Abstract
Objective
Reported prevalence of vasculitic neuropathy (VN) in antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) is highly variable, and associations with other organ manifestations have not been studied systematically while accounting for diagnostic certainty of VN.
Methods
Data of all patients with AAV within the Diagnostic and Classification criteria for primary systemic VASculitis study were analyzed cross-sectionally. VN was categorized as definite (histology proven), probable (multiple mononeuropathy or nerve biopsy consistent with vasculitis), or possible (all others). Associations with other organ manifestations were compared in patients with and without VN.
Results
Nine hundred fifty-five patients (mean age 57 years, range 18–91 years, 51% female) were identified. Of these, 572 had granulomatosis with polyangiitis (GPA), 218 microscopic polyangiitis (MPA), and 165 eosinophilic granulomatosis with polyangiitis (EGPA). The prevalence of VN was 65% in EGPA, 23% in MPA, and 19% in GPA. Nerve biopsy was performed in 32/269 (12%) patients, demonstrating definite vasculitis in 17/32 (53%) of patients. VN was associated with myeloperoxidase-ANCA positivity (p = 0.004) and skin (p < 0.001), musculoskeletal, (p < 0.001) and cardiovascular (p = 0.005) involvement. Patients with VN were less likely to have renal (p < 0.001), eye (p < 0.001), and gastrointestinal (p = 0.023) involvement.
Conclusions
Our study provides comprehensive insights into the prevalence and organ associations of VN in a large, systematically collected AAV cohort. VN is most commonly associated with skin, musculoskeletal, and cardiovascular manifestations. In routine clinical practice, diagnosis of VN is infrequently confirmed by the gold standard of nerve biopsy but rather supported by the clinical setting of active systemic AAV.
Epidemiologic data on the prevalence of vasculitic neuropathy (VN) in antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) are contradictory due to heterogeneous recruitment strategies, VN assessments, sample sizes, and length of follow-up across studies.1–4 Furthermore, de novo neuropathies in the context of AAV are often inferred to be vasculitic without acknowledging the diagnostic uncertainty.
The Diagnostic and Classification criteria for primary systemic VASculitis (DCVAS) study is a large multinational observational case-control study including more than 6,800 patients.5 Its primary goal is to define new diagnostic and classification criteria for the primary systemic vasculitides by a prospective analysis of the most discriminating features for each disorder. The DCVAS study captures all major organ systems affected by primary systemic vasculitic disorders including the central and the peripheral nervous system (PNS). As the world's largest prospective study of vasculitis and vasculitis mimics, DCVAS is best suited to answer epidemiologic questions on systemic VN.
In 2010, the Peripheral Nerve Society Guideline established case definitions for nonsystemic and systemic VN that were revised and adapted in 2017 by the Brighton Collaboration Vasculitic Peripheral Neuropathy Working Group into 3 definitions of VN with varying degrees of diagnostic certainty.6
For this study, we developed VN criteria compatible with the DCVAS data set, adapted from the previously published criteria, which stratified for the level of certainty (definite, probable, or possible) of the vasculitic origin of the neuropathy. Using these criteria, our study aimed to describe the prevalence and organ associations of de novo VN in AAV at diagnosis within the DCVAS cohort.
Methods
Study design and patients
A detailed description of the DCVAS study can be found elsewhere.5 In brief, 6,831 patients with a diagnosis of primary vasculitis or vasculitis mimics were recruited at 135 sites worldwide from January 2011 to August 2017. Primary vasculitides included but were not limited to polyarteritis nodosa, giant cell arteritis, Takayasu arteritis, and AAV. Baseline demographics, clinical features, radiography, EMG/nerve conduction study (NCS) findings, histology, and laboratory results were collected prospectively followed by a 6-month reevaluation for diagnostic certainty and completion of the Vasculitis Damage Index (VDI).7 Patients were included within the first 2 years of diagnosis. Symptoms were recorded if starting at or after onset of vasculitis and judged to be caused by vasculitis. ANCA measurements were performed according to the local laboratory protocol at each center. All data entries were evaluated for accuracy and consistency, and participating centers were contacted in case of inconsistent or missing data. Diagnoses were confirmed by an expert panel after finalization of data entry. We extracted all patients from the DCVAS database with a diagnosis of AAV confirmed by the expert panel.
Standard protocol approvals, registrations, and patient consents
The study was approved by the Berkshire Research Ethics Committee (10/H505/19). The DCVAS study is listed in the ClinicalTrials.gov database (clinical trial identifier number: NCT01066208). All sites obtained any additional ethical and institutional approvals required for their jurisdiction. All patients signed an informed consent form.
Definition of organ involvement
In the DCVAS case report form (CRF), organ categories (general, musculoskeletal, skin, eyes, ear-nose-throat, chest/pulmonary, cardiovascular, gastrointestinal [GI], genitourinary, and neurologic) were structured similar to the Birmingham Vasculitis Activity Scale.8 An organ was defined as involved if any of the organ-specific findings characteristic of vasculitis was recorded. Organ-specific items from the VDI were also included. Renal involvement was defined as definite vasculitis in kidney biopsy, red cell casts in the urine, or 24-hour urine protein concentration >1 g/L.
Definition of VN
The presence of neuropathy and its phenotype were determined by the investigator without further guidance by the CRF. Nerve biopsy was performed at the discretion of the treating physician. In the DCVAS CRF, all biopsy diagnoses were coded as normal, nondiagnostic, consistent with vasculitis but not definite, definite vasculitis, or unspecified tissue inflammation. Criteria for these findings were not further defined in the CRF and therefore at the discretion of the local pathologist.
