associated with reduced response to chemotherapy drugs and with a shorter life expectancy of patients 66. In ALL cases, LRP was shown to be related to the in vitro resistance against daunorubicin 67. Studies have also evaluated the contribution of LRP in MDR in children with ALL. These studies showed a positive correlation in pre‐B and pro‐B ALL and a negative correlation in the T‐cell ALL cases 63, 68. The results are still contradictory, since some analyses revealed no difference between LRP levels in the initial diagnosis and after relapses, while others found significantly larger expressions in samples with multiple relapses 65, 69.
Several studies related the detection of LRP, and of other resistance proteins that belong to the same cellular group, to the MDR phenotype. While LRP and ABCB1 are rarely overexpressed simultaneously, the frequent concomitant expression of LRP and ABCC1 is seen in ABCB1‐negative malignant cell strains. Due to the proximity in the location of genes lrp (16p11.2) and abcc1 (16p13.1), it was initially believed that the gene lrp was co‐amplified with gene abcc1. However, later studies reported the expression of LRP by itself, as well as determined that two proteins are rarely located in the same amplicon 58, 70.
Therefore, the contradictions seen in the studies published so far suggest the need for further investigations regarding LRP/MDR 63. The latest studies highlight the possibility of the involvement of LRP in MDR through the signaling regulation by kinase proteins traditionally regulated by extracellular growth factors (Ras/Raf/ERK) or by the phosphatidylinositol 3‐kinase/serine‐threonine kinase (PI3K/AKT) pathway. Among the phosphatases involved, Phosphatase and Tensin Homolog (PTEN) stands out, as it interacts with the pathways described above by promoting the regulation of gene expression and cellular growth 60. In this sense, mutations of the gene PTEN or the inhibition of this enzyme can silence it and promote the development of malignant neoplasms by the constitutive activation of AKT. In the hematopoietic strains, active Raf and AKT cause an MDR phenotype in cells by stimulating proliferation, apoptosis prevention, and increase of clonogenicity. In mammary cancer cells, Raf causes resistance against chemotherapy drugs by inducing the expression of ABCB1 and the antiapoptotic protein Bcl‐2 71, 72.
Uncertainties about the mechanism through which protein LRP is involved in the resistance against chemotherapy drugs reinforce the multifactorial nature of MDR. Several biochemical pathways can be simultaneously involved, as well as specific cell types are shown to be more susceptible to resistance against cancer treatment mediated by LRP 66.
FINAL CONSIDERATIONS
The development of new chemotherapy drugs, as well as the use of more aggressive therapeutic protocols, has improved the rates of AL cure. However, some patients do not respond to treatment and have relapses 73. It is believed that one of the causes for treatment failure takes place through the MDR mechanism. Extrusion of chemotherapeutic drugs out of the cell, mediated by overexpression or activation of transport proteins, is the most commonly involved mechanism of chemoresistance in hematologic neoplasms. Literature reports the increased expression of proteins ABCB1, ABCC1, and LRP would be related to the MDR phenotype, and several studies have been published aiming to elucidate the relation between the expression of proteins ABCB1, ABCC1, and LRP and the prognostic factors of ALs, such as age, WBC count at diagnosis, aberrant immunophenotypic markers in blasts cells, and CD34 expression (Table 1).
Table 1.
Studies on the clinical importance of the expression of genes abcb1, abcc1, and lrp and/or proteins ABCB1, ABCC1, and LRP in ALs
| Author | Method | Sample | Results/conclusions |
|---|---|---|---|
| Dhooge et al. (1999) 74 | Immunohistochemistry | de novo ALL (n = 102) and relapse ALL (n = 35) | The expression of protein ABCB1 negatively influenced the prognosis, especially in de novo ALL cases |
| Wutcher et al. (2000) 75 | Flow cytometry | ALL (n = 102) and AML (n = 121) | The expression of protein ABCB1 did not negatively influence the prognosis |
| Fujimaki et al. (2002) 73 | Flow cytometry and RT‐PCR | ALL (n = 18) and AML (n = 26) | The expression of gene and protein ABCB1 was more significant in AML patients, mainly in relapse cases. The expression of protein ABCC1 did not show clinical correlation |
| Schaich et al. (2004) 76 | RT‐PCR | De novo or secondary AML (n = 331) | The expression of ABCB1 and ABCC1 negatively influenced full disease remission after treatment, while LRP did not negatively influence the prognosis |
| Suarez et al. (2004) 77 | Flow cytometry | De novo AML (n = 90) | The expression of ABCB1, ABCC1, and LRP did not negatively influence the prognosis |
| Valera et al. (2004) 78 | RT‐PCR | ALL (n = 30) | Among the evaluation of proteins ABCB1, ABCC1, and LRP, only LRP negatively influence the prognosis |
| Benderra et al. (2005) 79 | Flow cytometry | De novo AML (n = 85) | The expression of ABCB1 was shown to influence treatment failure |
