Abstract
Background
Serum very low density lipoprotein (VLDL) levels increase during the early stages of insulin resistance; therefore, determination of VLDL levels would be useful for evaluating the progression of metabolic syndrome and diabetes mellitus. The aim of this study was to clarify the clinical utility of triglyceride in VLDL (VLDL‐TG) level, determined using a homogeneous assay kit (Shino‐test Corporation, Tokyo, Japan), as an index of insulin resistance.
Methods
We enrolled 74 subjects in this study (diabetic subjects, n = 42; nondiabetic subjects, n = 32). The levels of VLDL‐TG, remnant‐like lipoprotein particle cholesterol, preheparin lipoprotein lipase mass, and other biochemical markers were determined.
Results
VLDL‐TG levels were significantly higher in the diabetic group (1.04 ± 0.84 mmol/l vs. 0.64 ± 0.42 mmol/l, P < 0.01) than in the nondiabetic group. In the nondiabetic group, VLDL‐TG was significantly correlated with the homeostasis model assessment of insulin resistance (HOMA‐IR), the index for insulin resistance (r = 0.513, P = 0.003). VLDL‐TG levels, but not TG levels, were higher in the highest quartile (HOMA‐IR) of the nondiabetic group.
Conclusion
VLDL‐TG level was a useful early marker for insulin resistance, especially in nondiabetic subjects. The homogeneous VLDL‐TG assay is a simple, low‐cost method for determining insulin resistance.
Keywords: VLDL‐TG, homogeneous kit, insulin resistance, diabetes, HOMA‐IR
INTRODUCTION
Insulin resistance is associated with plasma lipid abnormalities including low level of high‐density lipoprotein (HDL), hypertriglyceridemia, and excessive postprandial lipemia 1, 2, 3. These atherogenic lipid abnormalities develop prior to other diabetic symptoms such as elevated blood glucose. Insulin resistance is an important clinical marker for early detection and follow‐up of type‐2 diabetes mellitus 4, 5. The early stage of insulin resistance is characterized by dyslipidemia and elevated triglyceride (TG) in very low density lipoprotein (VLDL‐TG; 6). VLDL plays a critical role in the transport of endogenous lipids, including TG and cholesterol, which are synthesized in the liver 7. VLDL synthesis is inhibited by insulin signaling, but this inhibition is disrupted in insulin resistance 8. Increased free‐fatty acid in the liver promotes VLDL synthesis in insulin resistance 9. Furthermore, hyperinsulinemia caused by insulin resistance induces the expression of sterol regulation element binding protein‐1c, a transcription factor related to lipid synthesis, thereby accelerating lipogenesis in the liver 10, 11. Many groups have reported the association between insulin resistance and hypersecretion of VLDL 12, 13, 14. VLDL‐TGs are hydrolyzed by lipoprotein lipase (LPL), resulting in the transformation of VLDL to low‐density lipoprotein (LDL; 15, 16). Insulin has an indirect influence on VLDL‐TG levels in serum because LPL activity is regulated by insulin 17, 18. Postheparin LPL activity or preheparin LPL mass levels in serum are inversely correlated with insulin resistance 19, 20.
Generally, fasting insulin levels in blood and homeostasis model assessment of insulin resistance (HOMA‐IR), which are calculated from the fasting insulin and blood glucose levels, are used as indices of insulin resistance. Fasting insulin and HOMA‐IR are measured only in diabetic patients and are not used as screening tools in healthy individuals. Conversely, many would argue that insulin resistance may lead to (and appears to be predictive of) type‐2 diabetes 21, 22, 23. A readily measurable index of insulin resistance should be developed for early detection and screening of diabetes mellitus.
VLDL‐TG is typically measured by ultracentrifugation 24 and agarose gel electrophoresis 25. There are few opportunities to measure VLDL‐TG in clinical practice because these methods are too complex for most clinical settings. However, a homogeneous method for VLDL‐TG was recently developed 26. The aim of this study was to clarify the clinical utility of VLDL‐TG, determined using the homogeneous kit, as an index of insulin resistance. The relation between lipid biomarkers, including VLDL‐TG levels and HOMA‐IR, was analyzed in diabetic and nondiabetic subjects.
