Abstract
Objective: Despite the achievement of blood glucose, blood pressure targets, the risk for kidney injury remains high among older adults. This observational retrospective study investigated whether high TG or high WC contribute to this high residual risk for kidney injury.
Methods: A total of 843 elderly from Dongli Community, Tianjin, China, we selected 666 individuals with a baseline estimated glomerular filtration rate (eGFR) ≥60 mL/min/1.73 m2 and negative microalbuminuria completing a 3-year follow-up. At baseline, subjects were grouped according to the levels of TG and WC. The primary outcome was the incidence of kidney injury, defined as low eGFR (eGFR <60 mL/min/1.73 m2) or reduced eGFR (eGFR reduced >25%) or UACR ≥30 mg/g.
Results: Overall, 6.01% developed low eGFR, 11.11% reduced eGFR, 25.98% UACR ≥30 mg/g, and 3.45% low eGFR and UACR ≥30mg/g after 3-year follow-up. TG ≥1.7 mmol/L increased the risk of eGFR <60 mL/min/1.73 m2 by 1.44-fold, of UACR ≥30 mg/g by 32%, and of developing both abnormality by 1.41-fold in model 1; further adjustment for potential confounders factors, the association is slightly weakened in model 2 and 3; WC (≥90 cm in men and ≥85 cm in women) were associated with a 1.68-fold higher risk of eGFR <60 mL/min/1.73 m2 and a 1.43-fold risk of UACR ≥30 mg/g and a 1.89-fold risk of developing both abnormality in model 1. Further adjustment for potential confounders factors, the association is slightly weakened in model 2 and 3.
Conclusions: In a population of Chinese community-dwelling older adults, high TG and central obesity were risk factors for the development of kidney injury over 3 years.
Keywords: Kidney injury, chronic kidney disease (CKD), glomerular filtration rate (GFR), triglyceride, central obesity, elderly population
Introduction
Kidney dysfunction is rising worldwide in parallel with population ageing and chronic kidney disease (CKD) is more common in older people [1]. It is not only a precursor of end-stage renal disease (ESRD), but also an important risk factor of cardiovascular disease (CVD), even in the early stage of renal impairment [2]. According to Kidney Disease: Improving Global Outcomes (K/DOQI) Work Group, CKD is interpreted as a structural or functional kidney abnormality lasting for 3 or more months [3]. The National Health and Nutrition Examination Survey showed that the prevalence of kidney injury is 38% among those aged >65 years, compared with 13% of the overall US population [4]. Similarly, a survey from Chinese general population, the prevalence of CKD among females increases from 7.4% among those aged 18–39 years to 18.0 and 24.2% among those aged 60–69 and 70 years, respectively [5]. As the prevalence of kidney injury has increased globally along with an aging population, it has become a major health problem incurring substantial healthcare costs [4].
Kidney injury is associated with developing metabolic syndrome (MetS). The number of patients with MetS is increasing and has become a risk factor for the development of kidney injury, therefore, predicting the development of CKD patients [6,7]. It is estimated that the MetS affects at least a quarter of the adult population worldwide [8]. In American adults, the prevalence of MetS increases with age, reaching 18.3% for people aged 20–39 and 46.7% for people over 60 [9]. In China, the overall prevalence of men is 19.2% and the prevalence of women is 27.0%, which is also higher in older groups [10]. Metabolic syndrome is a group of metabolic disorders centered on insulin resistance and basic characteristics include abnormal glucose metabolism, hypertension, central obesity, disorder of lipid metabolism [11]. At present, there is no universally recognized standard for diagnosing metabolic syndrome in the world and there are great differences in different regions. The diagnostic criteria for MetS in China are proposed in the 2017 China guidelines for the prevention and treatment of type 2 diabetes, (1) central obesity (WC ≥90 cm in men and ≥85 cm in women); (2) hypertriglyceridemia: fasting plasma TG ≥1.7 mmol/L; (3) elevated blood pressure: BP ≥130/85 mmHg, antihypertensive drug treatment in a patient with a history of hypertension is an alternate indicator; (4) hyperglycemia: fasting glucose level of ≥6.1 mmol/L, drug treatment of elevated glucose is an alternate indicator; (5) decreased HDL-C: fasting HDL-C<1.04 mmol/L. These risk factors can be isolated or intertwined to increase the severity of chronic kidney disease. Wong et al. [12] have shown that intensive glycemic control is associated with long-term development of ESKD and its benefits are in retaining renal function. The relationship between hypertension and the occurrence of adverse vascular events, including progression of kidney injury, is clear and independent of other confounding factors [13].
Although the increasing prevalence of kidney damage in older individuals seems to be partly because of an increasing prevalence of hyperglycemia and hypertension [14]. However, in spite of the achievement of recommended targets for blood glucose and blood pressure, the residual risk for kidney injury remains high. Additional components of the MetS-high triglyceride (TG) and high waist circumference (WC)-may be the factors responsible for this high residual risk [3]. TG are heritable risk factors for vascular disease [15], and more recently, it has been suggested that high TG is shown to accelerate the rate of renal damage [16]. It is reported that TG levels begin to increase in the early stage of kidney injury and reach a peak in CKD stage IV. Obesity also seems to increase the risk of developing major risk factors for CKD. Among the US population with CKD, the prevalence of obesity is even higher, at 44.1% [17]. The traditional definition of obesity is based on body mass index (BMI). However, obesity can be further assessed in terms of fat distribution via WC and WC is a measure of central obesity that reflects metabolically active visceral fat [18]. Therefore, high TG and central obesity have a certain impact on kidney injury, which may lead to a high residual risk of kidney injury. This article needs to further explore the effects of high TG and central obesity on elderly kidney function.