| Olson et al. (2005) 80 | Flow cytometry | Initial ALL (n = 295) | The overexpression of ABCB1, ABCC1, and LRP to diagnostics did not influence treatment failure |
| Anuchapreeda et al. (2006) 81 | RT‐PCR | ALL (n = 61) and AML (n = 14) | The expression of gene abcb1 was statistically similar in patients with relapse and patients who responded to treatment |
| Huh et al. (2006) 82 | Nested RT‐PCR | ALL (n = 32) and AML (n = 39) | The expression of ABCB1, ABCC1, and LRP influenced full remission and the survival rate in AL patients, especially ABCB1 and LRP |
| Styczynski et al. (2007) 83 | Flow cytometry | Initial ALL (n = 527), relapse ALL (n = 104), initial AML (n = 133), and relapse AML (n = 23) | The expression of ABCB1, ABCC1, and LRP represented an adverse prognostic factor with relevance in de novo ALL cases |
| Yasunami et al. (2007) 64 | Flow cytometry and real‐time RT‐PCR | ALL‐T (n = 11) | Among the evaluation of proteins ABCB1, ABCC1, and LRP, only LRP showed increased expression and function |
| Fedasenka et al. (2008) 84 | Real‐time RT‐PCR | Pre‐B ALL with differentiated responses to CT (n = 19) | The expression of ABCC1 and LRP did not have a direct relation with minimum residual disease |
| Figueiredo‐pontes et al. (2008) 85 | Flow cytometry | De novo AML CD34+ (n = 26) | The overexpression of ABCB1, ABCC1, and LRP in more immature leukemia cell strains influenced treatment failure |
| Grotel et al. (2008) 86 | Flow cytometry and real‐time RT‐PCR | ALL‐T (n = 72) | The expression of ABCB1, ABCC1, and LRP to diagnosis, in all cut‐offs adopted, did not negatively influence prognosis |
| Svirnovski et al. (2009) 87 | Flow cytometry and RT‐PCR | ALL (n = 65), relapse ALL (n = 42), AML (n = 53), and relapse AML (n = 16) | There was no significant difference between the expression of gene abcb1 and protein ABCB1 in patients with de novo and recently diagnosed AL |
| El‐Sharnouby et al. (2010) 88 | RT‐PCR | All (n = 34) | The expression of ABCC1 and LRP were associated with poorer outcomes and worse two‐year survival |
| Chauhan et al. (2012) 89 | Real‐time RT‐PCR | ALL (n = 40) and AML (n = 45) | High expression of ABCB1 in AML and ABCC1 in ALL was associated with poor response to induction chemotherapy |
| Scheiner et al. (2012) 90 | Flow cytometry | AML (n = 109) | ABCB1 expression did not show an impact on the response to remission induction therapy |
ALL, acute lymphoblastic leukemia; ALL‐T, acute lymphoblastic leukemia of lymphocytes T; Pre‐B ALL, acute lymphoblastic leukemia of pre‐B lymphocytes; AML, acute myeloid leukemia; CT, chemotherapy; RT‐PCR, reverse transcriptase–polymerase chain reaction.
However, many of these studies are controversial when regarding the relationship between the overexpression of these proteins with worse patient prognosis, emphasizing the need for elucidation of resistance mechanisms operated by them 78.
Nowadays, the resistance mechanism mediated by protein ABCB1 is the one that has been best established. Some studies, by evaluating ABCB1 expression in AL patients, showed that the correlation of this protein with patient prognosis has no clinical relevance 65, 77, 80, 81, 86, 87. On the other hand, Fujimaki and colleagues (2002) reported that the expression of proteins ABCB1 and ABCC1 is more relevantly related in the studies involving AML 73. According to Figueiredo‐Pontes and colleagues (2008), the heterogenicity of cells in AMLs and the increased expression of MDR transporters in most of the immature population of myeloid leukemia cells reinforces that MDR might be responsible for the failure in treating these neoplasms 85. However, these results must be interpreted with care, since in some studies the number of samples was small.
Other studies show that the isolated expression of protein LRP is determinant in the phenomenon of resistance against chemotherapy drugs in cell strains of ALL patients 64, 78. More specifically, Den Boer and colleagues (1998) suggest that LRP might contribute to MDR especially in pre‐B ALL in pediatric patients 67. Olson and colleagues (2005) did not find a correlation between LRP and the worst prognostic of ALL patients 80.
Another parameter often evaluated in the literature is the relationship between MDR proteins and the presence of CD34 antigen, an immature cell marker and an important factor in AL prognosis. Studies investigating AML patients showed the activity and expression of proteins ABCB1, ABCC1, and LRP were more relevant in CD34+ cells than in those with a negative phenotype for this marker 79, 85, 91. Grotel and colleagues (2008) showed that pediatric patients with LLA‐T CD34+ had shorter life expectancy 86. However, authors associated the short life expectancy of these patients with an increase in resistance protein activity, but not with an increased expression of genes abcb1, abcc1, and lrp. A possible explanation for this fact would be the existence of additional posttranscriptional regulatory mechanisms or the occurrence of posttranslational modifications necessary for protein activity.
