Abstract
Background
The efficacy of white blood cell (WBC) count and left shift in predicting bacterial infections has been controversial. The aim of this study was to prove that WBC count and left shift reflect a course of bacterial infection.
Materials
Six patients in whom the onset of bacterial infection had been determined and successful treatment had been done were selected. Manual 100‐cell differential counts were repeated at least every 24 hr.
Results
WBC count and left shift divided a course of bacterial infection into five phases. In the first phase of bacterial infection (0–10 hr after the onset), WBC count decreased to fewer than reference range without left shift. In the second phase (about 10–20 hr), low WBC count continued and left shift appeared. In the third phase (one to some days), WBC count increased above reference range with left shift. In the fourth phase (some to several days), high WBC count continued without left shift. In the fifth phase, WBC count went down into reference range without left shift.
Conclusions
A combination of WBC count and left shift real‐timely reflected a course of bacterial infection from the onset to healing. And we could judge which bacterial infection is adequately treated or not only by the above two routine laboratory tests.
Keywords: band neutrophil, immature neutrophil, leukocyte differential count, white blood cell
INTRODUCTION
The efficacy of manual band counts in predicting bacterial infections compared to the white blood cell (WBC) or absolute neutrophil counts has been controversial 1, 2, 3, 4. Several reports described the advantages of automated differential counts and stated that manual band counts added no useful information for diagnosis 5, 6, 7. Band counts, on the other hand, had greater sensitivity for predicting bacterial infection in infants and elderly patients 8. The combined information from automated and manual differential cell counts more accurately predicts bacterial infection than automated counting alone 5.
The maturation of WBCs in the bone marrow and their release into the circulating pool are influenced by colony‐stimulating factors, interleukins, tumor necrosis factor, and complement components, and an elevated neutrophil count typically reflects the normal response of bone marrow to an infectious or inflammatory process 6. Once neutrophils are released into the circulating pool, it remains in the blood only a few hours, and bone marrow continues to supply abundant neutrophils into the circulating pool (the volume in the circulating pool is replenished four to six times per day in a healthy person) 7. Therefore, the WBC (almost the same as absolute neutrophil) count changes quickly as the balance of neutrophil consumption and supply shifts.
Band cells and less mature cells appear in the circulating pool after the diminishment of segmented neutrophils (mature cells) stored in the bone marrow 7. This phenomenon is the left shift, and it signals an increased neutrophil production in the bone marrow. The WBC count and left shift frequently change during the course of bacterial infection. In this study, we showed real‐time changes of both parameters in six patients with bacterial infection from the onset of infection to the recovery from infection under adequate treatment.
MATERIALS AND METHODS
We retrospectively selected six patients with severe bacterial infection among 1,860 patients admitting to the Advanced Emergency & Critical Care Center of Shinshu University Hospital from January 2009 to December 2009. All six patients recognized the onset of bacterial infection and were admitted to our facility within 13 hr after the onset. They were adequately treated with antibiotics, surgery, or both immediately after admission and were clinically cured within eight days. WBC and 100‐cell manual differential counts were repeated at least every 24 hr. Left shift was defined as band ratio above 15% of WBC 8, 9.
We also evaluated C‐reactive protein (CRP) in each patient.
RESULTS
The characteristics of the six study patients are summarized in Table 1. The patients were five females and one male, and the average age was 76.5 (range 63–91) years old. Three patients were diagnosed as having peritonitis due to rupture of the sigmoid colon or ileus, two as having pyelonephritis with sepsis, and one as having aspiration pneumonia. All six patients were easily diagnosed as having bacterial infection by detection of pathogenic bacteria and clinical courses. The onsets of bacterial infection were determined by their symptoms, and all patients were admitted within 13 hr from the onset. They were treated with adequate antibiotics for the pathogens, surgery, or both, and all patients improved within several days.
Table 1.
