Abstract
Aim
To develop a new high‐performance liquid chromatography (HPLC) method for simultaneous determination of the combined drugs (ceftriaxone sodium, metronidazole, and levofloxacin) in human urine.
Methods
Ceftriaxone sodium, metronidazole, and levofloxacin were separated on a Kromasil 100–5 C18 (250 mm × 4.6 mm, 5 μm, AKZO NOBEL, Bohus, Sweden) analytical column, using the mobile phase consisted of 1.5 mM KH2PO4 (pH 4.5) with 0.0125% triethylamine–methnol (70:30, v/v). Ceftriaxone sodium, metronidazole, and levofloxacin were detected by a photodiode‐array detector at 247, 320, 292 nm, respectively.
Results
Under optimal conditions, the effective separation of ceftriaxone sodium, metronidazole, and levofloxacin was achieved. A good linearity with the correlation coefficients more than 0.999 was demonstrated. The detection limits of ceftriaxone sodium, metronidazole, and levofloxacin were 0.05, 0.01, and 0.25 μg/ml, respectively, and the average recoveries in human urine were in the range from 97.73 to 100.7% with the average relative standard deviation (RSD) in the range of 2.5% and 3.0%.
Conclusion
The proposed method was sensitive, accurate, and rapid. This work may provide a reference for clinical rational drug use and methodology for the pharmacokinetics study of the combined drugs.
Keywords: HPLC, ceftriaxone sodium, metronidazole, levofloxacin, human urine
INTRODUCTION
Ceftriaxone is a semisynthetic third‐generation cephalosporin antibiotic that is generally less active in vitro against susceptible strains of staphylococci than first‐generation cephalosporins 1. Metronidazole is an anti‐biotic, especially effective against anaerobic infections 2. Levofloxacin is the levo‐enantiomer of ofloxacin, a member of the fluoroquinolones, and a class of synthetic antimicrobial agents. It has been found to be involved in inhibition of bacterial topoisomerase IV and DNA gyrase that are important enzymes required for DNA replication, transcription, repair, and recombination, which was widely used in the treatment of respiratory tract and urinary tract infections 3. Levofloxacin is frequently combined with ceftriaxone and/or metronidazole with favorable synergistic sterilization action. Levofloxacin–ceftriaxone 4, 5, levofloxacin–metronidazole 6, metronidazole–ceftriaxone 7, 8, ciprofloxacin–metronidazole–ceftriaxone 9 have been used in clinical treatment test. The advantages of these drugs combination result in enhanced pharmacodynamics, which may improve patient outcomes against certain bacterial pathogens, especially penicillin‐resistant Streptococcus pneumoniae 8, 9, 10. These drugs can be administered perorally or intravenously, and are excreted mainly into the urine. In consequence, a great deal of attention has been paid to its determination in various biological fluids.
A series of analytical techniques for the determination of ceftriaxone 1, metronidazole 11, and levofloxacin 12 were reviewed. Among them, high‐performance liquid chromatography (HPLC) was widely used for the determination of the three drugs, such as ceftriaxone in plasma 13, 14, metronidazole in serum 15, 16, 17, and levofloxacin in biological samples 18, 19, 20, 21, 22. Some researchers investigated the compatible possibility of levofloxacin–ceftriaxone 23, levofloxacin–metronidazole 24, and metronidazole–ceftriaxone 25, 26. There is no report for simultaneously determining the three drugs in biological fluid. The development of analytical method for simultaneous determination of the three drugs in biological fluid is an important work for clinical rational drug use of the combined drugs and methodology for the pharmacokinetics studies.
The purpose of this work is to develop a new method for simultaneously determining the combined drugs in human urine. In this report, a simple, sensitive, and precise HPLC method is developed. It is useful and feasible for the determination of the combined drugs in human urine in most hospital laboratories.