The results in literature show that the methodology employed for the analysis of resistance proteins directly interferes on the interpretation of results. For example, while the study of Dhooge and colleagues (1999) showed by immunohistochemistry the existence of a strong correlation between the expression of protein ABCB1 and the adverse clinical course for ALL, Wutcher and colleagues (2000) used flow cytometry and obtained contrary results 74, 75. Fujimaki and colleagues (2002), in a study involving patients who were nonresponsive to the chemotherapy treatment, found higher levels of expression for gene abcb1, determined by reverse transcriptase–polymerase chain reaction (RT‐PCR), than of expression for protein ABCB1, determined by flow cytometry 73. This study shows that the results obtained by flow cytometry and RT‐PCR are not correlated in all cases. This is due both to the complexity of the mechanisms involved in translation of the functional protein and to the sensibility of the method and its standardization, since, according to some authors, the sensibility of RT‐PCR is higher for determining the MDR phenotype 64, 73.
As it can be seen, the influence of the MDR phenotype is controversial in AL prognosis. The contradictory results can be justified by use of different cut‐off values, existence of demographic variations among samples, involvement of other resistance mechanisms, and use of different analysis methodologies 78.
The use of methodologies that directly quantify the expression of the gene seems to be the most adequate for defining its participation in the resistance phenotype against chemotherapy drugs in oncohematologic diseases. However, RT‐PCR only evaluates the gene expression, while flow cytometry can bring up more information regarding AL prognosis as it allows for trials that evaluate the activity of MDR proteins and the phenotypic detection of additional markers typical of hematologic neoplasms.
Since the discovery of MDR proteins, investigations have been carried out to establish their role in the prognosis of hematological malignancies and discover drugs capable of antagonizing their role in chemoresistance. Although relative success was achieved in determining the biological role of MDR proteins 89, 90, 92, 93, little success has been obtained in demonstrating the benefits of their pharmacological modulation 93, 94, 95.
Among the possible target proteins related to the MDR phenotype, inhibitors of ABCB1 protein are the ones to have been most thoroughly investigated. Based on sequential refinements in the pharmacodynamic properties of ABCB1 competitive inhibitors, they are categorized into three generations of drugs 95. Studies that used cyclosporine A (CSA), a first‐generation ABCB1 inhibitor, showed a response and survival advantage for its use in relapsed and refractory AML 96. However, subsequent randomized trials of CSA or valdospar, a second‐generation ABCB1 inhibitor, failed to demonstrate an improvement in outcomes 94, 97. Zosuquidar, a third‐generation inhibitor, also demonstrated promising results at first 98, but Cripe and colleagues (2010) failed to demonstrate the same benefits from the addition of zosuquidar to standard induction chemotherapy 93. Despite the failure to find an ABCB1 inhibitor of proven clinical efficacy, studies that seek inhibitors of MDR proteins have not been completely abandoned, since it is impossible to ignore the connection between MDR proteins and therapy outcome, especially in AML patients 95.
In summary, MDR is a multifactorial phenomenon. The expression and activity profiles of ABCB1, ABCC1, and LRP, proteins capable of promoting the efflux of a variety of chemotherapeutic agents from the cell cytoplasm represent one of the greatest causes of failure in AL treatment. Although there is a consensus in reporting that the detection of these proteins, mainly ABCB1, is important in the AL prognosis, there is controversy in the literature regarding the methodology used for their detection.
CONFLICT OF INTEREST
The authors report no conflict of interest.
Grant sponsor: Coordenação de Aperfeiçoamento de Pessoal de Nível Superior‐CAPES.
REFERENCES
- 1. Swerdlow SH, Campo E, Harris NL, et al., editors. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues, fourth edition, Geneva: WHO Press; 2008. [Google Scholar]
