Abstract
Background
The autoimmune thyroid disease (AITD) is an organ‐specific autoimmune disease characterized by the breakdown of self‐tolerance to thyroid antigens. Some lymphocytes have been identified to be related notably to the pathogenesis of AITD. This article evaluated the distribution of the lymphocytic subpopulation in thyroid glands in order to develop the immunospecific forms of therapy for AITD.
Methods
Damaged thyroid specimens were obtained from 18 Graves' disease (GD) and 17 Hashimoto's thyroiditis (HT) patients. Normal thyroid specimens were obtained from unaffected glands of 17 patients who underwent parathyroidectomy. We evaluated the distribution of lymphocytic subpopulation by analyzing the expression difference and correlationship among CD4+ T lymphocyte, CD8+ T lymphocyte, CD20+ B lymphocyte as well as regulatory T cells(Tregs)' marker FoxP3 in the thyroid tissues via immunohistochemistry.
Results
Our research uncovered that no distinct lymphocyte infiltrated in the normal thyroid specimens. Scarcely any lymphocyte infiltration could be found in half of the totally 18 GD thyroid specimens. For the rest 9 GD specimens, CD8+ T cells and CD20+ B cells were expressed more or less in all of them, FoxP3+ Tregs were detected in 7 of them and CD4+ T cells were weakly expressed in only 2 of them. For the 17 HT thyroid specimens, CD20+ B cells were stained strongly in all of them, CD4+, CD8+ T cells were expressed more or less in most of them and FoxP3+ Tregs could be detected in 9 of them.
Conclusion
Based on CD20+ B cells predominantly infiltrating in all HT thyroid tissues we suggested CD20 antibody might be of help for HT treatment. Furthermore based on FoxP3+ Tregs abundantly infiltrating in some of the AITD thyroid specimens, we considered that activating the Tregs' function in comparison to increasing the Tregs' number only, may be a more effective approach to the treatment of AITD in some cases.
Keywords: CD4, CD8, FoxP3, CD20, Graves’ disease, Hashimoto's thyroiditis, regulatory T cells
INTRODUCTION
The autoimmune thyroid disease (AITD) is an organ‐specific autoimmune disease characterized by the breakdown of self‐tolerance to thyroid antigens and the occurrence in the serum of antibodies against thyroid self‐antigens 1. Two typical AITDs are Graves’ disease (GD) and Hashimoto's thyroiditis (HT). The thyroid tissues of patients with these diseases are infiltrated by lymphocytes, in which T cells and B cells are the cellular components of the adaptive immune response.
T cells are involved in cell‐mediated immunity. Several different subsets of T cells, for example, helper, cytotoxic, and regulatory T cells (Tregs), have been discovered, each with a distinct function. T‐helper cells, known as CD4+ cells because they express the CD4 protein on their surface, assist other cells in immunologic processes including maturation of B cells into plasma cells and activation of cytotoxic T cells and macrophages. Cytotoxic T cells, known as CD8+ cells since they express the CD8 glycoprotein at their surface, destroy virally infected cells and tumor cells, are also implicated in transplant rejection 2. Tregs, formerly known as suppressor T cells, are a specialized subpopulation of T cells that act to suppress activation of the immune system and thereby maintain immune system homeostasis and tolerance to self‐antigens 3, 4. Regulatory T cells exert their suppressor/regulatory activity on CD4+ T cells, CD8+ T cells, B cells, and DC cells. FoxP3 transcription factor is regarded as a hallmark of Tregs. It is expressed intracellularly and mediates the expression of many of the proteins required for Tregs suppressor function 5.
B cells are lymphocytes that play an important role in the humoral immune response. The principal functions of B cells are to make antibodies against antigens, perform the role of antigen‐presenting cells, and eventually develop into memory B cells after activation by antigen interaction. CD20 is a nonglycosylated phosphoprotein expressed on the surface of all mature B cells. It is expressed on all stages of B‐cell development except the first and last and is present from pre‐pre‐B cells through memory cells, but not on either pro‐B cells or plasma cells.
