Abstract
Background
Inflammation plays a role in the pathogenesis of carotid atherosclerosis. Although previous data demonstrated an association between inflammatory biomarkers and stroke, there is no publication reporting the relation of neutrophil to lymphocyte (N/L) ratio with ischemic stroke. We aimed to analyze the predictive ability of N/L ratio in acute ischemic cerebrovascular disease.
Methods
A total of 190 patients including 70 patients with first‐ever atherothrombotic acute ischemic stroke (AAIS), 50 patients with transient ischemic attack, and 70 healthy subjects were enrolled in this study. We analyzed the values of white blood cell (WBC), N/L ratio, C‐reactive protein (CRP), gamma‐glutamyltransferase (GGT), homocysteine (HCY), mean platelet volume (MPV) in patients with ischemic group and compared those with control individuals.
Results
WBC, CRP, HCY, N/L ratio were found to increase significantly in AAIS patients than the controls (P < 0.001). With respect to mortality, there were no significant differences between the values of CRP, GGT, HCY, and MPV in patients with AAIS. However, WBC and N/L ratio values were found to increase significantly in dead patients (P = 0.024 and P = 0.029, respectively). A comparison of receiver operating characteristic curves among WBC, CRP, GGT, HCY, MPV, and N/L ratio variables was made. No significant differences were obtained between area under curve values (P > 0.05). A cut‐off value of 4.1 for N/L ratio was detected in predicting mortality with a sensitivity of 66.7% and a specificity of 74.1% (κ = 0.299, P = 0.006).
Conclusions
These findings support the role of N/L ratio as a simple inexpensive and readily available marker of prognosis in acute ischemic stroke.
Keywords: ischemic stroke, neutrophil to lymphocyte ratio, prognosis
INTRODUCTION
An increasing body of evidence has linked inflammation with the pathogenesis of atherothrombotic stroke. Ischemic brain injury secondary to an arterial occlusion, which is characterized by acute local inflammation and changes in levels of inflammatory cytokines in body fluids of human patients, was demonstrated in the earlier studies 1, 2. To the present, only few prospective studies examined the association between white blood cell (WBC) count and incidence of stroke 3, 4, 5, 6.
Blood neutrophil to lymphocyte (N/L) ratio is a simple marker of subclinical inflammation that can be easily obtained from the differential WBC count. The N/L ratio has recently emerged as a prognostic marker in patients with cancer and coronary artery disease 7, 8. This marker has not yet been studied in ischemic stroke patients. In the present study, we aimed to analyze the predictive ability of N/L ratio in acute ischemic cerebrovascular disease against established risk factors.
METHODS
Study Population
This study was performed between January 2010 and December 2011 in the department of neurology in Kayseri Training and Research Hospital. A total of 190 patients including 70 patients with first‐ever atherothrombotic acute ischemic stroke (AAIS), 50 patients with transient ischemic attack (TIA), and 70 healthy subjects were enrolled in this study. The inclusion criteria of AAIS were based on the clinical evaluation (a focal neurological deficit of sudden onset that persisted beyond 24 hr) and magnetic resonance imaging (MRI) indicating the presence of anterior circulation infarction 9. TIA was defined as complete disappearance of signs and symptoms within 24 hr with negative finding on diffusion‐weighted MRI. All patients were screened according to a strict protocol consisting of a complete medical history, neurological examination, routine hematological and biochemical analysis on admission blood work, cerebral MRI and magnetic resonance angiography (MRA) performed in all cases within 24 hr from event, duplex scanning of the carotid arteries, and a cardiac analysis that included standard 12‐lead ECG and transthoracic echocardiography and, if indicated, 24‐hr ECG monitoring in order to exclude possible cardiac source of cerebral embolism. To avoid confounding factors, we excluded patients younger than 18 years, with history of recent clinical infection; concurrent major renal, hepatic, cardiac, and cancerous disease; known immunologic disease; surgery or major trauma in the previous month; the use of anticonvulsants or anticoagulants; uncontrolled hypertension (elevated systolic blood pressure of ≥140 mmHg and/or diastolic blood pressure of ≥90 mmHg, out of the acute phase, treated or not) 10 or diabetes mellitus (level of glycosylated hemoglobin >8%) 11, obvious signs and clinical evidence of acquired inhospital infection and pregnancy. For statistical analysis, carotid ultrasound measurements were grouped into three categories: absent, stenosis 0–50%, and 51% to occlusion on the symptomatic side 12.