- 2. Gol BJ, Zagozdzon R, Kaminski R, et al. Potentiated antitumor effectiveness of combined chemo‐immunotherapy with interleukin‐12 and 5‐fluorouracil of L1210 leukemia in vivo. Leukemia 2001;15(4):613–620. [DOI] [PubMed] [Google Scholar]
- 3. Filipits M. Mechanism of cancer: Multidrug resistance. Drug Discov Today 2004;1(2):229–234. [Google Scholar]
- 4. Larsen AK, Escargueil AE, Skladanowski A. Resistance mechanisms associated with altered intracellular distribution of anticancer agents. Pharmacol Ther 2000;85:217–229. [DOI] [PubMed] [Google Scholar]
- 5. Krishan A, Arya P. Monitoring of cellular resistance to cancer chemotherapy. Hematol Oncol Clin North Am 2002;16:357–372. [DOI] [PubMed] [Google Scholar]
- 6. Luqmani YA. Mechanisms of drug resistance in cancer chemotherapy. Med Princ Pract 2005;14(1):35–48. [DOI] [PubMed] [Google Scholar]
- 7. Van Der Deen M, De Vries EG, Timens W, Scheper RJ, Timmer‐Bosscha H, Postma DS. ATP‐binding cassette (ABC) transporters in normal and pathological lung. Respir Res 2005;6:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dean M, Rzhetsky A, Allikmets R. The human ATP‐binding cassete (ABC) transporter superfamily. Gen Res 2001;11:1156–1166. [DOI] [PubMed] [Google Scholar]
- 9. Juliano RL, Ling V. A surface glycoprotein modulating drug permeability in chinese hamster ovary cell mutants. Biochimica et Biophysica Acta 1976;455:152–162. [DOI] [PubMed] [Google Scholar]
- 10. Fojo AT, Whang‐Peng J, Gottesman MM, Pastan I. Amplification of DNA sequences in human multidrug‐resistance KB carcinoma cells. Proc Natl Acad Sci USA 1985;83(12):7661–7665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chen C, Chin JE, Ueda K, et al. Internal duplication and homology with bacterial transport proteins in the mdr1 (P‐Glycoprotein) gene from multidrug‐resistant human cells. Cell 1986;47(3):381–389. [DOI] [PubMed] [Google Scholar]
- 12. Ohtani H, Ikegawa T, Honda Y, et al. Effects of various methoxyflavones on vincristine uptake and multidrug resistance to vincristine in P‐Gp‐overexpressing K562/ADM cells. Pharm Res 2007;24(10):1936–1943. [DOI] [PubMed] [Google Scholar]
- 13. Shipley JL, Butera JN. Acute myelogenous leukemia. Exp Hematol 2009;37(6):649–658. [DOI] [PubMed] [Google Scholar]
- 14. Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: Role of ATP‐dependent transporters. Nat Rev Cancer 2002;2(1):48–58. [DOI] [PubMed] [Google Scholar]
- 15. Leslie EM, Deeley RG, Cole SPC. Multidrug resistance proteins: Role of P‐glycoprotein, MRP1, MRP2, and BCRP (ABCG2) in tissue defense. Toxicol Appl Pharmal 2005;204(3):216–237. [DOI] [PubMed] [Google Scholar]
- 16. Fromn MF. The influence of MDR1 polymorphisms on P‐glycoprotein expression and functions in humans. Adv Drug Deliv Rev 2002;54(10):1295–1310. [DOI] [PubMed] [Google Scholar]
- 17. Marzolini C, Paus E, Buclin T, Kim RB. Polimorphisms in human MDR1 (P‐glycoprotein) recent advances and clinical relevance. Clin Pharmacol Ther 2004;75(1):13–33. [DOI] [PubMed] [Google Scholar]
- 18. Jonhstone RW, Cretney E, Smyth MJ. P‐glycoprotein protects leukemia cells against caspase‐dependent, but not caspase‐independent cell death. Blood 1999;93(3):1075–1085. [PubMed] [Google Scholar]
- 19. Balcerkzac E, Panczyk M, Piaskowski S, Walczak GP, Salagacka A, Mirowski M. ABCB1/MDR1 gene polimorphisms as a prognostic factor in colorectal cancer. Int J Colorectal Dis 2010;25(10):1167–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Park SW, Lomri N, Simeoni LA, Fruehauf JP, Mechetner E. Analysis of P‐glycoprotein‐mediated membrane transport in human peripheral blood lymphocytes using the UIC2 shift assay. Citometry A 2003;53(2):67–78. [DOI] [PubMed] [Google Scholar]
- 21. Kourti M, Vavatsi N, Gombakis N, et al. Expression of multidrug resistance‐related protein 1 (MRP1), lung resistance protein (LRP), and breast cancer resistance protein (BCRP) genes and clinical outcome in childhood acute lymphoblastic leukemia. Int J Hematol 2007;86(2):166–173. [DOI] [PubMed] [Google Scholar]
- 22. Litman T, Druley TE, Stein WD, Bates SE. New understanding of multidrug resistance system, their properties and clinical significances. Cell Mol Life Sci 2001;58(7):931–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Higgins CF. Flip‐flop: The transmembrane translocation of lipids. Cell 1994;79(3):393–395. [DOI] [PubMed] [Google Scholar]
- 24. Dawson RJP, Locher KP. Structure of a bacterial multidrug ABC transporter. Nature 2006;443(7108):180–185. [DOI] [PubMed] [Google Scholar]