EXPERIMENTAL
Instrumentation
The chromatographic system consisted of a Shimadzu HPLC system equipped with an LC‐10A Multisolvent Delivery System, a DGU‐12A online degasser, an SCL‐10Avp gradient controller, a CTO‐10Avp column thermostat, and a multi‐wavelength SPD‐M10Avp photodiode‐array detector (DAD) covering the range 190–800 nm, which was interfaced to a computer for data acquisition using a CLASS‐VP workstation (Shimadzu, Kyoto, Japan). A Kromasil 100–5 C18 column (250 × 4.6 mm, 5 μm) and a six‐port valve with a 20 μl sample loop injector were also used. A centrifuge TGL‐16M (Xiangyi centrifuge Co., Hunan, China), ultrasonic cleaner (Ultrasonic Instrument Co., Kunshan, China), and PHS‐3C pH meter (Shanghai Precision & Scientific Instrument Co., Shanghai, China) were used in sample treatment.
Reagents and Chemicals
Levofloxacin, metronidazole, and ceftriaxone sodium were purchased from National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). The methanol was filtered through a 0.22‐μm microporous membrane of polyvinylidene fluoride before use.
Potassium dihydrogen phosphate, phosphoric acid, and triethylamine were of analytical grade. Purified water was prepared by an XGJ‐30 highly pure water machine (Yongcheng purification Science & Technology Co. Ltd., Beijing, China). Stock solution of ceftriaxone sodium (500 μg/ml), levofloxacin (500 μg/ml), and metronidazole (200 μg/ml) was prepared in water, and stored in refrigerator at −4°C. A series of standard solutions were prepared by diluting the stock solution with mobile phase, their concentrations were 0.98, 1.96, 3.91, 7.81, 15.62, 62.5, and 125 μg/ml for levofloxacin; 0.24, 1.95, 3.91, 7.81, 15.62, 62.5, and 250 μg/ml for ceftriaxone sodium; and 0.04, 0.78, 3.13, 6.25, 12.5, 25, and 100 μg/ml for metronidazole. All standard solutions and buffer solutions were prepared weekly, and filtered through 0.22‐μm cellulose acetate filters (Xinya Purification Material Factory, Shanghai, China) prior to injection.
Sample Treatment
The patient urine sample was provided by Hebei University Hospital. Since the patient urine sample contains slightly urine proteins, to achieve better baseline and recovery the proteins pretreatment was necessary. A 6 ml of acetonitrile was added in 2 ml of the urine sample or spiked urine sample to remove the protein. The mixture was shaken for 10 min, and then centrifuged at 13,420 × g force for 10 min, and 7.5 ml supernatant was obtained. The supernatant was diluted with a mobile phase to make analyte concentration within linear ranges and avoid interference. The diluted supernatant was filtered through 0.22‐μm microporous membrane of polyvinylidene fluoride. The urine samples and urine samples spiked at different concentrations were treated by same procedure above. The filtrate was applied for HPLC analysis and recovery test.
HPLC Analysis
The separation of levofloxacin, metronidazole, and ceftriaxone sodium was carried out using a Kromasil 100–5 C18 analytical column (250 × 4.6 mm id, 5 μm, AKZO NOBEL, Bohus, Sweden), and a six‐port valve with a 20 μl sample loop injector was used. A mobile phase composed of 1.5 mM potassium dihydrogen phosphate (adjust the pH to 4.5 with phosphoric acid) with 0.0125% triethylamine–methnol (70:30, v/v). The flow rate of the mobile phase was set at 1.0 ml/min. A 20 μl volume of sample solution was injected in the column. The elutions were monitored by a photodiode‐array detector at 247 nm for ceftriaxone sodium, at 320 nm for metronidazole, and 292 nm for levofloxacin. The linear equations for the relationship between the peak areas of analytes and their concentration were determined by least‐squares method. Quantification was carried out by using standard curve calibration based on peak area toward concentration.
RESULTS AND DISCUSSION
Optimization of Buffer pH
The pH value of running buffer influenced mainly the resolution and the apparent mobility of the target analytes. Generally, for compounds with lower pKa values, the dissociation ratio is higher and acidity is stronger. The pH of buffer influences ionization of the analytes. The mobile phase constituted of 1.5 mM KH2PO4 with 0.0125% triethylamine–methanol (70:30, v/v) was adjusted with phosphoric acid to different pH. The effect of pH on the peak of the analytes is shown in Figure 1.
Figure 1.