Materials and methods
Study participants
This was a retrospective observational study who subjects followed up at Dongli community, Tianjin, China participating in a physical examination. The analysis was performed using a data set of electronic medical records collected between 2011 and 2013. For the purpose of the analysis, we considered only individuals who were ≥60 years old and had at 3 years follow-up for data. The last visit with complete renal data was considered the 3-year evaluation. Among the total of 843 subjects identified, we excluded those with microalbuminuria positive, eGFR<60 mL/min/1.73 m2, urine positive RBC or WBC and those with missing data at baseline. A total of 666 subjects met the inclusion criteria and were included in the study.
Measurements
All TG and WC had been measured according to diagnostic criteria for MetS in Chinese guidelines for the prevention and treatment of type 2 diabetes in 2017. All samples were taken and measured in the clinical laboratory. The determination of TG and serum creatinine was performed on Abbott IC16000 and manufacturer provided reagents and had been standardized. The eGFR was evaluated using CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) formula, which is based on serum creatinine concentrations and age [19]. The quantification of urinary albumin was assessed by nephelometry using the Dade–Behring BNII special protein analyzer and manufacturer-provided reagents. Urinary creatinine was measured using the enzymatic method with the ABBOTT ARCHITECT C16000. ACR was then calculated. Smoking was defined as one or more cigarettes per day, including cessation within the past 3 months. Drinking was defined as 50 mL or more per day, including cessation within the past 3 months. The primary outcomes were (1) low eGFR(eGFR <60 mL/min/1.73 m2); (2) reduced eGFR (eGFR reduced >25%); (3) UACR ≥30 mg/g; (4) low eGFR and UACR ≥30mg/g. Low eGFR is defined as eGFR <60 mL/min/1.73 m2, and reduced eGFR is defined as eGFR reduced >25%. We only involved chronic kidney injury, CKD. The diagnosis of CKD is based on the criteria of the 2012 Kidney Disease: Improving Global Outcomes, that is, a single measurement showing eGFR <60 mL/min/1.73 m2 or proteinuria (albumin to creatinine ratio (ACR) ≥30 mg/g) or both [3].
The study was approved by the Health Bureau of Dongli District of Tianjin and all participants gave written informed consent. The study approval number was 2011-C01.
Statistical analysis
Baseline clinical and biochemical characteristics are presented as mean values ± standard deviation (SD), categorical variables are described as frequencies and percentages. The difference between groups was tested using the Chi-squared test for categorical variables. The main analysis aimed to evaluate the association between baseline TG and WC with renal outcomes during the study period. Associations of renal outcomes with risk factors, including TG and WC were expressed as an odds ratio (OR), which was calculated using logistic regression analyses. Analysis was built regression models based on the cognizance of different set of confounders, such as, model 1 adjusted for age, sex; model 2 adjusted for age, sex, TG, WC, BMI, SBP, DBP, FBG, TC; model 3 adjusted for above + lipid-lowering treatment, blood-pressure-lowering treatment, antidiabetic treatment. Analyses were performed by using SPSS 20 (IBM Corporation, Armonk, NY, USA) and GraphPad Prism 7 (GraphPad Software, Inc., La Jolla, CA, USA). p Values <.05 were considered statistically significant.
Results
Baseline characteristics of study subjects stratified by TG and WC values
Table 1 shows the baseline clinical characteristics of participants with subjects stratified by TG and WC values. As expected from the study design, renal function was preserved at baseline, with a mean eGFR of 83.79 mL/min/1.73 m2. Overall, most clinical data for subjects with or without high TG concentrations (≥1.7 mmol/l) showed small but significant differences. Subjects with high TG concentrations at baseline had a higher BMI (26.39 vs. 24.34 kg/m2) and WC (92.02 vs. 86.63 cm), slightly worse glucose control with higher FBG. Subjects in the high TG group were more frequently female and more use of lipid-lowering medications (67.95 vs. 3.24%) and had more hypertension or diabetes. Also, the percentages of the group who were smokers and who were receiving antihypertensive treatments and antidiabetic treatment were larger in the high TG group. Similar findings were observed when comparing the baseline characteristics of subjects with normal and high WC values (Table 1). Subjects with high WC had a higher BMI, worse glucose control, a higher hypertension or diabetes, a higher percentage of subjects taking lipid-lowering, antihypertensive medications and antidiabetic treatment. However, no differences were noted in basal use of antidiabetic medications when stratifying the study population.
Table 1.