- 25. Gottesman MM. Mechanisms of cancer drug resistance. Annu Rev Med 2002;53:615–627. [DOI] [PubMed] [Google Scholar]
- 26. Maia RC, Rumjanek VM. Clinical significance of multidrug resistance in acute leukemias. Rev Assoc Med Bras 1996;42(1):101–108. [PubMed] [Google Scholar]
- 27. Pirker R, Wallner T, Geissler K. MDR‐1 gene expression and treatment outcome in acute myeloid leukemia. J Natl Cancer Inst 1991;83:708–712. [DOI] [PubMed] [Google Scholar]
- 28. Goasguen JE, Dossot JM, Fardel O. Expression of the multidrug resistance associated p‐glycoprotein (P‐170) in 59 cases of de novo acute lymphoblastic leukemia. Prognostic implications. Blood 1993;81:2394–2399. [PubMed] [Google Scholar]
- 29. Penson RT, Oliva E, Skates SJ, et al. Expression of multidrug resistance‐1 protein inversely correlates with paclitaxel response and survival in ovarian cancer patients: A study in serial samples. Ginecol Oncol 2004;93:98–106. [DOI] [PubMed] [Google Scholar]
- 30. Leonessa F, Clarke R. ATP binding cassete transporters and drug resistance in breast cancer. Endocr Relat Cancer 2003;10(1):43–73. [DOI] [PubMed] [Google Scholar]
- 31. Rosenberg MF, Mao Q, Hoszenburgi A, Ford RC, Deeley RG, Cole SPC. The structure of the multidrug resistance protein 1 (MRP1/ABCC1) crystallization and single‐particle analysis. J Bioll Chem 2001;278(19):16076–16082. [DOI] [PubMed] [Google Scholar]
- 32. Kruh GD, Zeng H, Rea PA, et al. MRP subfamily transporters and resistance to anticancer agents. J Bioenerg Biomembr 2001;33(6):493–501. [DOI] [PubMed] [Google Scholar]
- 33. McGrath T, Center MS. Adriamycin resistance in HL60 cells in the absence of detectable P‐glycoprotein. Biochem Biophys Res Commun 1987;145(3):1171–1176. [DOI] [PubMed] [Google Scholar]
- 34. Mirski SEL, Gerlach JH, Cole SPC. Multidrug resistance in a human small cell lung cancer cell line selected in adriamycin. Cancer Res 1987;47(10):2594–2598. [PubMed] [Google Scholar]
- 35. Versantvoort CHM, Broxterman HJ, Pinedo HM, et al. Energy‐dependent processes involved in reduced drug accumulation in multidrug‐resistant human lung cancer cell lines without P‐glycoprotein expression. Cancer Res 1992;52(1):17–23. [PubMed] [Google Scholar]
- 36. Cole SPC, Bhardwaj G, Gerlach JH, et al. Overexpression of a transporter gene in a multidrug‐resistant human lung cancer cell line. Science 1992;258(5088):1650–1654. [DOI] [PubMed] [Google Scholar]
- 37. Borst P, Evers R, Kool M, Wijhholds J. A family of drug transporters: The multidrug resistance‐associated proteins. J Natl Cancer Inst 2000;92(16):1295–1302. [DOI] [PubMed] [Google Scholar]
- 38. Grant CE, Valdimarsson G, Hipfner DR, Almquist KC, Cole SP, Deeley RG. Overexpression of multidrug resistance‐associated protein (MRP) increases resistance to natural product drugs. Cancer Res 1994;54(2):357–361. [PubMed] [Google Scholar]
- 39. Kast C, Gros P. Topology mapping of the amino‐terminal half of multidrug resistance‐associated protein by epitope insertion and immunofluorescence. J Bioll Chem 1997;272(42):26479–26487. [DOI] [PubMed] [Google Scholar]
- 40. Haimeur A, Conseil G, Deeley RG, Cole SP. Mutations of charged amino acids in or near the transmembrane helices of the second membrane spanning domain differentially affect the substrate specificity and transport activity of the multidrug resistance protein MRP1 (ABCC1). Mol Pharmac 2004;65(6):1375–1385. [DOI] [PubMed] [Google Scholar]
- 41. Hipfner DR, Deeley RG, Cole SPC. Structural, mechanistic and clinical aspects of MRP1. Biochim Biophys Acta 1999;1461(2):359–376. [DOI] [PubMed] [Google Scholar]
- 42. Cole SP, Deeley RG. Transport of glutathione and glutathione conjugates by MRP1. Trends Pharmacol Sci 2006;27(8):438–446. [DOI] [PubMed] [Google Scholar]
- 43. Rao VV, Dahlheimer JL, Bardgett ME, et al. Choroid plexus epithelial expression of MDR1 P glycoprotein and multidrug resistance‐associated protein contribute to the blood‐cerebrospinal‐fluid drug‐permeability barrier. Proc Natl Acad Sci USA 1999;96(7):3900–3905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. St‐Pierre MV, Serrano MA, Macias RIR, et al. Expression of members of the multidrug resistance protein family in human term placenta. Am J Physiol 2000;279(4):R1495–R1503. [DOI] [PubMed] [Google Scholar]
- 45. Mao Q, Deeley RG, Cole SPC. Functional reconstitution of substrate transport by purified multidrug resistance protein MRP1 (ABCC1) in phospholipid vesicles. J Bioll Chem 2000;275(44):34166–34172. [DOI] [PubMed] [Google Scholar]