T cells and B cells have been identified to be related notably to the pathogenesis of AITD. It is generally considered that T cells elaborate various lymphokines and exhibit a mitogenic response when exposed to thyroid antigens or to peptide sequences from the antigens while B cells secrete thyroid autoantibody. In this paper, we evaluated the distribution of lymphocytic subpopulation and analyzed the expression difference and correlationship among CD4+ T lymphocyte, CD8+ T lymphocyte, CD20+ B lymphocyte as well as regulatory T cells’ marker FoxP3 via immunohistochemistry in the thyroid specimens sourced from 18 GD and 17 HT patients who received the operational administration from 2002 to 2009. Based on the experimental results and analysis, we deduced some new opinions and thoughts that should be helpful for the development of the immunospecific therapy forms for AITD in the future.
MATERIALS AND METHODS
Patients
Surgical thyroid tissues were obtained from 18 GD and 17 HT patients. Diagnosis was established on commonly accepted clinical, laboratory, and histological criteria. All GD patients had clinical and biochemical features of hyperthyroidism and underwent surgery when they were in a controlled, almost euthyroid state as a result of treatment with methimazole or propylthiouracil. All had received iodine preoperatively. Patients with HT who were operated had long‐standing, very large compressive goiters with obstructive symptoms. All were under treatment with thyroid hormone replacement, and thyroid hormone levels were normal. Normal thyroid tissues were obtained from 17 unaffected glands of patients undergoing parathyroidectomy. In all cases, an informed consent was obtained, and this study was approved by the local hospital ethical committee.
Immunohistochemical Analysis
After thyroidectomy, 4‐μm thyroid slices were histologically examined with hematoxylin‐eosin. Immunohistochemical reactions in paraffin specimens with monoclonal antibodies against T‐cell markers CD4, CD8 (ZYMED, San Francisco, CA) as well as against B cells CD20 (ZYMED) and the antigen‐presenting FoxP3 antibodies (ABGENT, San Diego, CA) were performed. The specimens were dewaxed in xylene, hydrated by rinsing with numerous alcohols and distilled water. Next, antigens were revealed in citrate buffer at pH 6.0. After the specimens had been washed in distilled water and Tris Buffered Saline (TBS) buffer, endogenous peroxidase was blocked with 3% H2O2, which was followed by a subsequent wash of the specimens in TBS buffer. Thus, prepared thyroid specimens were incubated with a primary antibody and then with dextran marked with horseradish peroxidase and with the chromogene of DAB horseradish. When the immunohistochemical reaction was finished, cell nuclei were stained with (Mayer's) hematoxylin; the specimens were dehydrated in alcohol, exposed to xylene, and embedded in Canada (neutral) balsam.
The immunoreactivity of CD4, CD8, CD20, and FoxP3 was scored semiquantitatively as the staining intensity and the frequency of staining of nuclear and cytoplasmic components and the whole. The intensity of staining was graded as 0 (negative), 1 (weak), 2 (moderate), and 3 (strong). The frequency was graded from 0 to 4 by percentage of positive cells as follows: grade 0, <3%; grade 1, 3–25%; grade 2, 25–50%; grade 3, 50–75%; and grade 4, >75%. The index score was the product of multiplication of the intensity and frequency grades, which was then binned into a 4‐point scale: index score 0 (−), product of 0; index score 1 (+), products 1 and 2; index score 2 (++), products 3 and 4; and index score 3 (+++), products 6–12 6. Comparison of immunoreactivity of CD4, CD8, CD20, and FoxP3 in GD and HT patients was carried out using the Friedman rank test with SPSS 13.
RESULTS
Immunohistochemical investigations showed a significant infiltration of lymphatic cells in the thyroid specimens from all HT and some GD patients, while no distinct infiltration of lymphatic cells in the normal thyroid specimens were found. CD4+, CD8+, CD20+, and FOXP3+ cells were mostly stained between the thyroid follicles. CD4+, CD20+, and FOXP3+ cells were presented in the proliferation centers of lymphatic follicles, while CD8+ cells were at the peripheries of lymphatic follicles.