MATERIALS AND METHODS
Subjects
Seventy‐four subjects were enrolled (diabetic subjects, n = 42; nondiabetic subjects, n = 32). All the subjects provided informed consent and the protocol was approved by the Kitasato University Medical Ethics Committee (B10–44). Diabetic subjects had high fasting plasma glucose (FPG; >7.0 mmol/l) and/or high hemoglobin A1c (HbA1c; >6.5%). Nondiabetic subjects had normal FPG, normal HbA1c, and were not on any medications. We excluded patients with type‐1 diabetes and those who were undergoing insulin therapy. Other exclusion criteria included hemoglobinopathy, hepatic disorder, renal dysfunction, and serious systemic disease such as acute/chronic inflammations and malignancies.
Biochemical Assays
In each subject, the following biochemical markers were determined after an overnight fast: FPG, HbA1c, fasting insulin, total cholesterol (TC), cholesterol in remnant‐like lipoprotein particle (RLP‐C), TG, HDL cholesterol (HDL‐C), and LDL cholesterol (LDL‐C). RLP‐C levels were determined by a detergent‐based method (MetaboLead RemL‐C, Kyowa Medex Co., Ltd., Tokyo, Japan). The serum samples were analyzed on a Hitachi Autoanalyzer 7600 (Hitachi, Tokyo, Japan). HOMA‐IR was calculated from fasting glucose and insulin levels using the formula HOMA‐IR = FPG × fasting insulin/22.5 27.
Determination of VLDL‐TG Using the Homogeneous Assay
The homogeneous assay kit for VLDL‐TG was provided by Shino‐test Corporation (Tokyo, Japan). The assay was performed according to the manufacturer's instructions. In this method, a specific combination of surfactants plays a key role in stabilizing the polar lipids of VLDL and intermediate‐density lipoprotein (IDL) via exclusive interaction with the apolipoprotein. Therefore, the VLDL‐TG level determined using this kit also includes the TG levels in IDL.
Preheparin LPL Mass Assay
LPL mass in preheparin serum was measured by the sandwich enzyme‐linked immunosorbent assay using a specific monoclonal antibody against bovine milk LPL 28. A commercial kit from Sekisui Medical (Tokyo, Japan) was used according to the manufacturer's instructions. The primary antibodies specifically bind human LPL, but not hepatic lipase. Without any pretreatment, diluted serum samples were incubated with the monoclonal antibody against bovine milk LPL coated on the wells for 2 h. The trapped LPL was incubated with chicken polyclonal antibody against bovine LPL for 1 h, and then with horseradish peroxidase‐labeled antibodies. Finally, LPL was detected with substrate solution at 492 nm. The coefficients of variation within and between runs were <6% and <10%, respectively.
Statistics
Significant differences between the diabetic and nondiabetic group were determined by Student's unpaired t‐test or Mann–Whitney U‐test. Significant differences within lipid parameters in the nondiabetic group were determined using one‐way ANOVA and the Steel–Dwass multiple comparison posttest analysis. The relationships between variables were analyzed using Spearman's correlation coefficient. Ky Plot 5.0 (Kyens Lab, Inc., Tokyo, Japan) was used for all analyses. Significance was set at P < 0.05. Results are expressed as the mean ± SD.
RESULTS
Clinical Characteristics of the Study Subjects
Biochemical characteristics in serum were compared between the diabetic and nondiabetic groups (Table 1). As expected, FPG, HbA1c, fasting insulin, and HOMA‐IR were higher in the diabetic group than in the nondiabetic group. TG and RLP‐C was significantly higher and HDL‐C was significantly lower in the diabetic group than those in the nondiabetic group. Serum VLDL‐TG and LPL mass levels significantly differed between groups. There were no significant differences in TC and LDL‐C.
Table 1.