Informed consent was obtained from all patients included. Our institutional ethical committee approved the study. We were able to receive current information on all included patients. Particular effort was made to assess the presence of a 30‐day mortality as measure of prognosis in ischemic stroke group.
Statistical Analysis
Shapiro–Wilk's test was used, histograms, q‐q plots were examined to check the data normality, and Levene's test was used to test the variance homogeneity. One‐way analysis of variance and Kruskal–Wallis analysis followed by Tukey and Dunn's post hoc tests were used for the comparison of continuous variables and χ2 analysis for the comparison of categorical variables. Values are expressed as frequencies and percentages, mean ± standard deviation or median and 25th–75th percentiles. Receiver operating characteristic (ROC) curves were drawn for the WBC, C‐reactive protein (CRP), gamma‐glutamyltransferase (GGT), homocysteine (HCY), mean platelet volume (MPV), and N/L variables. Area under curve (AUC) was calculated with 95% CI for these variables and compared with each other. Cut‐off values were determined for each variable, statistical diagnostic measures were calculated with 95% CI, and Kappa test was performed. P < 0.05 was considered statistically significant. MedCalc (Version 9.2.0.1) and SPSS 15.0 (SPSS Inc., Chicago, IL, USA) softwares were used for statistical analysis.
RESULTS
Baseline characteristics of participants are summarized in Table 1. The mean age in control subjects and TIA group was 60 and 64 years, respectively, with no significant differences between the two groups. But in AAIS patients, the mean age was 69 years, which was significantly different from control group (P < 0.001). Men and women were distributed equally in the TIA and AAIS groups, but most patients were women in control group. As shown in Table 1, carotid stenosis >50% was significantly high in AAIS patients than control and TIA groups (P < 0.001).
Table 1.
Baseline Characteristics and Parameters of Participants
| Feature | Controls (n = 70) | TIA (n = 50) | AAIS (n = 70) | P |
|---|---|---|---|---|
| Age (years) | 60.33 ± 10.23a | 64.82 ± 15.93ab | 69.53 ± 13.42b | <0.001 |
| Sex (male/female) | 20/50 | 24/26 | 33/37 | 0.037 |
| Carotid stenosis | ||||
| Absent (n, %) | 48 (53.9) | 20 (22.5) | 21 (23.6) | <0.001 |
| ≤50% (n, %) | 19 (23.2) | 28 (34.1) | 35 (42.7) | |
| >50% (n, %) | 3 (15.8) | 2 (10.5) | 14 (73.7) | |
| Parameters | ||||
| WBC (103/μl) | 6.50 (5.70–7.70)a | 6.75 (5.60–8.70)ab | 7.60 (6.40–10.50)b | 0.006 |
| CRP (mg/l) | 3.30 (3.30–4.90)a | 3.60 (3.50–10.00)a | 5.00 (3.40–10.30)b | <0.001 |
| GGT (IU/l) | 22.00 (17.00–29.00) | 20.00 (16.00–30.00) | 22.50 (15.00–36.00) | 0.688 |
| HCY (μmol/l) | 13.70 (11.40–17.20)a | 16.20 (13.20–19.80)ab | 18.00 (13.40–24.00)b | <0.001 |
| MPV (fL) | 10.21 ± 1.12a | 9.31 ± 1.30b | 9.77 ± 1.16ab | <0.001 |
| TG (mg/dl) | 135.00 (112.00–205.00) | 144.50 (109.00–198.00) | 137.50 (107.00–200.00) | 0.913 |
| TC (mg/dl) | 208.50 (185.00–231.00)a | 195.00 (173.00–217.00)ab | 189.50 (169.00–210.00)b | 0.010 |
| HDL‐C (mg/dl) | 46.00 (41.00–51.00)a | 41.50 (36.00–48.00)b | 40.00 (36.00–49.00)b | 0.002 |
| LDL‐C (mg/dl) | 128.50 (109.00–157.00) | 119.50 (103.00–142.00) | 117.00 (102.00–142.00) | 0.103 |
| N/L | 1.85 (1.50–2.30)a | 2.30 (1.60–3.10)b | 3.30 (2.30–4.80)c | <0.001 |
Values are expressed as n (%), mean ± SD, or median (25th and 75th percentiles). Different subscripts in a row indicate statistically significance difference. WBC, white blood cells; CRP, C‐reactive protein; GGT, gamma‐glutamyltransferase; HCY, homocysteine; MPV, mean platelet volume; N/L, neutrophil to lymphocyte ratio; TG, triglycerides; TC, total cholesterol; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol.