For the 18 GD thyroid specimens, scarcely any lymphocyte infiltration could be found in half of them (Fig. 1A–E). CD8+ and CD20+ cells were expressed more or less in rest of the nine GD thyroid specimens and FoxP3+ cells were detected in seven GD thyroid specimens. CD4+ cells were relatively the least frequently occurring among the lymphatic cell subsets investigated in our laboratory, which were weakly expressed in only two GD thyroid specimens (Table 1). More enhanced immunological reaction was observed in 17 HT thyroid specimens (Fig. 2F–J). CD20+ cells were stained strongly in all of them. CD4+ and CD8+ cells were expressed more or less in most of them with only one exception for each, respectively. FoxP3+ cells could also be found in nine of them (Table 2). Statistical analysis apparently showed that the predominant lymphocytes were CD20+ B cells in both GD and HT thyroid specimens (data not shown).
Figure 1.
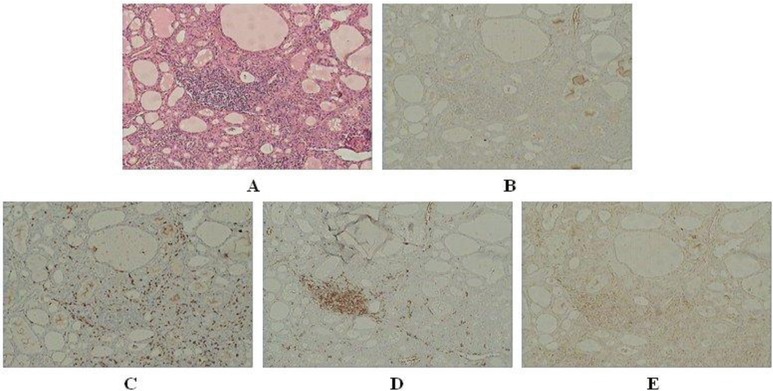
HE staining (×40) (A) and immunoreactivity of CD4 (B), CD8 (C), CD20 (D), FoxP3 (E) in the thyroid specimen sourced from no. 14 GD patient.
Table 1.
Immunoreactivity of Lymphocytes Subpopulations in Vision Fields of 18 GD Patients’ Thyroid Tissues
| CD4 | Foxp3 | CD8 | CD20 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient | Intensity | Frequency | Score | Intensity | Frequency | Score | Intensity | Frequency | Score | Intensity | Frequency | Score |
| 1 | 0 | 0 | − | 0 | 1 | − | 1 | 1 | + | 2 | 2 | ++ |
| 2 | 0 | 0 | − | 0 | 0 | − | 1 | 1 | + | 2 | 2 | ++ |
| 3 | 0 | 0 | − | 3 | 3 | +++ | 2 | 3 | +++ | 2 | 3 | +++ |
| 4 | 1 | 1 | + | 1 | 1 | + | 1 | 3 | ++ | 1 | 1 | + |
| 5 | 0 | 0 | − | 0 | 0 | − | 0 | 0 | − | 0 | 0 | − |
| 6 | 0 | 0 | − | 0 | 0 | − | 0 | 1 | − | 0 | 1 | − |
| 7 | 0 | 1 | − | 3 | 3 | +++ | 1 | 3 | ++ | 1 | 3 | ++ |
| 8 | 1 | 1 | + | 3 | 3 | +++ | 1 | 3 | ++ | 2 | 3 | +++ |
| 9 | 0 | 1 | − | 3 | 2 | +++ | 1 | 3 | ++ | 2 | 3 | +++ |
| 10 | 0 | 0 | − | 0 | 0 | − | 0 | 0 | − | 0 | 0 | − |
| 11 | 0 | 0 | − | 0 | 0 | − | 0 | 0 | − | 0 | 0 | − |
| 12 | 0 | 0 | − | 0 | 0 | − | 0 | 0 | − | 0 | 0 | − |
| 13 | 0 | 0 | − | 0 | 0 | − | 0 | 1 | − | 0 | 0 | − |
| 14 | 0 | 1 | − | 2 | 1 | + | 1 | 3 | ++ | 3 | 3 | +++ |
| 15 | 0 | 0 | − | 0 | 0 | − | 0 | 0 | − | 0 | 0 | − |
| 16 | 0 | 0 | − | 0 | 0 | − | 0 | 0 | − | 0 | 0 | − |
| 17 | 0 | 1 | − | 2 | 3 | +++ | 1 | 3 | ++ | 2 | 3 | +++ |
| 18 | 0 | 0 | − | 0 | 1 | − | 0 | 1 | − | 0 | 1 | − |
Intensity: staining intensity grades; frequency: staining frequency grades. Score: index score, product of multiplication of the intensity and frequency grades.