Baseline characteristics of study subjects stratified by TG and WC values.
| TG ≥1.7 mmol/l |
WC ≥90 cm(men) or ≥85 cm(women) |
||||||
|---|---|---|---|---|---|---|---|
| ALL (n = 666) |
No (n = 432) |
Yes (n = 234) |
p | No (n = 276) |
Yes (n = 390) |
p | |
| Male sex (%) | 279 (41.89%) | 202 (46.76%) | 77 (32.91%) | .001 | 126 (45.65%) | 153 (39.23%) | .098 |
| Age (year) | 65.58 ± 5.40 | 66.42 ± 5.62 | 64.03 ± 4.61 | <.001 | 66.26 ± 5.61 | 65.10 ± 5.21 | .006 |
| SBP (mmHg) | 133.64 ± 18.85 | 132.09 ± 18.70 | 136.50 ± 18.83 | .004 | 130.07 ± 16.69 | 136.17 ± 19.87 | <.001 |
| DBP (mmHg) | 85.06 ± 9.23 | 84.48 ± 8.92 | 86.14 ± 9.71 | .026 | 83.55 ± 8.55 | 86.13 ± 9.56 | <.001 |
| TC (mmol/l) | 5.17 ± 2.18 | 5.03 ± 2.55 | 5.40 ± 1.20 | .023 | 5.22 ± 3.18 | 5.14 ± 1.98 | .616 |
| TG (mmol/l) | 1.69 ± 1.10 | 1.10 ± 0.32 | 2.79 ± 1.19 | <.001 | 1.31 ± 0.67 | 1.96 ± 1.26 | <.001 |
| BMI (kg/m²) | 25.06 ± 3.48 | 24.34 ± 3.49 | 26.39 ± 3.05 | <.001 | 22.73 ± 2.88 | 26.71 ± 2.86 | <.001 |
| WC (cm) | 88.65 ± 9.36 | 86.63 ± 9.37 | 92.02 ± 8.36 | <.001 | 80.04 ± 5.80 | 94.74 ± 6.01 | <.001 |
| FBG (mmol/l) | 5.18 ± 1.46 | 5.10 ± 1.42 | 5.33 ± 1.53 | .053 | 5.07 ± 1.42 | 5.26 ± 1.49 | .089 |
| Scr (mmol/l) | 71.97 ± 10.45 | 71.79 ± 10.13 | 72.31 ± 11.03 | .541 | 71.22 ± 10.02 | 72.51 ± 10.72 | .116 |
| Urea nitrogen (mmol/l) | 6.02 ± 2.13 | 6.04 ± 1.56 | 5.98 ± 2.92 | .742 | 5.96 ± 1.59 | 6.06 ± 2.45 | .569 |
| Smoking (%) | 287 (43.09%) | 180 (41.67%) | 107 (45.73%) | .312 | 138 (50%) | 149 (38.21%) | .002 |
| Hypertension (%) | 344 (51.65%) | 197 (45.60%) | 147 (62.82%) | <.001 | 116 (42.03%) | 228 (58.46%) | <.001 |
| Diabetes (%) | 69 (10.36%) | 37 (8.56%) | 32 (13.68%) | .039 | 22 (7.97%) | 47 (12.05%) | .089 |
| Drinking (%) | 132 (19.82%) | 100 (23.15%) | 32 (13.68%) | .003 | 57 (20.65%) | 75 (19.23%) | .65 |
| Lipid-lowering treatment (%) | 173 (25.98%) | 14 (3.24%) | 159 (67.95%) | <.001 | 21 (7.61%) | 152 (38.97%) | <.001 |
| Blood-pressure-lowering treatment (%) |
345 (51.80%) | 194 (44.91%) | 144 (61.54%) | <.001 | 111 (40.22%) | 227 (58.21%) | <.001 |
| Antidiabetic treatment (%) | 71 (10.66%) | 34 (7.87%) | 32 (13.68%) | .017 | 21 (7.61%) | 45 (11.54%) | .095 |
| eGFR (ml/min/1.73 m²) | 83.79 ± 10.46 | 84.26 ± 10.33 | 82.93 ± 10.65 | .115 | 84.70 ± 10.46 | 83.16 ± 10.42 | .061 |
Data are mean ± SD or absolute frequency (percentage).
eGFR: estimated glomerular filtration rate; ACR: albumin-to-creatinine ratio; SBP: systolic blood pressure; DBP: diastolic blood pressure; BMI: body mass index; TG: triglyceride; TC: total cholesterol; FBG: fasting blood-glucose; WC: waist circumference; Scr: serum creatinine.
Clinical characteristics by renal outcome at 3 years of follow-up
At the end of the 3 years of follow-up, among 666 study subjects, 40 (6.01%) developed low eGFR values, 74 (11.11%) showed reduced eGFR, 173 (25.98%) developed UACR ≥30 mg/g, and 23 (3.45%) developed both low eGFR and UACR ≥30 mg/g (Table 2). As shown in Figure 1, all renal outcomes were significantly worse in subjects with high TG (Figure 1(A)) and/or high WC (Figure 1(B)) values than in subjects with baseline TG and WC values in the normal range.
Table 2.