Characteristics of Six Patients
| Age | Sex | Diagnosis | Pathogenic bacteria | Antibiotics | Operation | Duration | Underlying disease |
|---|---|---|---|---|---|---|---|
| 68 | Female | Peritonitis (Rupture of sigmoid colon) | Polymicrobial infection (Enterobacteriaceae, etc.) | MEPM | + | 13 hr | Hyperthyroidism |
| 91 | Female | Peritonitis (Rupture of sigmoid colon) | Polymicrobial infection (Enterobacteriaceae, etc.) | MEPM | + | 4 hr | Alzheimer's disease |
| 63 | Male | Peritonitis (Ileus) | Polymicrobial infection (Enterobacteriaceae, etc.) | FMOX | + | 5 hr | Chronic obstructive pulmonary disease |
| 76 | Female | Pyelonephritis with sepsis | Escherichia coli | ABPC | − | 10 hr | Alzheimer's disease |
| 85 | Female | Pyelonephritis with sepsis | Escherichia coli | CTM | − | 4 hr | Hypertension |
| 76 | Female | Aspiration pneumonia | Klebsiella pneumoniae | IPM/CS | − | 4 hr | Sjögren's syndrome |
Duration: Duration from the onset of bacterial infection to the admission.
Changes of WBC counts showed almost the same ones of absolute neutrophil counts (Figs. 1 and 2). Changes of WBCs of the six patients are summarized in Figure 1. In all cases, WBCs decreased to fewer than 3,000/μl in an early phase of bacterial infection (within 10 hr from the onset) and then increased in number, 10 to 36 hr after the onset. WBC count started to go down 40 to 150 hr after the onset in three patients, and they continued to increase in the remaining three patients. In the two patients with pyelonephritis and sepsis, on the other hand, WBCs decreased remarkably 72 hr after the onset, while one patient with peritonitis and one with pneumonia maintained high WBC counts.
Figure 1.
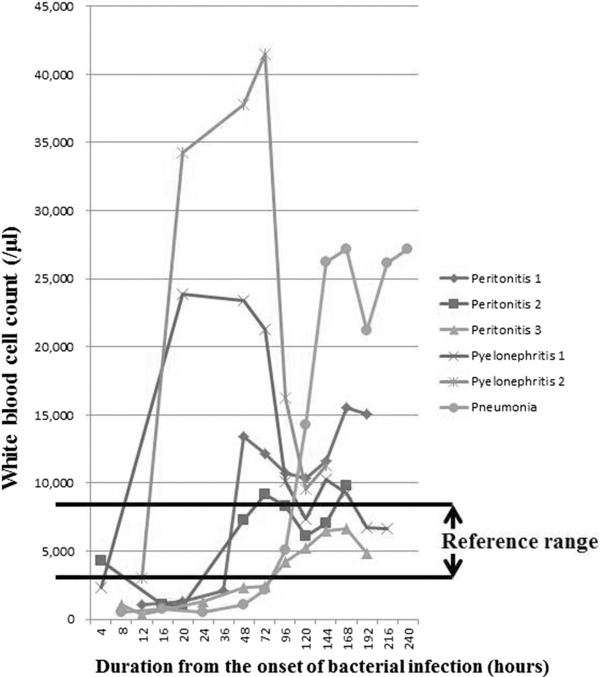
White blood cell (WBC) count. WBC counts decreased to fewer than 3,000/μl in an early phase of bacterial infection, and then increased in number. They continued to increase in three patients after the recovery of bacterial infection. Reference range (used by Nagano Association of Medical Technologists, Japan): 2,970–9,130/μl.
Figure 2.