Baseline parameters of WBC, CRP, GGT, HCY, MPV, N/L ratio, and lipids among the three groups are also listed in Table 1. WBC, CRP, HCY, N/L were found to increase significantly in AAIS patients than the controls whereas the values were similar between TIA group and control subjects except for N/L ratio (P < 0.001). No significant differences in GGT, MPV, and lipid parameters were found between the study groups except that high‐density lipoprotein cholesterol level was detected to decrease significantly in ischemic group (AAIS/TIA) as compared to controls (P = 0.002).
The correlation between serum parameters and mortality in AAIS group are presented in Table 2. There were 12 deaths among the 70 cases of AAIS. Five patients died within 30 days (early mortality) whereas seven of the deaths occurred after one month (late mortality). There were no significant differences between the values of CRP, GGT, HCY, and MPV in terms of mortality. However, WBC and N/L ratio values were found to increase significantly in dead patients (P = 0.024 and P = 0.029, respectively). The results were similar for early and late mortality.
Table 2.
Correlation Between Serum Parameters and Mortality in AAIS Group
| Parameters | Absent (n = 58) | Early mortality (n = 5) | Late mortality (n = 7) | P |
|---|---|---|---|---|
| WBC (103/μl) | 7.15 (6.10–9.70)a | 7.80 (7.50–9.10)ab | 11.50 (7.70–15.10)b | 0.024 |
| CRP (mg/l) | 4.75 (3.40–9.80) | 10.30 (8.60–14.50) | 4.70 (4.30–9.50) | 0.095 |
| GGT (IU/l) | 23.00 (15.00–36.00) | 13.00 (11.00–32.00) | 20.00 (15.00–50.00) | 0.569 |
| HCY (μmol/l) | 17.05 (13.10–23.40)a | 25.10 (18.80–32.30)b | 22.30 (20.40–27.10)ab | 0.041 |
| MPV (fL) | 9.70 (9.00–10.50) | 10.10 (8.50–10.10) | 10.40 (9.90–10.90) | 0.171 |
| N/L | 3.15 (2.20–4.10)a | 5.30 (4.20–5.60)b | 5.90 (2.70–10.00)b | 0.029 |
Values are expressed as n (%), mean ± SD, or median (25th and 75th percentiles). Different subscripts in a row indicate statistically significance difference.
A plot for comparison of ROC curves among WBC, CRP, GGT, HCY, MPV, and N/L ratio variables is shown in Figure 1A. AUC values were found as 0.73 (0.61–0.83), 0.65 (0.53–0.76), 0.56 (0.43–0.68), 0.73 (0.61–0.83), 0.62 (0.50–0.74), 0.74 (0.62–0.84), respectively, and there was not any significant difference between any two AUC values (P > 0.05). However, an additional analysis was made to determine the optimal cut‐off value for N/L ratio in Figure 1b. A cut‐off value of 4.1 for N/L ratio was obtained to predict mortality with a sensitivity of 66.7% and a specificity of 74.1% as shown in Table 3 (κ = 0.299, P = 0.006).
