Abstract
Background
The sialylation of serum proteins and lipids changes in liver diseases of different etiologies and could change the total sialic acid (TSA), lipid‐bound SA (LSA), and free SA (FSA) levels in the sera. However, little is known of the relationship of serum SAs concentrations and the severity of liver disease. Therefore, the aim of this study was to investigate the SAs concentrations (TSA, LSA, and FSA) in liver cirrhosis in relation with the severity of liver disease.
Methods
Tested group consisted of 91 consecutive patients suffering from liver cirrhosis. For each patient, the Child‐Pugh score was calculated. TSA and LSA were determined by the enzymatic method on microplate reader, and FSA using the thiobarbituric method.
Results
Among the SA forms, only the serum FSA level in liver cirrhosis appears to be different according to the severity of liver damage evaluated by the Child‐Pugh score. It was the highest in score C, and was higher than that in scores B and A. The elevated levels of FSA significantly positively correlated with the Child‐Pugh score.
Conclusion
In conclusion, the sialylation of serum proteins and lipids changes in liver cirrhosis, but only the serum concentrations of FSA are stage‐related and reflect the severity of liver disease.
Keywords: sialic acid, sialylation, severity of liver diseases
Sialic acid (SA) is acetylated derivative of neuraminic acid that mainly occupies the terminal position on the carbohydrate chains of glycoproteins and glycolipids 1. SA may also exist as free form but this form is scarce in organisms 2. Total SA (TSA) is a sum of protein‐bound SA (PBSA), lipid‐bound SA (LSA), and free SA (FSA). The sialylation patterns of glycoproteins and glycolipids are highly variable and depend on the liver status 3. Glycosylation of proteins and lipids mediates many types of glycosyltransferases, but the most important for SA is α2,6‐sialyltransferase, which transfers SA to a terminal galactose 4. The previous works demonstrated that α2,6‐sialyltransferase is downregulated in human alcoholics and is caused by downregulation of its gene expression 5. Other authors found that the activity and mRNA for this enzyme can undergo up‐ or downregulation in different hepatocellular carcinoma, but not in cirrhosis 4. On the other hand, there are evidence that the chronic stress associated with chronic liver disease decreases the activity of sialyltransferase in the liver 6. Our previous work demonstrated that LSA concentration in liver cirrhosis does not differ from that in the healthy subjects, but we did not evaluate the severity of liver damage 7. Other authors also did not estimate the LSA and FSA according to the stage of the liver disease 8, 9, 10. In turn, TSA concentration was decreased in liver cirrhosis of nonalcoholic origin and FSA increased in cirrhosis of alcoholic origin 11. The biochemical mechanism that links stress and the sialyltransferase activity in the liver disposed us to test the serum SA concentrations in liver cirrhosis, taking into account the degree of organ damage. Therefore, the aim of this study was to investigate the SAs concentrations (total, lipid‐bound, and free form) in liver cirrhosis in relation with the severity of disease.
The tested group consisted of 91 consecutive patients (30 females and 61 males; mean age: 59 years, range: 20–84 years) admitted to the Department of Infectious Diseases and Hepatology. The diagnosis of liver cirrhosis was performed on the basis of signs and symptoms of the disease, physical and clinical exam (abdominal ultrasound, liver biopsy in selected cases), and biochemical liver panel. The serological tests (surface antigen of hepatitis B virus (HBsAg), hepatitis C antibody (anti‐HCV)) were also used to support the diagnosis of viral infections. Because alcoholic cirrhosis was diagnosed in 58 patients, all patients were interviewed regarding their use of alcohol and examined for recent alcohol use by the determination of blood alcohol concentration. The causes of nonalcoholic cirrhosis were infections of HBV in 14 patients, infections of HCV in 9 patients, and unidentified factors in the remaining patients. For each patient, the Child‐Pugh score was calculated. Eighteen patients were classified as Child‐Pugh class A, forty patients as class B, and thirty‐three patients as class C. The diagnosis of hepatic encephalopathy was based on results of a physical examination, electroencephalography, and blood ammonia concentration. The fluid accumulation in the abdominal cavity (ascites) was diagnosed on the basis of physical examination and confirmed by ultrasound or CT scan of the abdomen. Before admission to the hospital the patients were not treated with drugs. The control group consisted of 50 healthy subjects recruited from hospital workers (mean age: 55 years, range: 22–65 years). All subjects (healthy and sick) gave their consent to participate in the studies. Blood samples for laboratory tests were taken by vein puncture once, after admittance before treatment. The study was approved by the Bioethical Committee working at the Medical University in Bialystok. All patients provided informed written consent.