Figure 2.
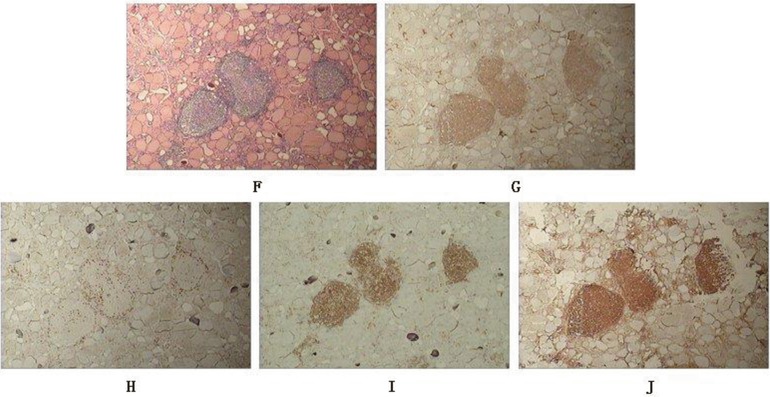
HE staining (×40) (F) and immunoreactivity of CD4 (G), CD8 (H), CD20 (I), FoxP3 (J) in the thyroid specimen sourced from no. 3 HT patient.
Table 2.
Immunoreactivity of Lymphocytes Subpopulations in Vision Fields of 17 HT Patients’ Thyroid Tissues
| CD4 | Foxp3 | CD8 | CD20 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient | Intensity | Frequency | Score | Intensity | Frequency | Score | Intensity | Frequency | Score | Intensity | Frequency | Score |
| 1 | 3 | 2 | +++ | 3 | 3 | +++ | 2 | 3 | +++ | 3 | 3 | +++ |
| 2 | 3 | 3 | +++ | 3 | 3 | +++ | 0 | 1 | − | 3 | 3 | +++ |
| 3 | 3 | 3 | +++ | 3 | 3 | +++ | 1 | 3 | ++ | 3 | 3 | +++ |
| 4 | 3 | 3 | +++ | 0 | 0 | − | 2 | 3 | +++ | 3 | 3 | +++ |
| 5 | 2 | 2 | ++ | 1 | 1 | + | 2 | 3 | +++ | 3 | 3 | +++ |
| 6 | 2 | 2 | ++ | 0 | 1 | − | 1 | 3 | ++ | 3 | 3 | +++ |
| 7 | 1 | 2 | + | 0 | 0 | − | 1 | 3 | ++ | 3 | 3 | +++ |
| 8 | 1 | 2 | + | 0 | 1 | − | 1 | 3 | ++ | 3 | 3 | +++ |
| 9 | 2 | 2 | ++ | 0 | 1 | − | 1 | 3 | ++ | 3 | 3 | +++ |
| 10 | 2 | 3 | +++ | 3 | 3 | +++ | 1 | 3 | ++ | 3 | 3 | +++ |
| 11 | 2 | 2 | ++ | 1 | 1 | + | 1 | 3 | ++ | 3 | 3 | +++ |
| 12 | 2 | 2 | ++ | 2 | 2 | ++ | 1 | 3 | ++ | 3 | 3 | +++ |
| 13 | 1 | 2 | + | 0 | 0 | − | 1 | 3 | ++ | 3 | 3 | +++ |
| 14 | 1 | 2 | + | 0 | 0 | − | 1 | 3 | ++ | 3 | 3 | +++ |
| 15 | 1 | 1 | + | 0 | 0 | − | 1 | 3 | ++ | 3 | 3 | +++ |
| 16 | 1 | 1 | + | 1 | 1 | + | 1 | 3 | ++ | 3 | 3 | +++ |
| 17 | 0 | 0 | − | 1 | 2 | + | 1 | 3 | ++ | 3 | 3 | +++ |
Intensity: staining intensity grades; frequency: staining frequency grades. Score: index score, product of multiplication of the intensity and frequency grades.