Renal outcomes in the study population at the 3-year follow-up.
| Study participants n(%) | |
|---|---|
| eGFR <60 ml/min/1.73 m² or UACR ≥30 mg/g | 190 (28.53%) |
| eGFR <60ml/min/1.73 m² and UACR ≥30 mg/g | 23 (3.45%) |
| eGFR <60 ml/min/1.73 m² | 40 (6.01%) |
| UACR ≥30 mg/g | 23 (13.29%) |
| UACR <30 mg/g | 17 (3.45%) |
| eGFR reduction >25% than baseline | 74 (11.11%) |
| eGFR <60 ml/min/1.73 m² | 36 (90%) |
| eGFR ≥60 ml/min/1.73 m² | 38 (6.07%) |
| UACR ≥30 mg/g | 33 (19.08%) |
| UACR <30 mg/g | 41 (8.32%) |
| UACR ≥30 mg/g | 173 (25.98%) |
| eGFR <60 ml/min/1.73 m2 | 23 (57.50%) |
| eGFR ≥60 ml/min/1.73 m2 | 150 (23.97%) |
Figure 1.
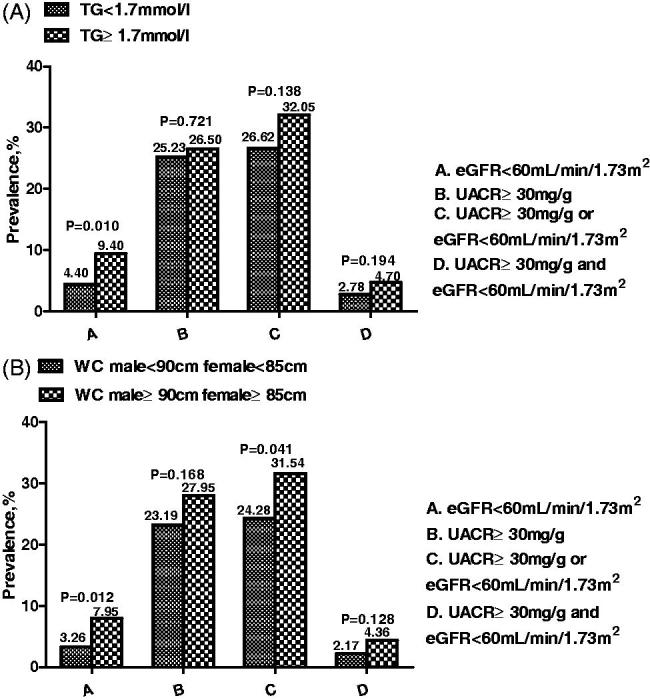
(A) Renal outcomes incidence according to baseline TG ≥1.7 mol/L. (B) Renal outcomes incidence according to baseline WC (≥90 cm in men; ≥85 cm in women).
Table 3 shows baseline characteristics according to the development of renal outcomes after 3-year follow up. Subjects developing low eGFR within 3 years of follow-up (n = 40) were more frequently female and older. They also had higher baseline blood pressure(BP), FBG, TG and higher hypertension or diabetes. Similar findings were noted when comparing baseline variables of patients who developed UACR ≥30 mg/g (n = 173) at follow-up versus those who remained UACR<30 mg/g, with the exception of the percentage of drinkers, which was larger among those developing UACR ≥30 mg/g.
Table 3.
Baseline clinical characteristics by renal outcome after 3 years.
| eGFR ≥60 ml/min/1.73 m2 (626) |
eGFR<60 ml/min/1.73 m2 (40) |
p | UACR <30 mg/g (493) | UACR ≥30 mg/g (173) | p | |
|---|---|---|---|---|---|---|
| Male sex (%) | 266 (42.49%) | 13 (32.5%) | .214 | 220 (44.62%) | 59 (34.10%) | .016 |
| Age (year) | 67.20 ± 5.23 | 71.05 ± 6.90 | <.001 | 67.16 ± 5.22 | 68.21 ± 5.90 | .028 |
| SBP (mmHg) | 131.60 ± 19.50 | 138.25 ± 20.15 | .037 | 130.18 ± 18.24 | 137.18 ± 22.26 | <.001 |
| DBP (mmHg) | 82.40 ± 12.09 | 83.13 ± 15.45 | .718 | 82.00 ± 12.65 | 83.71 ± 11.22 | .115 |
| TC (mmol/l) | 5.25 ± 2.84 | 5.31 ± 1.38 | .898 | 5.14 ± 1.02 | 5.20 ± 1.02 | .503 |
| TG (mmol/l) | 2.28 ± 6.84 | 3.92 ± 12.25 | .167 | 2.02 ± 5.14 | 1.82 ± 1.13 | .61 |
| BMI (kg/m²) | 25.62 ± 6.61 | 27.50 ± 11.03 | .098 | 25.30 ± 5.48 | 25.50 ± 3.52 | .66 |
| WC (cm) | 86.54 ± 11.55 | 85.58 ± 16.53 | .618 | 86.23 ± 12.03 | 87.22 ± 11.52 | .347 |
| FBG (mmol/l) | 6.25 ± 11.52 | 8.72 ± 22.61 | .225 | 6.50 ± 13.43 | 6.12 ± 9.14 | .732 |
| Scr (mmol/l) | 76.62 ± 10.25 | 102.57 ± 16.36 | <.001 | 77.86 ± 11.62 | 79.12 ± 14.31 | .252 |
| Urea Nitrogen (mmol/l) | 5.86 ± 2.67 | 7.94 ± 2.91 | <.001 | 6.01 ± 3.04 | 5.93 ± 1.50 | .769 |
| Smoking (%) | 275 (43.93%) | 19 (47.50%) | .666 | 232 (47.06%) | 62 (35.84%) | .011 |
| Drinking (%) | 149 (23.80%) | 6 (15%) | 0.202 | 119 (24.14%) | 36 (20.81%) | .373 |
| Hypertension (%) | 361 (57.67%) | 36 (90%) | <.001 | 280 (56.80%) | 117 (67.63%) | .012 |
| Diabetes(%) | 83 (13.26%) | 9 (22.50%) | .101 | 57 (11.56%) | 35 (20.23%) | .004 |
| Lipid-lowering treatment (%) | 228 (36.42%) | 21 (52.5%) | .042 | 183 (37.12%) | 65 (37.57%) | .916 |
| Blood-pressure-lowering treatment (%) |
362 (57.83%) | 37 (92.50%) | <.001 | 280 (56.80%) | 117 (67.63%) | .012 |
| Antidiabetic treatment (%) | 82 (13.10%) | 9 (22.50%) | .093 | 57 (11.56%) | 34 (19.65%) | .008 |
| eGFR (ml/min/1.73 m²) | 78.94 ± 9.09 | 53.28 ± 6.49 | <.001 | 78.25 ± 10.31 | 74.94 ± 11.93 | .001 |
Data are mean ± SD or absolute frequency (percentage).