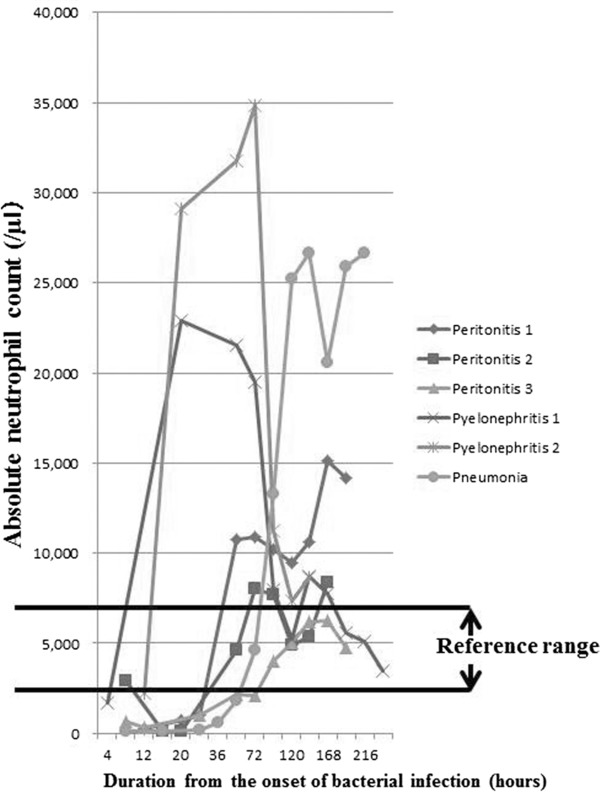
Absolute neutrophil count. Absolute neutrophil showed almost the same pattern as the WBC. Reference range (used by Nagano Association of Medical Technologists, Japan): 1,140–5,660/μl.
Changes in band counts are summarized in Figure 3. The changes in band counts were almost the same as those in immature cell counts. Band or immature counts increased 10 to 20 hr after the onset in all patients, and they decreased 48 to 120 hr after the onset.
Figure 3.
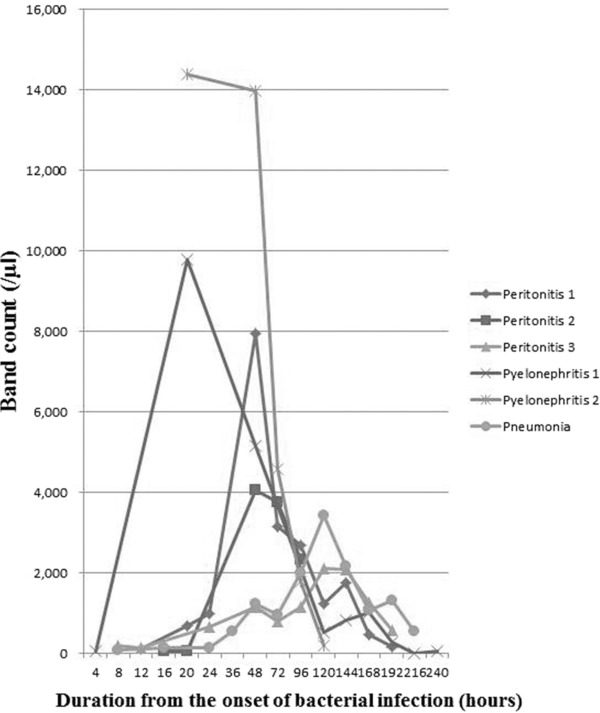
Band count. Band counts increased 10 to 20 hr after the onset in all patients, and they decreased 48 to 120 hr after the onset.
Ratios of band/WBC count and immature cell/WBC count are summarized in Figures 4 and 5, respectively. The ratios of band/WBC count were above 15% in two patients within 8 hr after the onset of bacterial infection, in five patients within 20 hr, and in all patients within 48 hr. On the other hand, the ratios of immature cell/WBC count were above 15% in two patients within 8 hr and in all patients within 20 hr. Both ratios started to decrease 48 hr after the onset.
Figure 4.
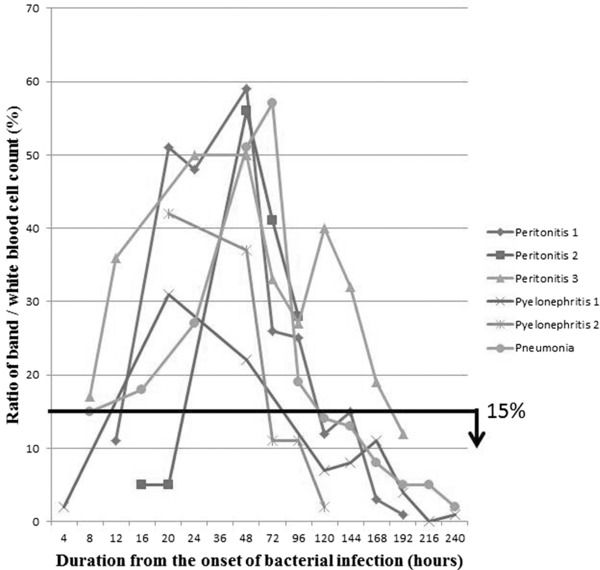
Ratios of band/WBC count. The ratios of band/WBC count were above 15% within 8 to 48 hr after the onset of bacterial infection. And they were below 15% after the recovery (150 hr after the onset).