DISCUSSION
A lot of studies on regulatory T cells and other lymphocyte subpopulations in AITD have been done recently in order to find out the relationship among lymphocytic subtypes. Deshun Pan et al. reported that the number of CD4+CD25+ cells was similar in active GD patients and control subjects 7. Nevertheless, Marazuela et al. reported that a significantly higher proportion of CD4+Foxp3+ was detected in AITD patients than in healthy controls 8. Considering AITD is an organ‐specific autoimmune disease, it is more important to detect the lymphocyte subsets in thyroid than in peripheral blood. However, the related reports on lymphocyte subsets in the thyroid are very few by now because it is not so easy to obtain the thyroid specimen of AITD patient since thyroid operation is not the preferred treatment for AITD in most cases. Marazuela et al. found that CD69+Foxp3+ lymphocytes were significantly enriched in thyroid tissue of GD but no apparent differences of the cell subsets were observed in tissues from HT compared to GD patients 8. Iwona Ben‐Skowronek et al. detected that in the thyroid specimen from GD patients, T lymphocytes constituted 17.73% of the cells in the specimens. A total of 6.49% of T lymphocytes were CD8+ T lymphocyte and 2.95% CD4+ T lymphocyte. In addition, they also reported that lymphocytes infiltrations were dominated by CD79a+ B lymphocytes, which constituted 23.21% of the observed cells 9. However none of them detected the amount of the effect T cell, B cell, and regulatory T cells, respectively, in each thyroid specimen of AITD and discussed the correlationship among the three lymphocyte subsets.
In this study, a significant infiltration of lymphatic cells was observed in our thyroid specimens of HT and some of GD patients, and as was expected, we could not find distinct infiltration of lymphatic cells in all the normal thyroid tissues. However, we were a little curious why lymphocyte infiltration could scarcely be found in nine GD specimens either. According to Weetman's report, thyroid autoantibody levels could be declined after antithyroid drug treatment because the thyroid gland itself is the major site of thyroid autoantibody secretion in AITD 10. Therefore, we deduced that the alleviation of immune response may be owing to the drug's fact considering that all patients had received the treatment with methimazole or propylthiouracil prior to surgery. However, we observed that CD8+ T lymphocytes were more frequently detected than CD4+ cells in GD thyroid specimens. This was in accordance with the report of Iwona Ben‐Skowronek et al. who found that using an electron microscope, CD8+ T lymphocyte were more frequently observed (0.65%) than CD4+ cells (0.19%) 9. Furthermore, nonstaining of CD4+ cells was found in 16 of 18 GD thyroid specimens. According to Yukiyo Nakamura et al.'s report, we considered that the increased Fas expression in intrathyroidal CD4+ cells may be the cause of CD4+ cells’ reduction in GD thyroid tissues 11.