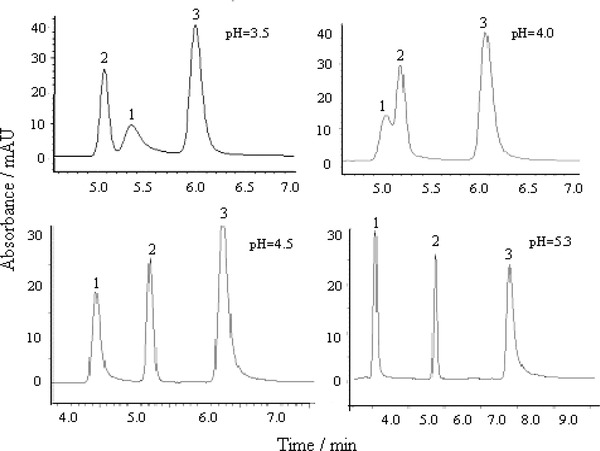
Effect of different pH on the separation of the three drugs. (1) Ceftriaxone sodium, 10 μg/ml; (2) metronidazole, 2 μg/ml; (3) levofloxacin, 5 μg/ml.
It has been found that when pH was 3.5 and 4.0, ceftriaxone sodium and metronidazole could not be separated, and when pH was 4.5 and 5.3, the three compounds can be separated with the resolution of greater than 2. Some effects of pH on retention time of the three compounds with different degrees were observed. Metronidazole is a basic compound with pKa value of 2.62 27, the effect of pH in the range of 3.5–5.3 on its retention time is weak. Ceftriaxone is a basic compound with pKa value of 1.72, 3.15, and 4.34 28, its retention time decreased with the increase of pH because ceftriaxone molecule contains the ionizable groups (–OH, –COOH, –NH2). Levofloxacin is a zwitterion at physiological pH, possessing a carboxylic group with pKa = 5.5, a piperazinyl group with pKa = 8.0, and another proton accepting function with pKa = 6.8 ± 0.3 29. When pH increases from 3.5 to 4.5, its retention time increased from 6.0 to 6.25 min, but when pH increases to 5.3 its retention time increased to 7.4 min. The buffer of pH 4.5 was used with high sensitivity and resolution, so it was selected in further tests.
Effects of Buffer Concentration
The effects of buffer concentration from 5 to 30 mM on retention time and the peak area were further tested. It was observed that with increasing concentration of buffer, the peak area and resolution for the three analytes increased slightly, and retention time was increased. Using high‐concentration buffer, the possibility of barrage jamming to the analytical column would be increased 30. So, 15 mM buffer was selected for further tests.
Selection of Proportions of Methanol in the Mobile Phase
The effects of different proportions of methanol in the mobile phase on the retention time and the peak area of three analytes were investigated, as shown in Figure 2.
Figure 2.

Effect of different proportions of methanol in the mobile phase. (1) ceftriaxone sodium, 10 μg/ml; (2) metronidazole, 2 μg/ml; (3) levofloxacin, 5 μg/ml; buffer concentration, 1.5 mM, pH 4.5; triethylamine, 0.0125%.
With the proportion of methanol varying from 20 to 35%, the retention time for three analytes decreased obviously, from 12.2 to 3.4 min for ceftriaxone sodium, from 7.8 to 4.5 min for metronidazole, and from 17.8 to 4.5 min for levofloxacin. Otherwise, both peak area and peak height increased with increasing proportion of methanol, but when methanol proportion reaches up to 35%, the peaks of metronidazole and levofloxacin could not be separated. The proportion of methanol in the mobile phase was selected to be 30%, with higher sensitivity and resolution for the three analytes.
Effect of Triethylamine
Using a mobile phase of 1.5 mM potassium dihydrogen phosphate buffer (pH 4.5) and methanol (70:30, v/v), peak tails for levofloxacin, ceftriaxone sodium, and metronidazole were observed because the three analytes with nitrogenous group reacts with unreacted silanol on 18 alkyl bonded silica gel column, resulting in spreading effect of chromatographic peak. Addition of triethylamine in mobile phase could improve peak face because the hydrophobic amine preferentially adsorbed on the unreacted silanol on the silica carrier, but with increasing dosage of diethylamide the retention time extended and baseline instability was observed. Use of 0.0125% triethylamine overcame peak tails and improved separation effect.