Subject Characteristics and VLDL‐TG Levels
| Nondiabetes | Diabetes | ||
|---|---|---|---|
| n = 32 | n = 42 | P * | |
| Sex (male, %) | 57.3 | 26.2 | – |
| Age (years) | 57.3 ± 13.5 | 59.0± 12.1 | ns |
| BMI (kg/m2) | 22.6 ± 4.56 | 25.4 ± 5.19 | <0.05 |
| Fasting glucose (mmol/l) | 5.66 ± 0.53 | 10.15 ± 2.52 | <0.001 |
| Hemoglobin A1c (%) | 5.70 ± 0.31 | 8.27 ± 1.15 | <0.001 |
| Fasting insulin (mU/l) | 6.47 ± 5.01 | 15.98 ± 20.74 | <0.01 |
| HOMA‐IR | 1.66 ± 1.34 | 8.36 ± 13.34 | <0.001 |
| Total cholesterol (mmol/l) | 5.28 ± 0.94 | 5.23 ± 0.92 | ns |
| HDL‐cholesterol (mmol/l) | 1.89 ± 0.67 | 1.53 ± 0.43 | <0.05 |
| LDL‐cholesterol (mmol/l) | 3.10 ± 0.77 | 3.20 ± 0.81 | ns |
| Triglycerides (mmol/l) | 1.05 ± 0.46 | 1.63 ± 1.13 | <0.01 |
| RLP‐C (mmol/l) | 0.16 ± 0.09 | 0.28 ±0.23 | <0.01 |
| Pre‐heparin LPL mass (ng/ml) | 69.4 ± 20.9 | 50.7 ± 26.6 | <0.01 |
| VLDL‐TG (mmol/l) | 0.64 ± 0.42 | 1.04 ± 0.84 | <0.01 |
HOMA‐IR, homeostasis model assessment of insulin resistance; ns, not significant; RLP‐C, remnant‐like lipoprotein particle cholesterol; LPL, lipoprotein lipase; VLDL‐TG, VLDL‐ triglycerides. Results are given as mean ± SD.
*Student's unpaired t‐test or Mann–Whitney U‐test, as appropriate.
Correlation Between HOMA‐IR and Lipid Parameters Containing VLDL‐TG in Nondiabetic and Diabetic Subjects
We analyzed the relationship between HOMA‐IR and each lipid parameter in both groups (Table 2). In the nondiabetic group, VLDL‐TG, TG, HDL‐C, and RLP‐C were significantly correlated with HOMA‐IR. In the diabetic group, on the other hand, only HDL‐C was negatively correlated with HOMA‐IR. Serum LPL mass levels were negatively correlated with HOMA‐IR in the diabetic group, but not in nondiabetic group.
Table 2.
Correlation Between HOMA‐IR and the Clinical Parameters Related to Lipid Disorder in Diabetic or Nondiabetic Group
| (vs. HOMA‐IR) | ||||
|---|---|---|---|---|
| Nondiabetes | Diabetes | |||
| (n = 32) | (n = 42) | |||
| R | P * | R | P * | |
| VLDL‐TG | 0.513 | 0.003 | 0.240 | 0.124 |
| Triglycerides | 0.509 | 0.003 | 0.220 | 0.160 |
| RLP‐C | 0.438 | 0.013 | 0.149 | 0.342 |
| HDL‐cholesterol | −0.402 | 0.023 | −0.372 | 0.016 |
| Pre‐heparin LPL mass | −0.080 | 0.669 | −0.512 | <0.001 |
| LDL‐cholesterol | 0.203 | 0.262 | 0.118 | 0.449 |
| Total cholesterol | 0.020 | 0.910 | −0.087 | 0.591 |
HOMA‐IR, homeostasis model assessment of insulin resistance; R, correlation coefficient; LPL, lipoprotein lipase; VLDL‐TG, VLDL‐triglycerides; RLP‐C, remnant‐like lipoprotein particle cholesterol. Results are given as mean ± SD.
*Spearman's test.
Relationship Between HOMA‐IR Index and Lipid Parameters in Nondiabetic Subjects
Nondiabetic subjects were separated into four groups based on HOMA‐IR (group I, 0.28–0.69; group II, 0.71–1.04; group III, 1.17–1.78; group IV, 3.11–5.14; Supporting Information Table 1), and their serum lipid parameters were examined. In the highest quartile (group IV), VLDL‐TG concentrations were 162% higher (P < 0.05) than in the group I, whereas TG and RemL‐C did not show a significant increase (84% and 104% increase, respectively; Fig. 1).
Figure 1.