eGFR: estimated glomerular filtration rate; ACR: albumin-to-creatinine ratio; SBP: systolic blood pressure; DBP: diastolic blood pressure; BMI: body mass index; TG: triglyceride; TC: total cholesterol; FBG: fasting blood-glucose; WC: waist circumference; Scr: serum creatinine.
Regression models associations of baseline TG and WC levels and renal outcomes at follow-up
Table 4 shows regression models associations of high TG and high WC with renal outcomes at follow-up. TG >1.7 mmol/L and WC ≥ 90 cm(men) or ≥80 cm (women) were associated with eGFR <60 mL/min/1.73 m2 (OR2.44 and 2.68) in model one. Further adjustment for factors which were potential confounders and unlikely to be in the causal pathway between the high TG and high WC and eGFR <60 mL/min/1.73 m2 had an impact on the odd ratios, high TG and high WC were still significantly associated with eGFR <60 mL/min/1.73 m2. The ORs for eGFR<60 mL/min/1.73 m2 were 2.01 and 2.66 in model two. When adjusted for lipid-lowering treatment, blood-pressure-lowering treatment and antidiabetic treatment, the association of high TG and high WC and eGFR <60 mL/min/1.73 m2 were slightly weakened (OR 1.71 and 2.48) in model three. High TG and high WC were associated with UACR ≥30 mg/g (OR 1.32 and 2.43) in model one. Further adjustment for potential confounders factors, high TG and high WC were associated with UACR ≥30 mg/g is slightly weakened, the ORs for UACR ≥30 mg/g were 1.12 and 1.59 in model two. When adjusted for lipid-lowering treatment, blood-pressure-lowering treatment and antidiabetic treatment, the association between high TG and high WC and UACR ≥30 mg/g is still slightly weakened in model three. The associations between high TG, WC and eGFR <60 mL/min/1.73 m2 and UACR ≥30 mg/g were true.
Table 4.
Associations between baseline TG and WC and renal outcomes after 3-year followup.
| Model 1 |
Model 2 |
Model 3 |
||||
|---|---|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | |
| eGFR <60 ml/min/1.73 m² | ||||||
| TG ≥1.7 mmol/l | 2.44 (1.27,4.68) | .007 | 2.01 (1.00,4.04) | .05 | 1.71 (0.72,4.05) | .221 |
| WC ≥90 cm (men) or ≥85 cm (women) | 2.68 (1.25,5.75) | .012 | 2.66 (1.08,6.58) | .034 | 2.48 (0.98,6.31) | .057 |
| UACR ≥30 mg/g | ||||||
| TG ≥1.7 mmol/l | 1.32 (0.56,3.11) | .528 | 1.12 (0.78,1.60) | .552 | 0.71 (0.23,2.26) | .566 |
| WC ≥90 cm (men) or ≥85 cm (women) | 2.43 (0.95,6.19) | .063 | 1.59 (0.54,4.70) | .406 | 1.37 (0.45,4.18) | .579 |
| eGFR<60 ml/min/1.73 m² and UACR ≥30 mg/g | ||||||
| TG ≥1.7 mmol/l | 2.41 (1.28,4.56) | .007 | 1.88 (0.95,3.72) | .068 | 1.63 (0.69,3.81) | .263 |
| WC ≥90 cm (men) or ≥85 cm (women) | 2.89 (1.35,6.17) | .006 | 2.53 (1.03,6.19) | .042 | 2.35 (0.93,5.95) | .07 |
Model 1: Adjusted for age, sex.
Model 2: Adjusted for age, sex, TG, WC, BMI, SBP, DBP, FBG, TC.