Figure 1.
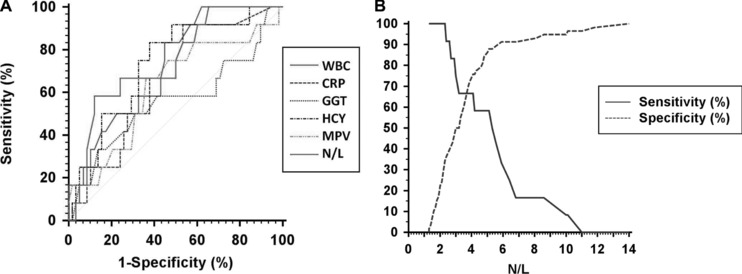
(A) A plot for comparison of ROC curves among WBC, CRP, GGT, HCY, MPV, and N/L variables. AUC values were 0.73 (0.61–0.83), 0.65 (0.53–0.76), 0.56 (0.43–0.68), 0.73 (0.61–0.83), 0.62 (0.50–0.74), 0.74 (0.62–0.84), respectively, and there were not any significant difference between any two AUC values (P > 0.05). (B) A plot to show the determination of the optimal cut‐off value for N/L.
Table 3.
Statistical Diagnostic Measures for the Determined Cut‐Off Values of Serum Parameters in the Detection of Mortality
| Diagnosing measures | Kappa test | ||||||
|---|---|---|---|---|---|---|---|
| Variables | SEN (95% CI) | SPE (95% CI) | PPR (95% CI) | NPR (95% CI) | AR (95% CI) | κ | P |
| WBC (>7.4 × 103/μl) | 83.3 (51.6–97.9) | 55.2 (41.6–68.3) | 27.8 (14.2–45.2) | 94.1 (80.3–99.3) | 60.0 (47.6–71.5) | 0.215 | 0.015 |
| CRP (>4.2 mg/l) | 91.7 (61.5–99.8) | 46.6 (33.3–60.1) | 26.2 (13.9–42.0) | 96.4 (81.7–99.9) | 54.3 (41.9–66.3) | 0.192 | 0.014 |
| GGT (>13 IU/l) | 66.7 (34.9–90.1) | 13.8 (0.06–25.4) | 13.8 (0.06–0.25) | 66.7 (34.9–90.1) | 22.9 (13.7–34.5) | ‒0.078 | 0.102 |
| HCY (18.7 μmol/l) | 83.3 (51.6–97.9) | 62.1 (48.4–74.5) | 31.3 (16.1–50.0) | 94.7 (82.3–99.4) | 65.7 (53.4–76.7) | 0.273 | 0.004 |
| MPV (10 fL) | 66.7 (34.9–90.1) | 63.8 (50.1–76.0) | 27.6 (12.7–47.2) | 90.2 (76.9–97.3) | 64.3 (51.9–75.4) | 0.195 | 0.051 |
| N/L (>4.1) | 66.7 (34.9–90.1) | 74.1 (61.0–84.7) | 34.8 (16.4–57.3) | 91.5 (79.6–97.6) | 72.9 (60.9–82.8) | 0.299 | 0.006 |
SEN, sensitivity; SPE, specifity; PPR, positive predictive rate; NPR, negative predictive rate; AR, accuracy rate.
DISCUSSION
The main finding of our study relates to the identification of N/L ratio as a novel noninvasive marker of prognosis in patients with AAIS. Our results demonstrate that: (a) N/L ratio is higher in patients with AAIS compared with TIA and control subjects, (b) a cut‐off value of 4.1 for this ratio can be used to predict mortality in patients with AAIS.