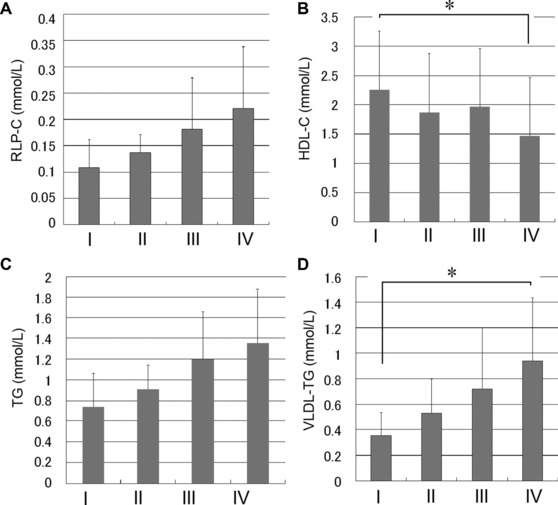
Lipid parameters in serum for each of the four quartiles defined only by homeostasis model assessment of insulin resistance (HOMA‐IR) in nondiabetic subjects. The relationship between each lipid parameter (VLDL‐TG, TG, RLP‐C, and HDL‐C) in the quartile (HOMA‐IR 0.28–0.69, group I; 0.71–1.04, group II; 1.17–1.78, group III; 3.11–5.14, group IV) in nondiabetic subjects is shown. Each lipid level was determined using enzymatic assay kits. Results are expressed as the mean ± SD *, P < 0.05.
DISCUSSION
VLDL‐TG in serum is correlated to the development of cardiovascular disease 29, 30 and insulin resistance. In this study, we analyzed the relationship between VLDL‐TG levels determined using a new homogeneous kit and HOMA‐IR, the index of insulin resistance.
Serum VLDL‐TG levels were significantly higher in the diabetic subjects than in the nondiabetic subjects. However, a major limitation of this study is that the male‐to‐female ratios of the two studied groups differed considerably. Interestingly, VLDL‐TG levels were positively correlated with HOMA‐IR in the nondiabetic group. For early detection of potential diabetes, it is important to identify insulin resistance in nondiabetic individuals. We assigned the nondiabetic subjects to four categories according to their HOMA‐IR; subjects with high HOMA‐IR had higher levels of VLDL‐TG than those with low HOMA‐IR. Thus, VLDL‐TG was the most useful marker of insulin resistance in persons with normal FPG and HbA1c. While insulin‐resistant patients with normoglycemia have increased secretion of basal VLDL in the liver and decreased ability of insulin to inhibit VLDL synthesis, they have normal insulin inhibition of endogenous glucose secretion 31. This suggests that elevation in VLDL‐TG levels occurs earlier than in FPG and HbA1c in nondiabetic subjects with insulin resistance. In the diabetic group, HOMA‐IR showed no correlation with lipid parameters, including VLDL‐TG. Because the pancreas appropriately augments its secretion of insulin to offset insulin resistance in diabetic patients, the beta‐cell fails to maintain its high rate of insulin secretion 32. Therefore, serum insulin levels do not properly reflect the condition of insulin resistance in diabetic patients.
Serum LPL mass levels are negatively correlated with insulin sensitivity 19, 20. In this study, LPL mass was significantly correlated with HOMA‐IR in the diabetic group, but not in the nondiabetic group. The increase of VLDL‐TG in insulin resistance was caused not only by the decreased lipolysis of TG by LPL, but also by the enhanced secretion of VLDL in the liver. It would appear that enhanced secretion of VLDL‐TG in the liver precedes decreased lipolysis of TG by LPL in insulin resistance.
Measuring VLDL‐TG levels is less susceptible to the effects of food intake than TG, fasting insulin, and FPG. Total TG concentrations increased by 247% 4 h after consuming a fatty meal, whereas VLDL‐TG concentrations increased by 176% 26. It is difficult to control patients for accurate test by restricting their food consumption. Thus, a method that is unaffected by dietary habits is important for a good screening test.
In conclusion, VLDL‐TG was significantly correlated with HOMA‐IR in nondiabetic subjects. Previous indices for insulin resistance have several limitations, primarily in their complexity and cost. The homogeneous VLDL‐TG assay is a simple, low‐cost method for determining insulin resistance in comparison with fasting insulin, HOMA‐IR, and LPL mass. VLDL‐TG levels should be evaluated in healthy subjects as a screening tool for early detection of insulin resistance.
CONFLICT OF INTEREST
The authors have declared no conflict of interest.
Supporting information
Disclaimer: Supplementary materials have been peer‐reviewed but not copyedited.