Specificity
Under the following optimal conditions—a mobile phase of 1.5 mM potassium dihydrogen phosphate buffer (pH 4.5) and methanol (70:30, v/v), and flow rate of 1.0 ml/min—the chromatograms of urine samples before and after the administration for a patient, and the urine sample after the administration spiked with 0.04 μg/ml of metronidazole were observed. The representational chromatograms are shown in Figure 3.
Figure 3.
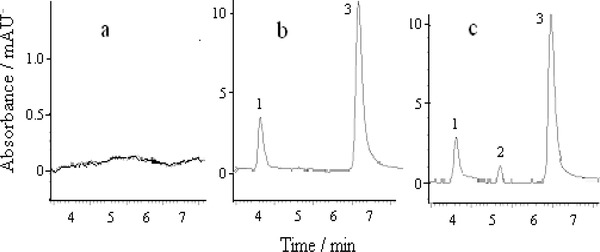
Chromatograms of patient urine samples. (A) Urine sample before the administration. (B) Urine sample after the administration of 1 g ceftriaxone sodium and 0.1 g levofloxacin. (C) b + 0.04 μg/ml metronidazole; (1) ceftriaxone sodium; (2) metronidazole; (3) levofloxacin.
The effective baseline separation of the analytes was observed in different samples. No interfering peaks were observed at the retention times of three analytes. Quantification could be carried out by using standard curve calibration.
Precision
The stability of combined solution of metronidazole–ceftriaxone 7, ceftriaxone sodium–levofloxacin 22, and metronidazole–levofloxacin 23 was investigated. The combined solution is clear, and its pH retains unchanged at room temperature within 8 h. In this work, precision was determined as both repeatability and intermediate precision, in accordance with ICH recommendations. Repeatability was determined as intraday variation and intermediate precision was determined by measurement of interday variation. The results of the repeatability and intermediate precision experiments for analyzing the three analytes in blank urines spiked at three different concentrations are shown in Table 1.
Table 1.
Precision for the Determination of the Three Drugs in Spiked Urine Sample
| Repeatability | Intermediate | |||
|---|---|---|---|---|
| (intra day | precision | |||
| Combined | Concentration | precision)a | (inter day)a | |
| solution | Drug | (μg/ml) | RSD (%) | RSD (%) |
| 1 | Ceftriaxone sodium | 0.98 | 1.55 | 4.90 |
| Metronidazole | 0.39 | 1.55 | 1.92 | |
| Levofloxacin | 0.98 | 1.83 | 3.79 | |
| 2 | Ceftriaxone sodium | 7.81 | 1.15 | 4.59 |
| Metronidazole | 3.13 | 0.19 | 2.81 | |
| Levofloxacin | 7.81 | 1.41 | 4.60 | |
| 3 | Ceftriaxone sodium | 62.50 | 0.44 | 1.84 |
| Metronidazole | 25.00 | 0.42 | 0.89 | |
| Levofloxacin | 62.50 | 0.77 | 0.52 |
Indicates mean of six determinations.
The data in Table 1 show that the average intraday variability (relative standard deviation [RSD]) of peak area obtained for six determinations at three levels was in the range of 0.44–1.55% for ceftriaxone sodium, 0.42–1.55% for metronidazole, and 0.77–1.83% for levofloxacin. The variabilities (RSDs) are less than 2%, it was shown that that the variation of the results within the same day was analyzed and statistically validated. The combined solutions of the three drugs stored in a refrigerator at −4°C was determined within 3 days, the interday RSD of peak area was 1.84–4.90% for ceftriaxone sodium, 0.89–2.81% for metronidazole, and 0.52–4.6% for levofloxacin. Among them, for a combined solution of the three drugs with high concentration the intermediate precisions (RSD) are less than 2%, while for the others with low concentration the intermediate precisions (RSD) are more than 2%, which is possible due to the lack of sufficient stability to the combined solutions within 3 days.
Linearity and Detection Limit
Linearity of the method was evaluated by determining the three drugs in seven concentration points. The calibration curves of the peak area toward analyte concentration were constructed by least‐squares linear regression. The equations of calibration curves obtained based on three parallel measurements are listed in Table 2. It can be seen that the linearity is very satisfactory.