TSA concentration in the serum was measured according to the enzymatic method (EnzyChrom Sialic Acid Assay Kit, BioAssay System, Hayward, CA) using the colorimetric procedure. LSA concentration was measured according to the same enzymatic method followed by the precipitation step. All reagents for the first step were delivered by Sigma‐Aldrich Chemie GmbH (Steinheim, Germany). FSA was determined using the thiobarbituric method of Skoza and Mohos 12. Total bilirubin and albumin in the sera were determined by Abbott methods (Abbott Wiesbaden) on the Architect c8000 analyzer (Abbott Laboratories, Abbott Park, IL). Ammonia in the plasma was assayed by an enzymatic method (Abbott, Wiesbaden, Germany) on the Architect c8000 analyzer. The prothrombin time was measured on the STA Compact Hemostasis Analyzer (Diagnostica Stago, Gennevilliers, France).
To test the effect of severity of liver diseases on the concentrations of TSA, LSA, and FSA, the ANOVA rank Kruskal–Wallis test was performed. Because the chance of finding one or more significant differences in three tested groups (class A, B, and C) was only 14.26% (Bonferroni correction factor), we performed the nonparametric multiple comparison test (posthoc analysis). The differences between tested and control groups were evaluated by Mann–Whitney U test. To analyze the correlation, the Spearman's rank correlation coefficient was used.
There were differences in TSA concentration when compared to the control group. In scores A (1.82 ± 0.40 mmol/l), B (1.90 ± 0.47 mmol/l), and C of Child‐Pugh (1.73 ± 0.53 mmol/l), TSA levels were significantly lower than that in the control group (2.06 ± 0.21 mmol/l; P = 0.011, P = 0.013, and P = 0.016, respectively). There were no significant differences in the serum TSA and LSA concentrations between degrees of liver damage (H = 1.484, P = 0.476 for TSA and H = 1.391, P = 0.499 for LSA). The serum level of FSA in liver cirrhosis appears to be different according to the severity of liver damage (H = 8.662, P = 0.013; Fig. 1). Posthoc analysis revealed that the mean FSA concentration was highest in score C (mean ± SD, 183 ± 38 μmol/l), and was higher than that in score B (164 ± 31 μmol/l, P = 0.042) and score A (160 ± 36 μmol/l, P = 0.034). FSA levels in score C were significantly higher than that in the control group (157 ± 21 μmol/l, P = 0.001). The analysis of correlation revealed that only the FSA levels significantly positively correlated with the Child‐Pugh score (R = 0.317, P = 0.002).
Figure 1.
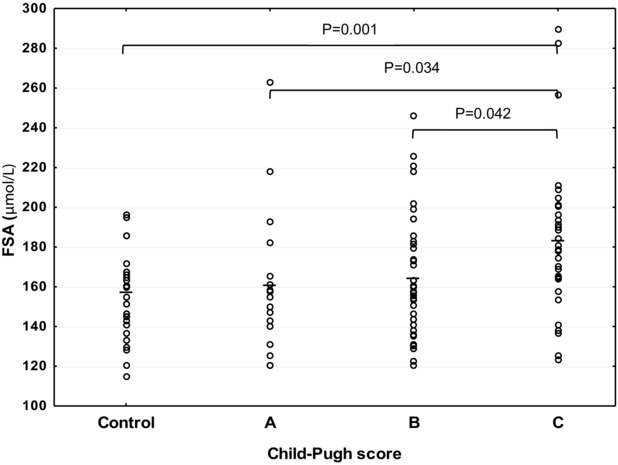
Serum FSA levels classified according to the severity of liver cirrhosis.
In our previous works, the changes in TSA, LSA, and FSA in liver disease of different etiologies were documented 7, 11. However, little is known of the relationship of these markers and the severity of liver diseases. Our results suggest that the FSA may become a marker for the degree of liver impairment in the course of liver cirrhosis. When the degree of cirrhosis reaches a Child‐Pugh score C, the level of FSA became the highest and is higher than that in scores A and B. This relation may be explained by the decreased sialyltransferase activity, which accompanied chronic liver diseases. It has been reported that chronic stress during chronic liver diseases is associated with the decreased activity of sialyltransferase in the liver 6. The relation between FSA and the severity of liver cirrhosis is supported by the positive correlation between FSA and Child‐Pugh score. The second possibility of explanation is the effect of alcohol abuse, because FSA can be a useful marker of chronic alcohol consumption 13. However, there was no correlation between FSA and weekly alcohol intake, and the duration of dependence (data not presented). Therefore, the relation of FSA with the severity of liver cirrhosis should be interpreted as an alteration in sialylation of proteins and lipids in liver disease.