Model 3: Adjusted for above + lipid-lowering treatment, blood-pressure-lowering treatment, antidiabetic treatment.
Renal outcome risk associated with high TG and high WC levels according to age, sex, and common risk factors
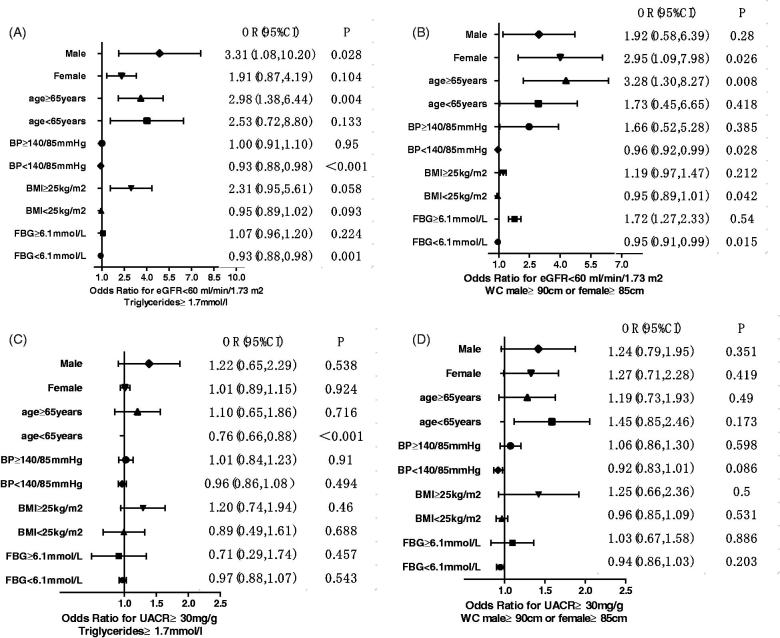
As shown in Figure 2, high TG and/or high WC levels significantly increased the risk of developing renal outcomes after factoring for sex, age, blood pressure, BMI, FBG levels.
Figure 2.
Multivariate associations of high TG and WC levels with renal outcomes after stratification for sex, age, and several risk factors. Data are shown as ORs with 95% CI for eGFR<60 mL/min/1.73 m2 (A and B) and for albuminuria (C and D).
Discussion
Although the incidence of kidney damage is gradually increasing among the elderly, the therapeutic progress has led to a larger number of subjects reaching the recommended targets for blood glucose and blood pressure, which can further reduce the burden on the kidneys. However, in addition to the known factors affecting kidney injury, there may be other confounding factors for its residual risk. Therefore, our longitudinal cohort study aimed to investigate the effects of high TG and central obesity on renal function in elderly subjects with preserved renal function in the north China community. In short, our objective is to more effectively find out the elderly kidney injury patients.
In our cohort of 666 subjects with normal kidney at baseline, 6.01% developed low eGFR values, 11.11% showed reduced eGFR, 25.98% developed UACR ≥30 mg/g and 3.45% developed both low eGFR and UACR ≥30 mg/g. This incidence is lower than the previous study by Russo et al. [20], they reported that 12.8% developed low eGFR, 7.6% showed reduced eGFR, 23.2% albuminuria, and 4% albuminuria and either low eGFR or an eGFR reduction >30%. This may depend on selection bias, their enrolled population is diabetic, but we chose elderly who participated in community hospital physical examinations and who were healthy people and also combined with various diseases such as diabetes and hypertension.
Lipoprotein abnormalities have been identified as possible causes of kidney damage, especially glomerular injury [21]. Dyslipidemia is associated with nephropathy and has been shown to play a key role in the development and progression of kidney disease. The mechanism behind this effect has not been fully elucidated, but it has been suggested that lipids may damage the blood vessels of the kidney, mesangial cells and tubular cells. Moorhead et al. first proposed the “lipid nephrotoxicity hypothesis.” [22] High TG is one of the clinical components of the MetS and may be a consequence of the underlying kidney damage. Furthermore, the TG to HDL-C ratio is a better marker for identifying renal insufficiency [23]. Our data also clearly indicate high TG levels are important risk factors for the development of kidney and the adjusted risk for developing any renal event associated with higher TG levels was still higher. Although it seems that the risk of glomerular injury is slightly lower than the risk of decreased renal function. These associations were partly attenuated by multivariate adjustment. Lipid-lowering drugs used by the elderly in our study included fibrates and statins. Antihypertensive therapy, including oral antihypertensive drugs such as nifedipine, amlodipine, compound reserpine. Antidiabetic treatment, including oral hypoglycemic drugs, such as acarbose, metformin or subcutaneous injection of insulin. Although the fibrates, statins, acarbose and metformin might have damaged the kidneys, they are only contraindicated in patients with severe renal impairment. All subjects had a normal renal function at the start of the study and they were dosed strictly in accordance with the drug instructions. Therefore, there was no potential confusion about medical treatment. Recent systematic reviews concluded that lipid-lowering therapy decreases death events in patients with CKD [24]. For example, statins might be more potent to block the development of kidney disease [25]. The target range of dyslipidemia in cases of CKD, however, remains to be determined and there may be individual differences. Thus, evaluation and the target range of treatment of dyslipidemia should be individualized.