Table 2.
Analytical Performance of the Method
| Analyte | Linearity regression equation | Correlation coefficient | Linearity range (μg/ml) | LOD (μg/ml) | LOQ (μg/ml) |
|---|---|---|---|---|---|
| Ceftriaxone sodium | A = 2.83 × 104 C – 3.02 × 104 | 0.99986 | 0.24–250 | 0.05 | 0.17 |
| Metronidazole | A = 7.04 × 104 C + 2.31 × 104 | 0.99967 | 0.04–100 | 0.01 | 0.03 |
| Levofloxacin | A = 9.63 × 104 C – 8.75 × 104 | 0.99989 | 0.98–125 | 0.25 | 0.83 |
The limit of detection (LOD) was considered the minimum analyte concentration yielding an S/N = 3, and the limit of quantification (LOQ) was considered the minimum analyte concentration yielding an S/N = 10. The LOD values of the method were 0.05, 0.01, and 0.25 μg/ml for ceftriaxone sodium, metronidazole, and levofloxacin, respectively, and their LOQ values were 0.17, 0.03, and 0.83 μg/ml. The LOD value of this method is lower than that of cephalosporin by HPLC 13 and capillary electrophoresis 31, metronidazole by HPLC 16, and levofloxacin by HPLC 18, 19, 22.
Application
The three drugs can be administered perorally or intravenously, and they are excreted mainly into the urine. Based on these information and the LODs of this method, it was suggested that the effective detection for the three drugs in patient urine after the administration could be achieved. However, there is no a compound preparation of the three drugs, it is difficult to take patient urine sample after the administration with all the three drugs. Patient urine was obtained at 12 h after the administration of 1 g ceftriaxone sodium and 0.1 g levofloxacin. The urine collected before dosing was employed as a blank. The recovery test of the assays for the three drugs was determined by adding known amounts of these drugs to the urine samples. The results are listed in Table 3.
Table 3.
Determination of Ceftriaxone Sodium, Metronidazole and Levofloxacin in a Patient Urine Sample
| RSD | |||||
|---|---|---|---|---|---|
| Concentrationa | Added | Detected | Recovery | (n = 6) | |
| Drug | (μg/ml) | (μg/ml) | (μg/ml) | (%) | (%) |
| Ceftriaxone | 2.18 | 1.74 | 4.32 | 110 | 1.9 |
| sodium | 2.18 | 4.29 | 98.4 | 3.4 | |
| 2.62 | 4.44 | 92.5 | 3.8 | ||
| Metronidazole | N | 0.04 | 0.04 | 100 | 5.4 |
| 20.00 | 20.31 | 102 | 1.2 | ||
| 75.00 | 75.16 | 100 | 1.0 | ||
| Levofloxacin | 2.23 | 1.78 | 3.85 | 96.0 | 2.6 |
| 2.23 | 4.39 | 98.4 | 3.3 | ||
| 2.68 | 4.85 | 98.8 | 2.7 |
For urine sample diluted by 37.5‐fold. N, not detected.
The concentration of the two drugs in the diluted urine sample was 2.18 and 2.23 μg/ml for ceftriaxone sodium and levofloxacin, respectively. Considering dilution factor (urine sample diluted by 37.5‐fold), the concentration of ceftriaxone sodium and levofloxacin in the patient urine was 81.75 and 83.63 μg/ml, respectively.
The data showed that the average recovery was 100.3, 100.7, and 97.73% for ceftriaxone sodium, metronidazole, and levofloxacin, respectively, and their average RSD was 3.0, 2.5, and 2.9%. The high percentage of recovery shows that the method can be used for the determination of analyzed drugs in human urine samples.
CONCLUSION
A new method for effective separation and simultaneous determination of ceftriaxone sodium, metronidazole, and levofloxacin by HPLC is proposed in the first time. The present method offers advantages of speediness, simplicity, sensitivity, and accuracy, and is applicable to the selective or simultaneous determination of the studied drugs. The proposed method can be applied for routine determination of these drugs in human urine samples in clinical studies, and may provide a reference for clinical rational drug use of the combined drugs and methodology for the pharmacokinetics studies by standard equipment.