Figure 5.
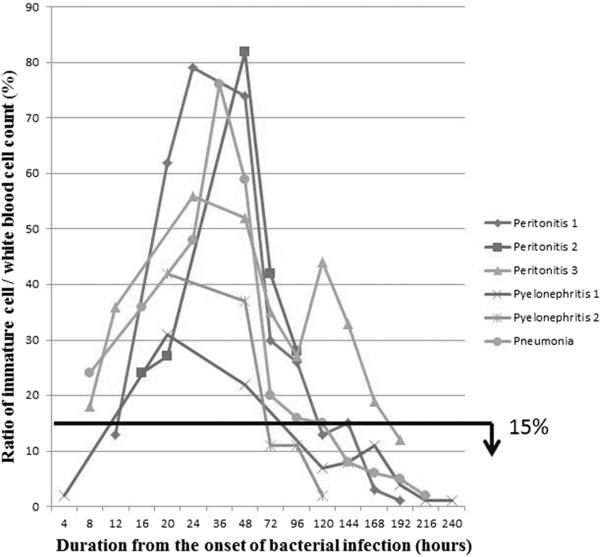
Ratios of immature cell/WBC count. The ratios of immature cell/WBC count showed almost the same pattern as the ratios of band/WBC count.
WBC count and left shift divided a course of bacterial infection treated adequately into five phases. In the first phase of bacterial infection (0–10 hr after the onset), the WBC count decreased to fewer than reference range without left shift. In the second phase (about 10–20 hr), low WBC count continued and left shift appeared. In the third phase (one to some days), WBC increased into or above reference range with left shift. In the fourth phase (some to several days), high WBC count continued without left shift. In the fifth phase, WBC went down into reference range without left shift.
CRP values are summarized in Figure 6. Four patients showed CRP values under 3.0 mg/dl within 8 hr after the onset of bacterial infection, and two patients showed CRP value under 8.0 mg/dl within that time. CRP reached peak values 20 to 72 hr after the onset of bacterial infection and then decreased. However, CRP was above 5 mg/dl in five cases after the recovery from the bacterial infection (150 hr after the onset).
Figure 6.
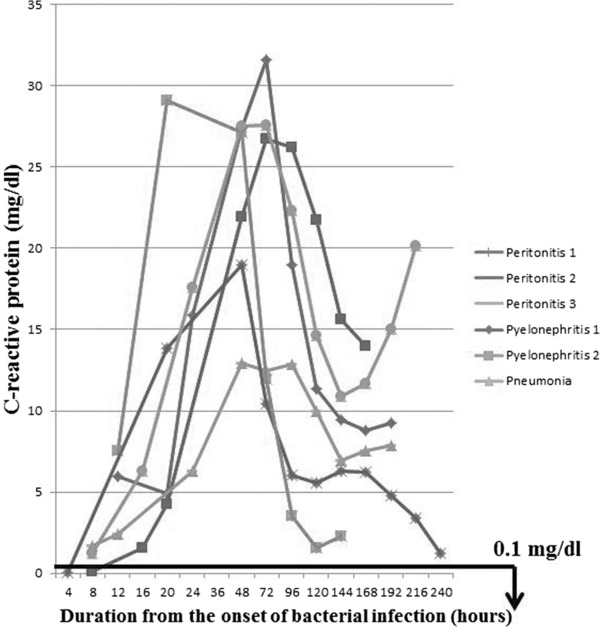
C‐reactive protein (CRP). CRP reached peak values 20 to 72 hr after the onset of bacterial infection, and it continued to be above 5 mg/dl in all cases after the recovery. Reference range (used by Nagano Association of Medical Technologists, Japan): less than 0.1 mg/dl.