Unlike many other noninvasive markers of AAIS, N/L ratio can be easily calculated from the differential WBC count, which is routinely performed on admission and is universally available. The Asian study has newly reported that N/L ratio is an indicator of the overall inflammatory status of the body 13. Inflammation plays a fundamental role in the development and progression of atherosclerosis 14. Previous data have suggested a role for leukocytes in chronic atherosclerosis 15. It has been reported that leukocytes participate in the formation of atheromas, even in the earliest stage of the disease process 16. There is evidence that leukocytosis may also participate in plaque destabilization, thereby inducing acute thrombotic events 17, 18. Thus, leukocytosis has been suggested as a risk factor for stroke and its subtypes 6. However, there is no publication studying the relation of N/L ratio with acute ischemic cerebrovascular disease.
In recent years, N/L ratio has been validated as a predictor of outcome in multiple studies in patients with colorectal cancer and coronary artery disease 7, 8. Walsh et al. examined 230 patients diagnosed with colorectal cancer over a two‐year period and found a positive correlation of preoperative N/L ratio with poor prognosis in these patients 7. Likewise, Azab et al. mentioned that N/L ratio is the strongest WBC predictor of adverse outcomes in stable and unstable coronary artery syndromes 19. They also concluded that N/L ratio is an independent predictor of short‐ and long‐term mortalities in patients with non‐ST‐segment elevation myocardial infarction. In another study, Tamhane et al. studied 2,833 patients admitted with diagnosis of acute coronary syndrome and found that admission N/L ratio was an independent predictor of in‐hospital and six‐month mortality 8. We similarly found this ratio higher in patients with AAIS as compared to TIA and control subjects maybe due to the similar pathogenetic mechanisms of vascular ischemic origin with coronary syndromes. In our study, there were 12 deaths among 70 stroke patients in which the causes were found to be related to the type of central lesions such as stroke volume and/or localization. We observed that only WBC and N/L ratio were found to increase significantly in dead subjects, having similar results in both early and late mortality group, among the other popular biomarkers of inflammation such as CRP, GGT, HCY, and MPV. The possible mechanism is that, these biomarkers—particularly WBC and N/L ratio—may be involved in the pathogenesis of atherothrombotic stroke resulting in a greater inflammatory response. An additional analysis was made to determine a cut‐off value of 4.1 for N/L ratio, which might be used as a predictive biomarker for worse outcome after AAIS beyond conventional clinical parameters.
This study has some potential limitations. First, the sample size is relatively small mainly due to the fact that the participants were selected from single institute only via strict exclusion criteria. Second, we used a single WBC measurement to predict mortality; multiple measurements over time and changes in those measurements may provide a more accurate mechanism for predicting mortality. Third, the data were obtained in an elderly group, and this may limit the applicability of the results to younger men and women. Fourth, we were not able to use clinical scales due to patient incompliance associated with the sociocultural level.
In conclusion, despite the methodological limitations, the present study is the first to show, in a prospective design, a positive association of N/L ratio with clinical outcome in first‐ever acute ischemic stroke. Further studies in larger cohorts are needed for the verification of these findings to better define the role of N/L ratio in evaluating risk stratification as well as recurrence in patients with acute ischemic cerebrovascular disease.
ACKNOWLEDGMENTS
There are no any proprietary interests or research funding.