ACKNOWLEDGMENTS
This work was supported by the Science Foundation Education Office of Hebei Province (B2008000583).
Grant sponsor: Science Foundation Education Office of Hebei Province; Grant number: B2008000583.
REFERENCES
- 1. Owens HM, Dash AK. Ceftriaxone sodium: Comprehensive profile. Profiles Drug Subst Excip Relat Methodol 2003;30:21–57. [DOI] [PubMed] [Google Scholar]
- 2. Zhou W‐C, Zhang X‐P. New progress of fluoroquinolone research. Chin J New Drugs 2000;9(10):667–669. [Google Scholar]
- 3. Wimer SM, Schoonover L, Garrison MW. Levofloxacin: A therapeutic review. Clin Ther 1998;20:1049–1070. [DOI] [PubMed] [Google Scholar]
- 4. Norrby SR, Petermann W, Willcox PA, et al. Comparative study of levofloxacin and ceftriaxone in the treatment of hospitalized patients with pneumonia. Scand J Infect Dis 1998;30(4):397–404. [DOI] [PubMed] [Google Scholar]
- 5. Flatz L, Cottagnoud M, Kühn F, et al. Ceftriaxone acts synergistically with levofloxacin in experimental meningitis and reduces levofloxacin‐induced resistance in penicillin‐resistant pneumococci. J Antimicrob Chemoth 2004;53:305–310. [DOI] [PubMed] [Google Scholar]
- 6. Matsumoto Y, Miki I, Aoyama N, et al. Levofloxacin‐versus metronidazole‐based rescue therapy for H. pylori infection in Japan. Digest Liver Dis 2005;37:821–825. [DOI] [PubMed] [Google Scholar]
- 7. Zhang Y‐C, Jia J‐F. The compatible stability of ceftriaxone sodium for injection and metronidazole injection. Chin J Hosp Pharm 1996;16(8):357–358. [Google Scholar]
- 8. Solomkin J, Zhao YP, Ma EL, et al. Moxifloxacin is non‐inferior to combination therapy with ceftriaxone plus metronidazole in patients with community‐origin complicated intra‐abdominal infections. Int J Antimicrob Ag 2009;34:439–445. [DOI] [PubMed] [Google Scholar]
- 9. Starakis I, Karravias D, Asimakopoulos C, et al. Results of a prospective, randomized, double blind comparison of the efficacy and the safety of sequential ciprofloxacin (intravenous/oral)+metronidazole (intravenous/oral) with ceftriaxone (intravenous)+metronidazole (intravenous/oral) for the treatment of intra‐abdominal infections. Int J Antimicrob Ag 2003;21:49–57. [DOI] [PubMed] [Google Scholar]
- 10. Sun H‐W, Wei L‐J, Zuo Y‐L, et al. Effective separation and simultaneous detection of gatifloxacin, aminomethylbenzoic acid, cefazolin and cefminox in human urine by capillary zone electrophoresis. J Iran Chem Soc 2011;8:1043–1051. [Google Scholar]
- 11. Wang J‐C, Wang D‐J, Yang Z‐J, et al. Research progress on analytical methods of nitroimidazoles residues. Chin J Vet Drug 2007;41(8):31–33. [Google Scholar]
- 12. Wang W‐J, Xie Y‐C, Liu Q, et al. Recent development in related substances of levofloxacin. Chin J Antibiot 2010;35(11):820–525. [Google Scholar]
- 13. McWhinneya BC, Wallis SC, Hillister T, et al. Analysis of 12 beta‐lactam antibiotics in human plasma by HPLC with ultraviolet detection. J Chromatogr B 2010;878:2039–2043. [DOI] [PubMed] [Google Scholar]
- 14. Granich GG, Krogstad DJ. Ion pair high‐performance liquid chromatographic assay for ceftriaxone. Antimicrob Agents Chemother 1987;31:385–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Galmier MJ, Frasey AM, Bastide M, et al. Simple and sensitive method for determination of metronidazole in human serum by high‐performance liquid chromatography. J Chromatogr B 1998;720:239–243. [DOI] [PubMed] [Google Scholar]