- 46. Wong IL, Faichan K, Tsang KH, et al. Modulation of multidrug resistance protein 1 (MRP/ABCC1) – Mediated multidrug resistance by bivalent apigenin homodimers and there derivatives. J Med Chem 2009;52:5211–5322. [DOI] [PubMed] [Google Scholar]
- 47. Ballatori N, Hammong CL, Cunningham JB, Krance SM, Marchan R. Molecular mechanisms of reduced glutathione transport: Role of the MRP/CFTR/ABCC and OATP/SLC21A families of membrane proteins. Toxico Ap Pharma 2005;204(3):238–255. [DOI] [PubMed] [Google Scholar]
- 48. Ishikawa T, Bao JJ, Yamane Y, et al. Coordinated induction of MRP/GS‐X pump and gamma‐glutamylcysteine synthetase by heavy metals in human leukemia cells. J Biol Chem 1996;271(25):14981–14988. [DOI] [PubMed] [Google Scholar]
- 49. Kuo MT, Bao J, Furuichi M, et al. Frequent coexpression of MRP/GS‐X pump and gamma‐glutamylcysteine synthetase mRNA in drug‐resistant cells, untreated tumor cells, and normal mouse tissues. Biochem Pharmaco 1998;55(5):605–615. [DOI] [PubMed] [Google Scholar]
- 50. Berger W, Setinek U, Hollaus P, et al. Multidrug resistance markers P‐glycoprotein, multidrug resistance protein 1, and lung resistance protein in non‐small lung cancer: Prognostic implications. J Cancer Res Clin Onco 2005;131:355–363. [DOI] [PubMed] [Google Scholar]
- 51. Young LC, Campling BG, Cole SP, Deeley RG, Gerlach JH. Multidrug resistance proteins MRP3, MRP1, and MRP2 in lung cancer: Correlation of protein levels with drug response and messenger. Clin Cancer Res 2001;7:1798–1804. [PubMed] [Google Scholar]
- 52. Munoz M, Henderson M, Haberand M, Norris M. Role of the MRP1/ABCC1 multidrug transporter protein in cancer. Life 2007;59(12):752–757. [DOI] [PubMed] [Google Scholar]
- 53. Plasschaert SL, De Bont ES, Boezen M, et al. Expression of multidrug resistance‐associated proteins predicts prognosis in childhood and adult acute lymphoblastic leukemia. Clin Cancer Res 2005;11(24):8661–8668. [DOI] [PubMed] [Google Scholar]
- 54. Gottesman NM, Fojo T, Bates SE. Multidrug resistance in cancer: Role of ATP‐dependent transporters. Nature Rev Cancer 2002;2:48–58. [DOI] [PubMed] [Google Scholar]
- 55. Mahjoubi F, Golalipour M, Ghavamzadeh A, Alimoghaddam K. Expression of MRP1 gene in acute leukemia. São Paulo Med J 2008;126(3):172–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Deeley RG, Westlake C, Cole SP. Transmembrane transport of endo‐ and xenobiotics by mammalian ATP‐binding cassette multidrug resistance proteins. Physio Rev 2006;86(3):849–899. [DOI] [PubMed] [Google Scholar]
- 57. Meschini S, Marra M, Calcabrini A, et al. Role of the lung resistance‐related protein (LRP) in the drug sensitivity of cultured tumor cells. Toxicol in vitro 2002;16:389–398. [DOI] [PubMed] [Google Scholar]
- 58. Izquierdo MA, Scheffer GL, Flens MJ, Schroeijers AB, van der Valk P, Scheper RJ. Major vault protein LRP‐related multidrug resistance. Euro J Cancer 1996;32(6):979–984. [DOI] [PubMed] [Google Scholar]
- 59. Kolli S, Zito CI, Mossink MH, Wiemer EA, Bennett AM. The major vault protein is a novel substrate for the tyrosine phosphatase SHP‐2 and scaffold protein in epidermal growth factor signaling. J Biol Chem 2004;279:29374–29385. [DOI] [PubMed] [Google Scholar]
- 60. Kozlov G, Vavelyuk O, Minailiuc CO, Banville D, Gehring K, Ekiel I. Solution structure of a two‐repeat fragment of major vault protein. J Mol Biol 2006;356:444–452. [DOI] [PubMed] [Google Scholar]
- 61. Siva AC, Raval‐Fernandes S, Stephen AG, et al. Up‐regulation of vaults may be necessary but not sufficient for multidrug resistance. Internatl J Cancer 2001;92:195–202. [DOI] [PubMed] [Google Scholar]
- 62. Van Zon A, Mossink MH, Schoester M, Scheper RJ, Sonneveld P, Wiemer EA. Efflux kinetics and intracelular distribution of daunorubicin are not affected by major vault protein / lung resistance‐related protein (vault) expression. Cancer Res 2004;64:4887–4892. [DOI] [PubMed] [Google Scholar]
- 63. Swerts K, De Moerloose B, Dhooge C, Laureys G, Benoit Y, Philippe J. Prognostic significance of multidrug resistance‐related proteins in childhood acute lynphoblastic leukaemia. Euro J Cancer 2006;42:295–309. [DOI] [PubMed] [Google Scholar]
- 64. Yasunami T, Wang Y, Tsuji K, Takanashi M, Yamada Y, Motoji T. Multidrug resistance protein expression of adult T‐cell leukemia/lymphoma. Leukemia Res 2007;31:465–470. [DOI] [PubMed] [Google Scholar]