REFERENCES
- 1. Wang P, Kao C, Mui M, Wang SJ. Leukocyte infiltration in acute hemispheric ischemic stroke. Stroke 1993;2:236–240. [DOI] [PubMed] [Google Scholar]
- 2. Fassbender K, Rossol S, Kammer T, et al. Proinflammatory cytokines in serum of patients with acute cerebral ischemia: Kinetics of secretion and relation to the extent of brain damage and outcome of disease. J Neurol Sci 1994;122:135–139. [DOI] [PubMed] [Google Scholar]
- 3. Buck BH, Liebeskind DS, Saver JL, et al. Early neutrophilia is associated with volume of ischemic tissue in acute stroke. Stroke 2008;39:355–360. [DOI] [PubMed] [Google Scholar]
- 4. Kazmierski R, Guzik P, Ambrosius W, Ciesielska A, Moskal J, Kozubski W. Predictive value of white blood cell count on admission for in‐hospital mortality in acute stroke patients. Clin Neurol Neurosurg 2004;107:38–43. [DOI] [PubMed] [Google Scholar]
- 5. Nayak AR, Kashyap RS, Kabra D, et al. Evaluation of routinely performed hematological and biochemical parameters for the prognosis of acute ischemic stroke patients. Neurol Sci 2011;32:855–860. [DOI] [PubMed] [Google Scholar]
- 6. Elkind MS, Sciacca RR, Boden‐Albala B, Rundek T, Paik MC, Sacco RL. Relative elevation in baseline leukocyte count predicts first cerebral infarction. Neurology 2005;64:2121–2125. [DOI] [PubMed] [Google Scholar]
- 7. Walsh SR, Cook EJ, Goulder F, Justin TA, Keeling NJ. Neutrophil‐lymphocyte ratio as a prognostic factor in colorectal cancer. J Surg Oncol 2005;91:181–184. [DOI] [PubMed] [Google Scholar]
- 8. Tamhane UU, Aneja S, Montgomery D, Rogers EK, Eagle KA, Gurm HS. Association between admission neutrophil to lymphocyte ratio and outcomes in patients with acute coronary syndrome. Am J Cardiol 2008;102:653–657. [DOI] [PubMed] [Google Scholar]
- 9. Foulkes MA, Wolf PA, Price TP, Mohr JP, Hier DB. The Stroke Data Bank: Design, methods, and baseline characteristics. Stroke 1988;19:547–554. [DOI] [PubMed] [Google Scholar]
- 10. Sturgeon JD, Folsom AR, Jr Longstreth WT , Shahar E, Rosamond WD, Cushman M. Risk factors for intracerebral hemorrhage in a pooled prospective study. Stroke 2007;38:2718–2725. [DOI] [PubMed] [Google Scholar]
- 11. Ou L, Li RF. Effect of periodontal treatment on glycosylated hemoglobin levels in elderly patients with periodontal disease and type 2 diabetes. Chin Med J 2011;124:3070–3073. [PubMed] [Google Scholar]
- 12. Di Napoli M, Papa F, Bocola V. C‐reactive protein in ischemic stroke: An independent prognostic factor. Stroke 2001;32:917–924. [DOI] [PubMed] [Google Scholar]
- 13. Imtiaz F, Shafigue K, Mirza SS, Ayoob Z, Vart P, Rao S. Neutrophil lymphocyte ratio as a measure of systemic inflammation in prevalent chronic diseases in Asian population. Int Arch Med 2012;5:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Youn CS, Choi SP, Kim SH, et al. Serum highly selective C‐reactive protein concentration is associated with the volume of ischemic tissue in acute ischemic stroke. Am J Emerg Med 2012;30:124–128. [DOI] [PubMed] [Google Scholar]
- 15. Elkind MS, Cheng J, Boden‐Albala B, Paik MC, Sacco RL; Northern Manhattan Stroke Study . Elevated white blood cell count and carotid plaque thickness: the northern manhattan stroke study. Stroke 2001;32:842–849. [DOI] [PubMed] [Google Scholar]
- 16. Schillaci G, Pirro M, Pucci G, et al. Prognostic value of elevated white blood cell count in hypertension. Am J Hypertens 2007;20:364–369. [DOI] [PubMed] [Google Scholar]
- 17. Elkind MS. Inflammation, atherosclerosis, and stroke. Neurologist 2006;12:140–148. [DOI] [PubMed] [Google Scholar]
- 18. Stoll G, Bendszus M. Inflammation and atherosclerosis: Novel insights into plaque formation and destabilization. Stroke 2006;37:1923–1932. [DOI] [PubMed] [Google Scholar]
- 19. Azab B, Zaher M, Weiserbs KF, et al. Usefulness of neutrophil to lymphocyte ratio in predicting short‐ and long‐term mortality after non‐ST‐elevation myocardial infarction. Am J Cardiol 2010;106:470–476. [DOI] [PubMed] [Google Scholar]