It is intriguing that CD20+ B lymphocytes predominantly infiltrated in our thyroid specimens from all the HT and half of GD patients. Daniel El Fassi et al. tried to use Rituximab (RTX), a monoclonal human/mouse antibody directed against CD20, to deplete the circulating B lymphocytes in GD thyroid tissue 12 considering that B lymphocytes usually comprise around 9% of lymphocytes in GD thyroid gland 13. But the effects of RTX treatment on GD are still little convincing in actual clinical practice, in general. Lasting therapeutic effects of RTX were achieved in some patients with low‐thyroid‐stimulating hormone receptor (TSHR) antibodies but not in those with high‐TSHR antibodies 14, 15. In our investigation, we found that CD20+ could not be detected in nine of 18 GD thyroid specimens, which indicated that not all the GD thyroid tissues were stained with CD20 and as a result, the GD patients with CD20− B cells in thyroid tissue may not be affected by RTX treatment. Interestingly, we detected a striking expression of CD20+ cells in HT thyroid gland. Therefore, we suppose that RTX‐targeting B cell may be a more effective intervention immunotherapy on HT. Currently, RTX treatment was only used for HT patients complicated with lymphoma. According to the latest report by Toshio Kahara et al., the antithyroid peroxidase antibody turned negative or decreased, TSH levels decreased with the same levothyroxine dosage in three cases of HT complicated with thyroid mucosa‐associated lymphoid tissue (MALT) lymphoma after RTX monotherapy 16. However, no related report can be found by now on RTX monotherapy for normal HT patients. It still needs our further investigation to verify if RTX is helpful to prevent or reverse HT from being hypothyroidism.
In order to learn the role FoxP3+ regulatory T cells played in the pathology of AITD, we detected the expression of FoxP3 in AITD thyroid specimen and noticed that FoxP3+ cells were presented in seven of nine GD thyroid specimens, and in 9 of 17 HT thyroid specimens, in which abundant lymphocyte infiltration could be observed. FOXP3 is a key gene in the development of Tregs and it commits naive T cells to become Treg cells. Considering the decrease of self‐tolerance against thyroid antigens caused by defection of suppressor lymphocytes is an important reason in the pathogenesis of AITD, our initial expectation was that the number of Tregs might be decreased in AITD. However, it was not the case. FoxP3+ cells were actually expressed more or less in many of our AITD thyroid specimens. One possible explanation for this phenomenon is that Tregs are augmented as a physiological response to the development of an autoimmune process, in an attempt to control autoimmunity. Another explanation is that the function of Tregs might be apparently disabled although they are abundant in inflamed thyroid tissue, which was put forward by Monica Marazuela et al. 8. Defective function of Tregs may cause the destruction of immune tolerance and induce the pathobiology of AITD. The results obtained from our research indicated that activating the Tregs’ function, for example, Vitamin D3 17, would be probably a more effective approach to the treatment of AITD than increasing the Tregs’ number only, for example, Tregs‐based adoptive therapies, in some cases.
In conclusion, our study attempted to develop the immunospecific forms of therapy for AITD by analyzing the expression difference and correlationship among CD4+ T lymphocyte, CD8+ T lymphocyte, CD20+ B lymphocyte, and regulatory T cells’ marker FoxP3 in the thyroid specimens from GD, HT, and normal thyroid tissues. We disclosed that CD20+ cells predominantly infiltrated in HT thyroid tissues and suggested that CD20 antibody might be of help for HT treatment. Furthermore, we found that FoxP3+ Tregs were abundantly infiltrated in some of the AITD thyroid specimens. Thus, we considered that activating the Tregs’ function in comparison to increasing the Tregs’ number only, may be a feasible approach to the treatment of AITD in some cases.
Grant sponsor: Natural Science Foundation of Shanghai; Grant number: 12ZR1423600; Grant sponsor: Minhang District Natural Science Foundation of Shanghai; Grant number: 2010MHZ024; Grant sponsor: National Science Foundation of China; Grant number: 81302570.