DISCUSSION
The study patients showed that changes of WBC counts (almost the same as absolute neutrophil counts) and left shift could show five different phases of bacterial infection, which were satisfactorily treated from the onset to the recovery. WBC counts above the reference range indicate sufficient supply of neutrophils to bacterial infection site, and left shift means increase of neutrophil production in the bone marrow. And a combination of two routine laboratory tests as above could real‐timely predict whether a patient with bacterial infection is adequately treated or not.
The study patients showed changes of WBC count and left shift during the course of bacterial infection from the onset to the recovery. In an early phase of bacterial infection, WBCs decreased in number without left shift. After that, the low WBC count continued but was accompanied by an increased band count (left shift). WBCs started to increase with left shift 12–20 hr after the onset. High WBC counts continued and band counts gradually decreased under adequate treatment. When the bacterial infection was cured, WBC counts went back into the reference range without left shift. Therefore, combinations of WBC count and left shift faithfully reflected the course of bacterial infection in each study subject.
We selected six patients with severe bacterial infection, in whom the onset of infection was determined by the symptoms. All patients were treated with adequate antibiotics, surgery, or both immediately after admission, and they rapidly improved within a few days. Evidence for the courses of bacterial infection was supplied by changes in CRP values, which started to increase several hours after the onset of inflammation and reached a peak in 48–72 hr 10. However, CRP could not show a course of bacterial infection real‐timely because it remained low in the early phase of the bacterial infection and often continued to be above the reference range after the recovery.
Changes in WBC counts can result from altered rates of bone marrow release, changes in margination, and egress from the blood, or a combination of the two processes 11, 12, 13. In an early phase of bacterial infection, neutrophils in the circulating pool rapidly migrate into the infected site, and those stored in the marginal pool mobilize in the circulating pool, but the bone marrow does not supply neutrophils to the circulating pool. The WBC count falls momentarily below the reference range until bone marrow supplies a sufficient number of neutrophils (probably several hours after the onset of bacterial infection) 14.
In this study, bone marrow started to supply neutrophils to the circulating pool 12–20 hr after the onset of bacterial infection. When mature neutrophils stored in the bone marrow are diminished, the bone marrow supplies band and immature cells to the circulating pool instead of mature neutrophils (left shift) 11. Therefore, left shift indicates that abundant neutrophils are consumed at the site of bacterial infection, and the bone marrow increases the production of neutrophils. WBC (almost the same as neutrophil count), on the other hand, indicates whether bone marrow can supply sufficient neutrophils to the infected site. If the WBC count is above the reference range in a bacterial infection, neutrophils are being supplied in sufficient numbers. Low WBC count with left shift, however, demonstrates that bone marrow cannot produce enough neutrophils against the bacterial infection. A multivariate analysis reported that band count is most useful to diagnose a bacterial infection when the neutrophil count is low to normal 5.
When a bacterial infection improves, increased neutrophil production in the bone marrow is not necessary because of the decreased neutrophil demand in the infected site. A gradual decrease of the band and immature cell count indicates a lower consumption of neutrophils. When bacterial infection is completely cured, the WBC count goes into the reference range without left shift.
In our study patients, the ratios of immature cells (band, metamyelocyte, and myelocyte)/WBCs revealed the courses of bacterial infection, and they started to increase within 8 to 20 hr after the onset, reached a peak within 24 to 48 hr, and decreased to the reference range within 72 to 192 hr. The ratios of band cells/WBCs were almost the same as those of immature cells/WBCs, though the former changed several hours later than the latter. Band counts went up later compared with total immature cell counts.
It is not useful to debate whether the manual band count or the WBC count is better for predicting bacterial infection, because WBC and band counts dramatically change during the course of a bacterial infection, and it is difficult to predict bacterial infection by one point count. Some severe bacterial infections, including infectious endocarditis, meningitis, and abscesses often consume only a small number of neutrophils, and they are not accompanied by high WBC and band counts (http://www.uptodate.com). In infectious endocarditis, only circulating neutrophils can deal with the circulating bacteria without more neutrophils being supplied from the bone marrow. In meningitis and abscesses, on the other hand, circulating neutrophils cannot migrate to the infected sites and the neutrophils are not consumed.