- 65. Sauerbrey A, Voigt A, Wittig S, Hafer R, Zinti F. Messenger RNA analysis of the multidrug resistance related protein (MRP1) and the lung resistance protein (LRP) in de novo and relapsed childhood acute lymphoblastic leukemia. Leuk Lymphoma 2002;43:875–879. [DOI] [PubMed] [Google Scholar]
- 66. Filipts M. Mechanism of cancer: Multidrug resistance. Drug Discov Today 2004;1(2):229–234. [Google Scholar]
- 67. Den Boer ML, Pieters R, Kazemier KM, et al. Relationship between major vault protein/lung resistance protein, multidrug resistance‐associated protein, P‐glycoprotein expression, and drug resistance in childhood leukemia. Blood 1998;91:2092–2098. [PubMed] [Google Scholar]
- 68. Ramakers‐Vanwoerden NL, Beverloo HB, Veerrman AJP, et al. In vitro drug‐resistance profile in infant acute lymphoblastic leukemia in relation to age, MLL rearrangements and immunophenotype. Leukemia 2004;18:521–529. [DOI] [PubMed] [Google Scholar]
- 69. Ogretmen B, Barredo JC, Safa AR. Increased expression of lung resistance‐related protein and multidrug resistance‐associated protein messenger RNA in childhood acute lymphoblastic leukemia. J Ped Hematol Oncol 2000;22:45–49. [DOI] [PubMed] [Google Scholar]
- 70. Krishan A, Arya P. Monitoring of cellular resistance to cancer chemotherapy. Hematol Oncol Clin North Am 2002;16:357–372. [DOI] [PubMed] [Google Scholar]
- 71. Scherbakova EA, Stromskaya TP, Rybalkina EYU, Kalita OV, Stavrovskaya AA. Role of the PTEN protein in the multidrug resistance of prostate cancer cells. Cell Mol Biol 2008;42(3):430–435. [PubMed] [Google Scholar]
- 72. Steelman LS, Abrams SL, Whelan J, et al. Contributions of the Raf/MEK/ERK, PI3K/PTEN/AKT/mTOR and Jak/STAT pathways to leukemia signaling and apoptotic pathways and leukemia. Leukemia 2008;22:686–707. [DOI] [PubMed] [Google Scholar]
- 73. Fujimaki S, Funato T, Harigae H. Quantitative analysis of a MDR1 transcript for prediction of drug resistance in acute leukemia. Clin Chem 2002;48(6):811–817. [PubMed] [Google Scholar]
- 74. Dhooge C, De Moerloose B, Laureys G, et al. P‐glycoprotein is an independent prognostic factor predicting relapse in childhood acute lymphoblastic leukaemia: Results of a 6‐year prospective study. Br J Haematol 1999;105(3):676–683. [DOI] [PubMed] [Google Scholar]
- 75. Wuchter C, Leonid K, Ruppert V, et al. Clinical significance of P‐glycoprotein expression and function for response to induction chemotherapy, relapse rate and overall survival in acute leukemia. Haematologica 2000;85(7):711–721. [PubMed] [Google Scholar]
- 76. Schaich M, Soucek S, Thiede C, Ehninger G, Illmer T. MDR1 and MRP1 gene expression are independent predictors for treatment outcome in adult acute myeloid leukaemia. Br J Haematol 2004;128:324–332. [DOI] [PubMed] [Google Scholar]
- 77. Suarez L, Vidriales MV, Garcia‐Laraña J, et al. CD34 + Cells from acute myeloid leukemia, myelodysplastic syndromes and normal bone marrow display different apoptosis and drug resistance‐associated phenotypes. Clin Cancer Res 2004;10:7599–7606. [DOI] [PubMed] [Google Scholar]
- 78. Valera ET, Scrideli CA, Queiroz RGP, Mari BMO, Tone LG. Multiple drug resistance protein (MDR‐1), multidrug resistancerelated protein (MRP) and lung resistance protein (LRP) gene expression in childhood acute lymphoblastic leukemia. São Paulo Med J 2004;122(4):166–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Benderra S, Faussat AM, Sayada L, et al. MRP3, and P‐glycoprotein in activities are prognostic factors in adult acute myeloid leukemia. Clin Cancer Res 2005;11(21):7764–7772. [DOI] [PubMed] [Google Scholar]
- 80. Olson DP, Taylor BJ, La M, Sather H, Reaman GH, Ivy P. The prognostic significance of P‐glycoprotein, multidrug resistancerelated protein 1 and lung resistance protein in pediatric acute lymphoblastic leukemia: A retrospective study of 295 newly diagnosed patients by the Children's Oncology Group. Leuk Lymphoma 2005;45(5):681–691. [DOI] [PubMed] [Google Scholar]
- 81. Anuchapreeda S, Thanarattanakorn P, Sittipreechacharn S, Tima S, Chanarat P, Limtrakul P. Inhibitory effect of curcumin on gene expression in patient leukemic cells. Arch Pharm Res 2006;29(10):866–873. [DOI] [PubMed] [Google Scholar]
- 82. Huh HJ, Park CJ, Jang S, et al. Prognostic significance of multidrug resistance gene 1 (MDR1), multidrug resistance‐related protein (MRP) and lung resistance protein (LRP) mRNA expression in acute leukemia. J Korean Med Sci 2006;21:253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Styczynski J, Wysocki M, Debski R, et al. Predictive value of multidrug resistance proteins and cellular drug resistance in childhood relapsed acute lymphoblastic leukemia. J Cancer Res Clin Oncol 2007;133:875–893. [DOI] [PubMed] [Google Scholar]