REFERENCES
- 1. Weetman AP. Autoimmune thyroid disease: Propagation and progression. Eur J Endocrinol 2003;148:1–9. [DOI] [PubMed] [Google Scholar]
- 2. Sepulveda H, Cerwenka A, Morgan T, Dutton RW. CD28, IL‐2‐independent costimulatory pathways for CD8 T lymphocyte activation. J Immunol 1999;163:1133–1142. [PubMed] [Google Scholar]
- 3. Miyara M, Sakaguchi S. Natural regulatory T cells: Mechanisms of suppression. Trends Mol Med 2007;13:108–116. [DOI] [PubMed] [Google Scholar]
- 4. Baecher‐Allan C, Viglietta V, Hafler DA. Human CD4+CD25+ regulatory T cells. Semin Immunol 2004;16:89–98. [DOI] [PubMed] [Google Scholar]
- 5. Banham AH, Powrie FM, Suri‐Payer E. FOXP3+ regulatory T cells: Current controversies and future perspectives. Eur J Immunol 2006;36:2832–2836. [DOI] [PubMed] [Google Scholar]
- 6. Lee OJ, Hong SM, Razvi MH, et al. Expression of calcium‐binding proteins S100A2 and S100A4 in Barrett's adenocarcinomas. Neoplasia 2006;8:843–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pan D, Shin YH, Gopalakrishnan G, Hennessey J, De Groot LJ. Regulatory T cells in Graves’ disease. Clin Endocrinol (Oxf) 2009;71:587–593. [DOI] [PubMed] [Google Scholar]
- 8. Marazuela M, García‐López MA, Figueroa‐Vega N, et al. Regulatory T‐cells in human autoimmune thyroid disease. J Clin Endocrinol Metab 2006;91:3639–3646. [DOI] [PubMed] [Google Scholar]
- 9. Ben‐Skowronek I, Sierocinska‐Sawa J, Szewczyk L, Korobowicz E. Interaction of lymphocytes and thyrocytes in Graves’ disease and nonautoimmune thyroid diseases in immunohistochemical and ultrastructural investigations. Horm Res 2009;71:350–358. [DOI] [PubMed] [Google Scholar]
- 10. Weetman AP. The immunomodulatory effects of antithyroid drugs. Thyroid 1994;4:145–146. [DOI] [PubMed] [Google Scholar]
- 11. Nakamura Y, Watanabe M, Matsuzuka F, et al. Intrathyroidal CD4+ T lymphocytes express high levels of Fas and CD4+ CD8+ macrophages/dendritic cells express Fas ligand in autoimmune thyroid disease. Thyroid 2004;14:819–824. [DOI] [PubMed] [Google Scholar]
- 12. El Fassi D, Clemmensen O, Nielsen CH, Silkiss RZ, Hegedüs L. Evidence of intrathyroidal B‐lymphocyte depletion after rituximab therapy in a patient with Graves’ disease. J Clin Endocrinol Metab 2007;92:3762–3763. [DOI] [PubMed] [Google Scholar]
- 13. Segundo C, Rodríguez C, Aguilar M, et al. Differences in thyroid‐infiltrating B lymphocytes in patients with Graves’ disease: Relationship to autoantibody detection. Thyroid 2004;14:337–344. [DOI] [PubMed] [Google Scholar]
- 14. El Fassi D, Nielsen CH, Hasselbalch HC, Hegedüs L. The rationale for B lymphocyte depletion in Graves’ disease. Monoclonal anti‐CD20 antibody therapy as a novel treatment option. Eur J Endocrinol 2006;154:623–632. [DOI] [PubMed] [Google Scholar]
- 15. El Fassi D, Nielsen CH, Bonnema SJ, Hasselbalch HC, Hegedüs L. B lymphocyte depletion with the monoclonal antibody rituximab in Graves’ disease: A controlled pilot study. J Clin Endocrinol Metab 2007;92:1769–1772. [DOI] [PubMed] [Google Scholar]
- 16. Kahara T, Iwaki N, Kaya H, et al. Transition of thyroid autoantibodies by rituximab treatment for thyroid MALT lymphoma. Endocr J 2011;58:7–12. [DOI] [PubMed] [Google Scholar]
- 17. Penna G, Roncari A, Amuchastegui S, et a1. Expression of the inhibitory receptor ILT3 on dendritic cells is dispensable for induction of CD4+Foxp3+ regulatory T cells by 1,25‐dihydroxyvitamin D3. Blood 2005;106:3490–3497. [DOI] [PubMed] [Google Scholar]