Left shift with low WBC count also appears in viral infection and bleeding. In viral infection, circulating neutrophils migrate into a marginal zone pool 15, and then bone marrow increases neutrophil production. In cases of a large quantity of bleeding, circulating neutrophils go out of the body and bone marrow increases neutrophil production in addition to erythrocytes production. However, moderate to high CRP elevation is not observed in either of these situations as it is in cases of bacterial infection. Left shift was rarely observed in viral infection or large‐quantity bleeding, and CRP levels can differentiate them.
Left shift reliably shows a course of bacterial infection by neutrophil demand and supply. WBC count, on the other hand, demonstrated a real‐time ability of bone marrow to supply neutrophils. Therefore, a combination of both parameters can be used to assess a battle between neutrophils and bacteria and also to assist in the evaluation of antibiotics efficacy in the case of bacterial infection.
REFERENCES
- 1. Cornbleet PJ. Clinical utility of the band count. Clin Lab Med 2002;22(1):101–136. [DOI] [PubMed] [Google Scholar]
- 2. Kuppermann N, Walton EA. Immature neutrophils in the blood smears of young febrile children. Arch Pediatr Adolesc Med 1999;153(3):261–266. [DOI] [PubMed] [Google Scholar]
- 3. Ardron MJ, Westengard JC, Dutcher TF. Band neutrophil counts are unnecessary for the diagnosis of infection in patients with normal total leukocyte counts. Am J Clin Pathol 1994;102(5):646–649. [DOI] [PubMed] [Google Scholar]
- 4. Rimarenko S, Castella A, Salzberg MR, Strand CL. Evaluation of the automated leukocyte differential count in emergency department patients. Am J Emerg Med 1987;5(3):187–189. [DOI] [PubMed] [Google Scholar]
- 5. Wile MJ, Homer LD, Gaehler S, Phillips S, Millan J. Manual differential cell counts help predict bacterial infection. A multivariate analysis. Am J Clin Pathol 2001;115(5):644–649. [DOI] [PubMed] [Google Scholar]
- 6. Bokoch GM. Chemoattractant signaling and leukocyte activation. Blood 1995;86(5):1649–1660. [PubMed] [Google Scholar]
- 7. Abramson N, Melton B. Leukocytosis: basics of clinical assessment. Am Fam Physician 2000;62(9):2053–2060. [PubMed] [Google Scholar]
- 8. Al‐Gwaiz LA, Babay HH. The diagnostic value of absolute neutrophil count, band count and morphologic changes of neutrophils in predicting bacterial infections. Med Princ Pract 2007;16(5):344–347. [DOI] [PubMed] [Google Scholar]
- 9. Levy MM, Fink MP, Marshall JC, et al. Conference ISD. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Intensive Care Med 2003;29(4):530–538. [DOI] [PubMed] [Google Scholar]
- 10. Pepys MB, Hirschfield GM. C‐reactive protein: A critical update. J Clin Invest 2003;111(12):1805–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jandle J. Blood: Textbook of Hematology. New York: Williams & Wilkins; 1996. [Google Scholar]
- 12. Cartwright GE, Athens JW, Haab OP, Raab SO, Boggs DR, Wintrobe MM. Blood granulocyte kinetics in conditions associated with granulocytosis. Ann N Y Acad Sci 1964;113:963–967. [DOI] [PubMed] [Google Scholar]
- 13. Summers C, Rankin SM, Condliffe AM, Singh N, Peters AM, Chilvers ER. Neutrophil kinetics in health and disease. Trends Immunol 2010;31(8):318–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Christensen RD, Rothstein G, Anstall HB, Bybee B. Granulocyte transfusions in neonates with bacterial infection, neutropenia, and depletion of mature marrow neutrophils. Pediatrics 1982;70(1):1–6. [PubMed] [Google Scholar]
- 15. MacGregor RR, Friedman HM, Macarak EJ, Kefalides NA. Virus infection of endothelial cells increases granulocyte adherence. J Clin Invest 1980;65(6):1469–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]