- 84. Fedasenka UU, Shman TV, Savitski VP, Belevsev MV. Expression of MDR1, LRP, BCRP and BCL‐2 genes at diagnosis of childhood ALL: Comparison with mrd status after Induction therapy. Expl Oncol 2008;30(3):248–252. [PubMed] [Google Scholar]
- 85. Figueiredo‐Pontes LF, Pintão MC, Oliveira LCO, et al. Determination of P‐glycoprotein, MDR‐related protein 1, breast cancer resistance protein, and lung‐resistance protein expression in leukemic stem cells of acute myeloid leukemia. Cytometry Part B 2008;74B:163–168. [DOI] [PubMed] [Google Scholar]
- 86. Grotel MV, Van Den Heuvel‐Eibrink MM, Van Wering ER, et al. CD34 expression is associated with poor survival in pediatric T‐cell acute lymphoblastic leukemia. Ped Blood Cancer 2008;51:737–740. [DOI] [PubMed] [Google Scholar]
- 87. Svirnovski A, Shman TV, Serhiyenka T, et al. ABCB1 and ABCG2 proteins, the functional activity and gene expression concert with drug sensitivity of leukemia cells. Hematology 2009;14(4):204–212. [DOI] [PubMed] [Google Scholar]
- 88. El‐Sharnouby JA, Abou El‐Enein AM, El Ghannam DM, et al. Expression of lung resistance protein and multidrug resistance‐related protein (MRP1) in pediatric acute lymphoblastic leukemia. J Oncol Pharm Pract 2010;16(3):179–188. [DOI] [PubMed] [Google Scholar]
- 89. Chauhan PS, Bhushan B, Singh LC, et al. Expression of genes related to multiple drug resistance and apoptosis in acute leukemia: Response to induction chemotherapy. Exp Mol Pathol 2012;92(1):44–49. [DOI] [PubMed] [Google Scholar]
- 90. Scheiner MAM, Vasconcelos FC, Matta RR, Figueira RDB, Maia RC. ABCB1 genetic variation and P‐glycoprotein expression/activity in a cohort of Brazilian acute myeloid leukemia patients. J Cancer Clin Oncol 2012;138(6):959–969. [DOI] [PubMed] [Google Scholar]
- 91. Raaikimakers MHGP, Grouw EPLM, Reijden BAV, Witte TJM, Jansen JH, Raymakers RAP. ABCB1 modulation does not circumvent drug extrusion from primitive leukemic progenitor cells and may preferentially target residual normal cells in acute myelogenous leukemia. Clin Cancer Res 2006;12(11):3452–3458. [DOI] [PubMed] [Google Scholar]
- 92. Leith CP, Kopecky KJ, Chen IM, et al. Frequency and clinical significance of the expression of the multidrug resistance proteins MDR1/P‐glycoprotein, MRP1, and LRP in acute myeloid leukemia: A Southwest Oncology Group Study. Blood 1999;94:1086–1099. [PubMed] [Google Scholar]
- 93. Cripe LD, Uno H, Paietta EM, et al. Zosuquidar, a novel modulator of P‐glycoprotein, does not improve the outcome of older patients with newly diagnosed acute myeloid leukemia: A randomized, placebo‐controlled trial of the Eastern Cooperative Oncology Group 3999. Blood 2010;116:4077–4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Kolitz JE, George SL, Marcucci G, et al. P‐glycoprotein inhibition using valspodar (PSC‐833) does not improve outcomes for patients younger than age 60 years with newly diagnosed acute myeloid leukemia: Cancer and Leukemia Group B study 19808. Blood 2010;116:1413–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Shaffer BC, Gillet JP, Patel C, Baer MR, Bates SE, Gottesman MM. Drug resistance: Still a daunting challenge to the successful treatment of AML. Drug Resist Updat 2012;15(1–2):62–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. List AF, Kopecky KJ, Willman CL, et al. Benefit of cyclosporine modulation of drug resistance in patients with poor‐risk acute myeloid leukemia: A Southwest Oncology Group study. Blood 2001;98(12):3212–3220. [DOI] [PubMed] [Google Scholar]
- 97. Liu Yin JA, Wheatley K, Rees JK, Burnett AK. Comparison of “sequential” versus “standard” chemotherapy as re‐induction treatment, with or without cyclosporine, in refractory/relapsed acute myeloid leukaemia (AML): Results of the UK Medical Research Council AML‐R trial. Br J Haematol 2001;113(3):713–726. [DOI] [PubMed] [Google Scholar]
- 98. Gerrard G, Payne E, Baker R, et al. Clinical effects and P‐glycoprotein inhibition in patients with acute myeloid leukemia treated with zosuquidar trihydrochloride, daunorubicin and cytarabine. Haematologica 2004;89:782–790. [PubMed] [Google Scholar]
- 99. Sharom FJ. ABC multidrug transporters: Structure, function and role in chemoresistance. Pharmacogenomisc 2008;9(1):105–127. [DOI] [PubMed] [Google Scholar]