Because the metabolism (synthesis, degradation, storage, and glycosylation) of lipids and lipoproteins is attributed to the liver, the liver diseases may affect the serum level of SA bounded with glycolipids, thus LSA. The most characteristic glycolipids in the blood are gangliosides 14. Despite the fact that the LSA concentration differs significantly between liver diseases of different etiology, the severity of liver disease does not affect the LSA level. The reason for this phenomenon might be the lack of differences in light‐density lipoprotein (LDL) concentrations in relation with the severity of liver cirrhosis (data in authors). Because the LDL particles transport most of the gangliosides in the blood (66%), most of the SAs are bounded with them. It can be assumed that if the LDL concentration does not change, then LSA concentration does not change as well.
Total SA (TSA) is not changed in liver cirrhosis according to the severity of disease, although as we previously demonstrated, TSA concentration in nonalcoholic liver cirrhosis was significantly decreased in comparison to the control group 11. Among the various forms of SA, the PBSA is predominant and has the strongest influence on the total concentration of SA. Because the synthesis of some proteins in liver cirrhosis increases and other decreases, finally, the SA attached to proteins does not change. The multidirectional changes in proteins synthesis in the liver cirrhosis are stage‐related, therefore the level of SA bounded with these proteins does not change in relation with the severity of liver cirrhosis. Our findings show that the sialylation in liver cirrhosis is stage‐related, but only FSA, and not TSA and LSA, concentration reflects the severity of liver disease.
REFERENCES
- 1. Schauer R, Kamerling JP. Chemistry and biochemistry of sialic acid In: Montreuil J, Vliegenthart JFG, Schacter H, editors. Glycoproteins II, Amsterdam: Elsevier; 1997. p 243–402. [Google Scholar]
- 2. Waters PJ, Lewry E, Peunock CA. Measurement of sialic acid in serum and urine: Clinical applications and limitations. Ann Clin Biochem 1992;29:625–637. [DOI] [PubMed] [Google Scholar]
- 3. Blomme B, van Steenkiste C, Callewaert N, van Vlierberghe H. Alteration of protein glycosylation in liver diseases. J Hepatol 2009;50:592–603. [DOI] [PubMed] [Google Scholar]
- 4. Dall'Olio F, Chiricolo M, D'Errico A, et al. Expression of beta‐galactoside alpha2,6sialyltransferase and alpha2,6‐sialylated glycoconjugates in normal human liver, hepatocarcinoma, and cirrhosis. Glycobiology 2004;14:39–49. [DOI] [PubMed] [Google Scholar]
- 5. Gong M, Gargie M, Hirsch K, Lakshman MR. Liver Galbeta1,4GlcNAc alpha2,6‐sialyltransferase is down‐regulated in human alcoholics: Possible cause for the appearance of asialoconjugates. Metabolism 2007;56:1241–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dabelic S, Flögel M, Maravic G, Lauc G. Stress causes tissue‐spcific changes in the sialyltransferase activity. Z Naturforsch C 2004;59:276–280. [DOI] [PubMed] [Google Scholar]
- 7. Chrostek L, Cylwik B, Panasiuk A, Brodowska‐Adamusiak D, Gruszewska E. Lipid‐bound sialic acid in liver diseases of different etiologies. Ann Hepatol 2011;10:150–154. [PubMed] [Google Scholar]
- 8. Arif S, Haq N, Hanif R, Khan AS, Rehman J, Mufti TA. Variations of serum sialic acid level in liver cirrhosis. J Ayub Med Coll Abbottabad 2005;17:54–57. [PubMed] [Google Scholar]
- 9. Matsuzaki S, Itakura M, Iwamura K, Kamiguchi H. Serum sialic acid levels in liver cirrhosis and liver cancer. Jpn J Gastro‐Enterol 1981;78:2395–2401. [PubMed] [Google Scholar]
- 10. Stefenelli N, Klotz H, Engel A, Bauer P. Serum sialic acid in malignant tumors, bacterial infections, and chronic liver diseases. J Cancer Res Clin Oncol 1985;109:55–59. [DOI] [PubMed] [Google Scholar]
- 11. Cylwik B, Chrostek L, Panasiuk A, Szmitkowski M. Serum total and free sialic acid in patients with chronic liver diseases. Clin Chem Lab Med 2010;48:137–139. [DOI] [PubMed] [Google Scholar]
- 12. Skoza L, Mohos S. Stable thiobarbituric acid chromophore with dimethyl sulphoxide. Application to sialic acid assays in analytical De‐O‐acetylation. Biochem J 1976;159:457–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chrostek L, Cylwik B, Korcz W, et al. Serum free sialic acid as a marker of alcohol abuse. Alcohol Clin Exp Res 2007;31:996–1001. [DOI] [PubMed] [Google Scholar]
- 14. Senn HJ, Orth M, Fitzke E, Wieland H, Gerok W. Gangliosides in normal human serum. Concentration, pattern and transport by lipoproteins. Eur J Biochem 1989;181:657–662. [DOI] [PubMed] [Google Scholar]