Table S1. Subject characteristics of four categories of insulin resistance
ACKNOWLEDGMENTS
The authors thank Shino‐test Corporation and Kyowa Medex Co., Ltd. for providing the reagents and controls for use in this study. The authors specially thank the Health‐Science‐Center Foundation, Inc., (Kanagawa, Japan) for the sample collection.
Grant sponsor: Kitasato University Hospital; Grant number: 2010‐09.
REFERENCES
- 1. Fontbonne A, Eschwege E, Cambien F, et al. Hypertriglyceridaemia as a risk factor of coronary heart disease mortality in subjects with impaired glucose tolerance or diabetes: Results from the 11‐year follow‐up of the Paris Prospective Study. Diabetologia 1989;32:300–304. [DOI] [PubMed] [Google Scholar]
- 2. Watson KE, Horowitz BN, Matson G. Lipid abnormalities in insulin resistant states. Rev Cardiovasc Med 2003;4:228–236. [PubMed] [Google Scholar]
- 3. Ginsberg HN, Zhang YL, Hernandez‐Ono A. Metabolic syndrome: Focus on dyslipidemia. Obesity (Silver Spring) 2006;14(Suppl 1):41S–49S. [DOI] [PubMed] [Google Scholar]
- 4. American Diabetes Association . Diagnosis and classification of diabetes mellitus. Diabetes Care 2011;34(Suppl 1):S62–S69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Imamura F, Mukamal KJ, Meigs JB, et al. Risk factors for type 2 diabetes mellitus preceded by β‐cell dysfunction, insulin resistance, or both in older adults: The cardiovascular health study. Am J Epidemiol 2013;177:1418–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Taskinen MR. Diabetic dyslipidaemia: From basic research to clinical practice. Diabetologia 2003;46:733–749. [DOI] [PubMed] [Google Scholar]
- 7. Schaefer EJ, Eisenberg S, Levy RI. Lipoprotein apoprotein metabolism. J Lipid Res 1978;19:667–687. [PubMed] [Google Scholar]
- 8. Olofsson SO, Stillemark‐Billton P, Asp L. Intracellular assembly of VLDL: Two major steps in separate cell compartments. Trends Cardiovasc Med 2000;10:338–345. [DOI] [PubMed] [Google Scholar]
- 9. Lewis FG, Carpentier A, Adeli K, Giacca A. Disordered fat storage and mobilization in the pathogenesis of insulin resistance and type 2 diabetes. Endocr Rev 2002;23:201–229. [DOI] [PubMed] [Google Scholar]
- 10. Koo SH, Dutcher AK, Towle HC. Glucose and insulin function through two distinct transcription factors to stimulate expression of lipogenic enzyme genes in liver. J Biol Chem 2001;276:9437–9445. [DOI] [PubMed] [Google Scholar]
- 11. Browning JD, Horton JD. Molecular mediators of hepatic steatosis and liver injury. J Clin Invest 2004;114:147–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cummings MH, Watts GF, Pal C, et al. Increased hepatic secretion of very‐low‐density lipoprotein apolipoprotein B‐100 in obesity: A stable isotope study. Clin Sci (Lond) 1995;88:225–233. [DOI] [PubMed] [Google Scholar]
- 13. Gill JM, Brown JC, Bedford D, et al. Hepatic production of VLDL1 but not VLDL2 is related to insulin resistance in normoglycaemic middle‐aged subjects. Atherosclerosis 2004;176:49–56. [DOI] [PubMed] [Google Scholar]
- 14. Adeli K, Taghibiglou C, Van Iderstine SC, Lewis GF. Mechanisms of hepatic very low‐density lipoprotein overproduction in insulin resistance. Trends Cardiovasc Med 2001;11:170–176. [DOI] [PubMed] [Google Scholar]
- 15. Jansen H, Hülsmann WC. Enzymology and physiological role of hepatic lipase. Biochem Soc Trans 1985;13:24–26. [DOI] [PubMed] [Google Scholar]
- 16. Chappell DA, Fry GL, Waknitz MA, et al. Lipoprotein lipase induces catabolism of normal triglyceride‐rich lipoproteins via the low density lipoprotein receptor‐related protein/alpha 2‐macroglobulinreceptor in vitro. A process facilitated by cell‐surface proteoglycans. J Biol Chem 1993;268:14168–14175. [PubMed] [Google Scholar]