Obesity has become a worldwide epidemic and its prevalence has been projected to grow by 40% in the next decade. This increasing prevalence has implications for the risk of CKD [26]. Chen et al. [27] showed that increased WC was significantly related to microalbuminuria and reduced GFR, suggesting that central obesity might be an independent risk for kidney injury, which is in agreement with our data. However, Huh et al. [28] demonstrated that there was no significant association between abdominal obesity and kidney injury, this finding is inconsistent with our study. There are different versions of the diagnostic criteria for MetS and they consider that a possible explanation for this discrepancy is that the cutoff values of obesity for renal damage may be different from those of MetS. At present, there are some differences in the study of the effects of WC on kidney damage. Therefore, we need to explore this issue further.
Age is a major determinant of outcomes in kidney injury. Older patients with CKD are much more likely to die of complications related to aging and CVD before developing ESRD [29]. A review report that kidney function declines with age, people over the age of 40 experience a loss of GFR of about 1 mL/min/year and accelerates slightly in the later years. This steady decline occurs in the context of widespread age-related tissue damage seen in all organs of the body [30]. TG and WC are affected by many factors, while the elderly have more basic diseases, which have a great impact on the two. Just like our researchers, there are many subjects with hypertension and diabetes, and taking antihypertensive and hypoglycemic drugs, these underlying diseases may cause high WC and TG or mutual influence. Since the subject of this article is the elderly in northern China, most people in northern China have halophilic, which may be one of the reasons leading to higher hypertension. It is reported that long-term adherence to the Dietary Approaches to Stop Hypertension (DASH)-style diet may contribute to the prevention of CKD [31]. Moreover, sex may be a factor that potentially influences the association of kidney injury risk with dyslipidemia and central obesity. Many studies have shown that women have a higher prevalence of CKD than men. This simplest explanation is that the longer life expectancy of women combined with the natural decline of kidney function with aging contributes to an enlarged population at risk for CKD [29]. Furthermore, women's WC are higher than men's, which also causes the prevalence of women's kidney injury to be higher. In our study, women with high WC had a higher risk of kidney damage when low eGFR was a dependent variable.
Limitations of our present study should be considered when interpreting these results. First, the sample size of our study was relatively small, especially only 666 participants were included. Second, the electronic medical records reflect the patient's previous physical health status, such as the past history of the disease and family history, which has some guiding significance for the study, which may affect the assessment of clinical risk. Thirdly, positive urine protein only one test, multiple measurements are required to average. Finally, regarding dyslipidemia, we lack indicators such as HDL-C, which may not be perfect. However, we are longitudinal studies that allow us to infer causality between the high TG, WC and kidney injury risk.
Conclusion
In a population of Chinese community-dwelling older adults, high TG levels and central obesity were risk factors for the development of kidney injury over 3 years. Treatment of risk factors would help slow down the reduction of kidney function in elderly patients. At last, regular health check or selective screening of the elderly population is an effective method for early detection of kidney injury.
Glossary
Abbreviations
- CKD
chronic kidney disease
- eGFR
estimated glomerular filtration rate
- UACR
urinary albumin to creatinine ratio
- TG
triglyceride
- WC
waist circumference
- Lipid-lowering treatment
oral lipid-lowering, such as statins and fibrates
- Blood pressure-lowering treatment
oral antihypertensive drugs, such as nifedipine
- Antidiabetic treatment
oral hypoglycemic agents or subcutaneous insulin injections
Acknowledgements
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors. However, we appreciate Tianjin Jun Liangcheng Hospital for supporting this study.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- 1.de Boer IH, Rue TC, Hall YN, et al. . Temporal trends in the prevalence of diabetic kidney disease in the United States. JAMA. 2011;305:2532–2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Go AS, Chertow GM, Dongjie F, et al. . Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Eng J Med. 2004;351:1296. [DOI] [PubMed] [Google Scholar]
- 3.Inker LA, Astor BC, Fox CH, et al. . KDOQI US commentary on the 2012 KDIGO clinical practice guideline for the evaluation and management of CKD. Am J Kidney Dis. 2014;63:713–735. [DOI] [PubMed] [Google Scholar]
- 4.Coresh J, Selvin E, Stevens LA, et al. . Prevalence of chronic kidney disease in the United States. JAMA. 2007;298:2038. [DOI] [PubMed] [Google Scholar]
- 5.Zhang L, Wang F, Wang L, et al. . Prevalence of chronic kidney disease in China: a cross-sectional survey. Lancet. 2012;379:815–822. [DOI] [PubMed] [Google Scholar]