- 16. Tavakoli N, Varshosaz J, Dorkoosh F, et al. Development and validation of a simple HPLC method for simultaneous in vitro determination of amoxicillin and metronidazole at single wavelength. J Pharm Biomed Anal 2007;43:325–329. [DOI] [PubMed] [Google Scholar]
- 17. Maher HM, Youssef RM, Khalil RH, et al. Simultaneous multiresidue determination of metronidazole and spiramycin in fish muscle using high performance liquid chromatography with UV detection. J Chromatogr B 2008;876:175–181. [DOI] [PubMed] [Google Scholar]
- 18. Bottcher S, Baum HV, Hoppe‐Tichy T, et al. An HPLC assay and a microbiological assay to determine levofloxacin in soft tissue, bone, bile and serum. J Pharm Biomed Anal 2001;25:197–203. [DOI] [PubMed] [Google Scholar]
- 19. Siewert S. Validation of a levofloxacin HPLC assay in plasma and dialysate for pharmacokinetic studies. J Pharm Biomed Anal 2006;41:1360–1362. [DOI] [PubMed] [Google Scholar]
- 20. Neckel U, Joukhadar C, Frossard M, et al. Simultaneous determination of levofloxacin and ciprofloxacin in microdialysates and plasma by high‐performance liquid chromatography. Anal Chim Acta 2002;463:199–206. [Google Scholar]
- 21. Djabarouti S, Boselli E, Allaouchiche B, et al. Determination of levofloxacin in plasma, bronchoalveolar lavage and bone tissues by high‐performance liquid chromatography with ultraviolet detection using a fully automated extraction method. J Chromatogr B 2004;799:165–172. [DOI] [PubMed] [Google Scholar]
- 22. Watabea S, Yokoyama Y, Nakazawa K, et al. Simultaneous measurement of pazufloxacin, ciprofloxacin, and levofloxacin in human serum by high‐performance liquid chromatography with fluorescence detection. J Chromatog B 2010;878:1555–1561. [DOI] [PubMed] [Google Scholar]
- 23. Yin Y‐Q, Yang X‐Z, Fang L‐S, et al. Stability of compatibility of levofloxacin and 9 antibacterials. J Pharm Pract 2003;21(1):39–40. [Google Scholar]
- 24. Liang W‐H, Tan X‐Z. Stability of compatibility injection of levofloxacin and metronidazole. Chin Guangxi Med 2006;28:381–382. [Google Scholar]
- 25. Sun Y‐C, Fang Y, Qu J. Study on the stability of cefazolin and ceftriaxone combined with metronidazole for treating biliary tract infection. Anhui Med Pharma J 2003;7(1):10–13. [Google Scholar]
- 26. Zhao S‐J, Yuan J, Sun G‐H. Pharmacoeconomic study of two therapeutic schemes for infection of billiary tract. Guangdong Med J 2002;23(11):1126. [Google Scholar]
- 27. Abid YA, Gustafsson LL, Ericsson O, et al. Metronidazole in: Handbook of drugs for tropical parasitic infections, second edition, London: Taylor & Francis; 1995. p 100–105. [Google Scholar]
- 28. Nakai Y, Tokuyama E, Yoshida M et al. Prediction of incompatibility of ceftriaxone sodium with calcium ions using the ionic product. Yakugaku Zasshi 2010;130:95–102. [DOI] [PubMed] [Google Scholar]
- 29. Koeppe MO, Cristofoletti R, Fernandes EF, et al. Biowaiver monographs for immediate release solid oral dosage forms: Levofloxacin. J Pharm Sci 2011;100:1628–1636. [DOI] [PubMed] [Google Scholar]
- 30. Sun HW, Wang HL, Ge XS, et al. Simultaneous determination of the combined drugs ribavirin and ceftriaxone sodium in human urine by HPLC‐DAD. Int J Sci Innov Discov 2011;1:216–225. [Google Scholar]
- 31. Pajchel G, Tyski S. Adaptation of capillary electrophoresis to the determination of selected cephalosporins for injection. J Chromatogr A 2000;895:27–31. [DOI] [PubMed] [Google Scholar]