- 17. Ong JM, Kirchgessner TG, Schotz MC, Kern PA. Insulin increases the synthetic rate and messenger RNA level of lipoprotein lipase in isolated rat adipocytes. J Biol Chem 1988;263:12933–12938. [PubMed] [Google Scholar]
- 18. Panarotto D, Rémillard P, Bouffard L, Maheux P. Insulin resistance affects the regulation of lipoprotein lipase in the postprandial period and in an adipose tissue‐specific manner. Eur J Clin Invest 2002;32:84–92. [DOI] [PubMed] [Google Scholar]
- 19. Eriksson JW, Burén J, Svensson M, Olivecrona T, Olivecrona G. Postprandial regulation of blood lipids and adipose tissue lipoprotein lipase in type 2 diabetes patients and healthy control subjects. Atherosclerosis 2003;166:359–367. [DOI] [PubMed] [Google Scholar]
- 20. Hanyu O, Miida T, Obayashi K, et al. Lipoprotein lipase (LPL) mass in preheparin serum reflects insulin sensitivity. Atherosclerosis 2004;174:385–390. [DOI] [PubMed] [Google Scholar]
- 21. Lillioja S, Mott DM, Spraul M, et al. Insulin resistance and insulin secretory dysfunction as precursors of non‐insulin‐dependent diabetes mellitus: Prospective studies of Pima Indians. N Engl J Med 1993;329:1988–1992. [DOI] [PubMed] [Google Scholar]
- 22. Haffner SM, Miettinen H, Gaskill SP, Stern MP. Decreased insulin secretion and increased insulin resistance are independently related to the 7‐year risk of NIDDM in Mexican‐Americans. Diabetes 1995;44:1386–1391. [DOI] [PubMed] [Google Scholar]
- 23. Consensus Development Conference on Insulin Resistance . 5–6 November 1997. American Diabetes Association. Diabetes Care 1998;21:310–314. [DOI] [PubMed] [Google Scholar]
- 24. Carlson LA, Ericsson M. Quantitative and qualitative serum lipoprotein analysis. Part 1. Studies in healthy men and women. Atherosclerosis 1975;21:417–433. [DOI] [PubMed] [Google Scholar]
- 25. Sato I, Taniguchi T, Ishikawa Y, et al. The lipoprotein fraction between VLDL and LDL detected by biphasic agarose gel electrophoresis reflects serum remnant lipoprotein and Lp(a) concentrations. J Atheroscler Thromb 2006;13:55–61. [DOI] [PubMed] [Google Scholar]
- 26. Okada M, Saito T, Yoshimura H, et al. Surfactant‐based homogeneous assay for the measurement of triglyceride concentrations in VLDL and intermediate‐density lipoprotein. Clin Chem 2005;51:1804–1810. [DOI] [PubMed] [Google Scholar]
- 27. Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care 2004;27:1487–1495. [DOI] [PubMed] [Google Scholar]
- 28. Kobayashi J, Hashimoto H, Fukamachi I, et al. Lipoprotein lipase mass and activity in severe hypertriglyceridemia. Clin Chim Acta 1993;216:113–123. [DOI] [PubMed] [Google Scholar]
- 29. Hodson L, Bickerton AS, McQuaid SE, et al. The contribution of splanchnic fat to VLDL triglyceride is greater in insulin‐resistant than insulin‐sensitive men and women studies in the postprandial state. Diabetes 2007;56:2433–2441. [DOI] [PubMed] [Google Scholar]
- 30. Perona JS, Covas MI, Fitó M, et al. Reduction in systemic and VLDL triacylglycerol concentration after a 3‐month Mediterranean‐style diet in high‐cardiovascular‐risk subjects. J Nutr Biochem 2010;21:892–898. [DOI] [PubMed] [Google Scholar]
- 31. Sorensen LP, Sondergaard E, Nellemann B, Christiansen JS, Gormsen LC, Nielsen S. Increased VLDL‐triglyceride secretion precedes impaired control of endogenous glucose production in obese, normoglycemic men. Diabetes 2011;60:2257–2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. DeFronzo RA, Bonadonna RC, Ferrannini E. Pathogenesis of NIDDM. A balanced overview. Diabetes Care 1992;15:318–368. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Disclaimer: Supplementary materials have been peer‐reviewed but not copyedited.
Table S1. Subject characteristics of four categories of insulin resistance