- 6.Zomorrodian D, Khajavi-Rad A, Avan A, et al. . Metabolic syndrome components as markers to prognosticate the risk of developing chronic kidney disease: evidence-based study with 6492 individuals. J Epidemiol Community Health. 2015;69:594–598. [DOI] [PubMed] [Google Scholar]
- 7.Joyce T, Chirino YI, Natalia MT, et al. . Renal damage in the metabolic syndrome (MetSx): disorders implicated. Eur J Pharmacol. 2018;818:554–568. [DOI] [PubMed] [Google Scholar]
- 8.Hossain S, Fatema K, Ahmed KR, et al. . Prevalence and determinants of metabolic syndrome among newly diagnosed type 2 diabetic subjects according to different criteria. Diabetes Metab Syndr. 2015;9:120–123. [DOI] [PubMed] [Google Scholar]
- 9.Aguilar M, Bhuket T, Torres S, et al. . Prevalence of the metabolic syndrome in the United States, 2003–2012. JAMA. 2015;313:1973–1974. [DOI] [PubMed] [Google Scholar]
- 10.Li R, Li W, Lun Z, et al. . Prevalence of metabolic syndrome in Mainland China: a meta-analysis of published studies. BMC Public Health. 2016;16:296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang X, Cui X, Li F, et al. . Association between diabetes mellitus with metabolic syndrome and diabetic microangiopathy. Exp Ther Med. 2014;8:1867–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wong MG, Perkovic V, Chalmers J, for the ADVANCE-ON Collaborative Group, et al. . Long-term benefits of intensive glucose control for preventing end-stage kidney disease: ADVANCE-ON. Diabetes Care. 2016;39:694–700. [DOI] [PubMed] [Google Scholar]
- 13.Giunti S, Barit D, Cooper ME. Mechanisms of diabetic nephropathy: role of hypertension. Hypertension. 2006;48:519–526. [DOI] [PubMed] [Google Scholar]
- 14.Andrew S ,Levey JC. Chronic kidney disease. Lancet. 2012;379: [DOI] [PubMed] [Google Scholar]
- 15.Lanktree MB, Thériault S, Walsh M, et al. . HDL cholesterol, LDL cholesterol, and triglycerides as risk factors for CKD: a Mendelian randomization study. Am J Kidney Dis. 2018;71:166–172. [DOI] [PubMed] [Google Scholar]
- 16.Palazhy S, Viswanathan V. Lipid abnormalities in type 2 diabetes mellitus patients with overt nephropathy. Diabetes Metab J. 2017;41:128–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang AR, Grams ME, Navaneethan SD. Bariatric surgery and kidney-related outcomes. Kidney Int Rep. 2017;2:261–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang P, Wang R, Gao C, et al. . Prevalence of central obesity among adults with normal BMI and its association with metabolic diseases in Northeast China. PLOS One. 2016;11:e0160402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu X, Wang Y, Wang C et al. . A new equation to estimate glomerular filtration rate in Chinese elderly population PLOS One 2013;8:e79675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Russo GT, De Cosmo S, Viazzi F, et al. . Plasma triglycerides and HDL-C levels predict the development of diabetic kidney disease in subjects with type 2 diabetes_ the AMD annals initiative. Diabetes Care. 2016;39: 2278–2287. [DOI] [PubMed] [Google Scholar]
- 21.Hu PJ, Wu MY, Lin TC, et al. . Effect of statins on renal function in chronic kidney disease patients. Sci Rep. 2018;8:16276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moorhead JC, El-Nahas M, Z V. Lipid nephrotoxicity in chronic progressive glomerular and tubulo-interstitial disease. Lancet. 1982;2:1309–1311. [DOI] [PubMed] [Google Scholar]
- 23.Sun K, Lin D, Li F, et al. . Discordant associations of lipid parameters with albuminuria and chronic kidney disease: a population-based study. Lipids Health Dis. 2015;14:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Upadhyay A, Lamont JL, Haynes S, et al. . Lipid-lowering therapy in persons with chronic kidney disease_ a systematic review and meta-analysis. Ann Intern Med. 2012;157:251–262. [DOI] [PubMed] [Google Scholar]
- 25.Kimura G, Kasahara M, Ueshima K, et al. . Effects of atorvastatin on renal function in patients with dyslipidemia and chronic kidney disease: assessment of clinical usefulness in CKD patients with atorvastatin (ASUCA) trial. Clin Exp Nephrol. 2017;21:417–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kovesdy CP, Furth SL, Zoccali C. Obesity and kidney disease: hidden consequences of the epidemic. Braz J Med Biol Res. 2017;50:e6075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen J, Gu D, Chen CS, et al. . Association between the metabolic syndrome and chronic kidney disease in Chinese adults. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association. Eur Renal Assoc. 2007;22:1100–1106. [DOI] [PubMed] [Google Scholar]
- 28.Huh JH, Yadav D, Kim JS, et al. . An association of metabolic syndrome and chronic kidney disease from a 10-year prospective cohort study. Metabolism. 2017;67:54–61. [DOI] [PubMed] [Google Scholar]
- 29.Glassock R, Delanaye P, El Nahas M. An age-calibrated classification of chronic kidney disease. JAMA. 2015;314:559–560. [DOI] [PubMed] [Google Scholar]
- 30.R Lewis. An overview of chronic kidney disease in older people. Nurs Older People. 2013;25:31–38. [DOI] [PubMed] [Google Scholar]
- 31.Yuzbashian E, Asghari G, Mirmiran P, et al. . Adherence to low-sodium dietary approaches to stop hypertension-style diet may decrease the risk of incident chronic kidney disease among high-risk patients: a secondary prevention in prospective cohort study. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association. Europ Renal Assoc. 2018;33:1159–1168. [DOI] [PubMed] [Google Scholar]