VN was defined using 3, nonmutually exclusive items of the DCVAS CRF (“mononeuritis multiplex”, “sensory neuropathy”, and “motor neuropathy”) occurring in the context of the diagnosis of AAV, the nerve histology diagnosis, and the “peripheral neuropathy” entry in the VDI (completed by the investigator 6 months after diagnosis). Because of the low rate of biopsy-proven VN diagnosis, we performed a subset analysis by stratifying VN according to its level of diagnostic certainty into definite, probable, or possible categories. Definite VN required “definite” vasculitis by nerve biopsy. For probable VN, we required: (1) nerve biopsy “consistent with vasculitis but not definite” AND “sensory” or “motor neuropathy”; OR (2) “mononeuritis multiplex”. Possible VN was assumed if (1) “sensory neuropathy” without “diabetes mellitus” OR “motor neuropathy” was recorded in the CRF; OR (2) “peripheral neuropathy” was marked in the VDI.
Neuropathic phenotypes were classified as a multiple mononeuropathy (mononeuritis multiplex checked on CRF, with or without concomitant coding of sensory neuropathy and motor neuropathy), sensory neuropathy (only sensory neuropathy checked on the CRF), motor neuropathy (only motor neuropathy checked on the CRF), diffuse sensorimotor polyneuropathy (sensory and motor neuropathy but not mononeuritis multiplex checked on the CRF), or unspecified (appeared in the VDI but not the CRF). The sensorimotor vs sensory vs motor character of the multifocal neuropathies was not consistently detailed in the CRF, precluding an overall assessment of functional modality involvement. EMG/NCS confirmation was not required but performed in 153 (57%) VN cases. Neuropathy “due to radiculopathy” was excluded.
Statistical analysis
Categorical variables were calculated as frequencies and percentages. Continuous variables were calculated as mean with SD and medians with interquartile range. Logistic regression analyses were performed for all variables to determine confounding effects of age and sex. For categorical variables, between-group comparisons were calculated using chi-squared tests or Fisher exact tests; t tests or Mann-Whitney U tests were used to compare continuous variables. Multiple logistic regression was used to assess the combined effect of age, sex, myeloperoxidase- and proteinase-3 (PR3)-ANCA positivity on the associations of VN with other organ involvements. All data were analyzed with Stata/IC 14.1 (StataCorp).
Data availability
Raw data were not acquired as part of a clinical trial. Data from the DCVAS study used for analysis of this study are available from the corresponding author (T.D.) after consultation with the DCVAS steering committee on reasonable request. The data are not publicly available because of ethical restrictions.
Results
Patients
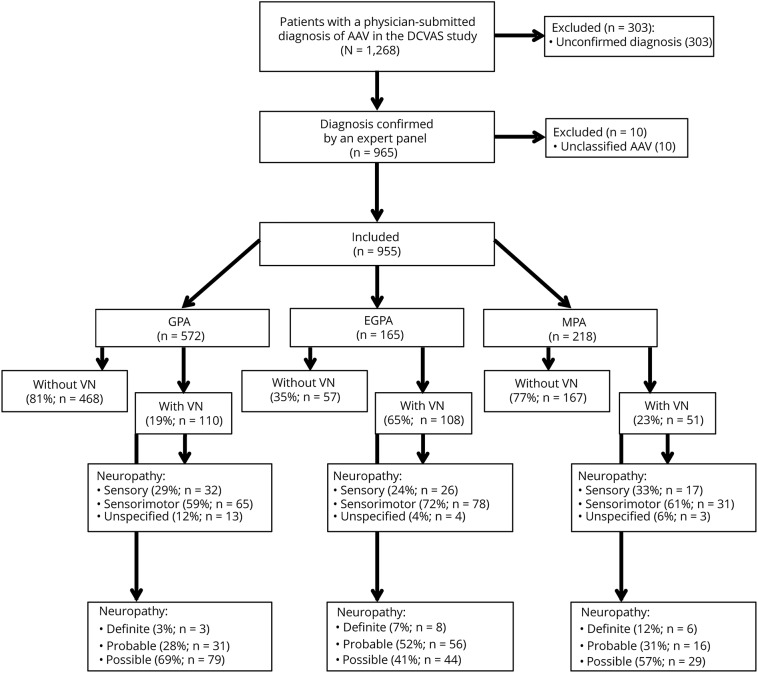
At databank closure in December 2017, 1,268 patients had a physician-submitted diagnosis of AAV (figure 1). Of these, 955 patients (mean age 57 years, range 18–91 years; 486 [51%] females) had the diagnosis confirmed by an expert panel and were included in the analysis. Of these, 572/955 patients were diagnosed as granulomatosis with polyangiitis (GPA), 165 patients as eosinophilic granulomatosis with polyangiitis (EGPA), and 218 patients as microscopic polyangiitis (MPA).
Figure 1. Flowchart of study recruitment.
AAV = ANCA-associated vasculitis; DCVAS = Diagnostic and Classification criteria for primary systemic VASculitis study; EGPA = eosinophilic granulomatosis with polyangiitis; GPA = granulomatosis with polyangiitis; MPA = microscopic polyangiitis; VN = vasculitic neuropathy.
Vasculitic neuropathy
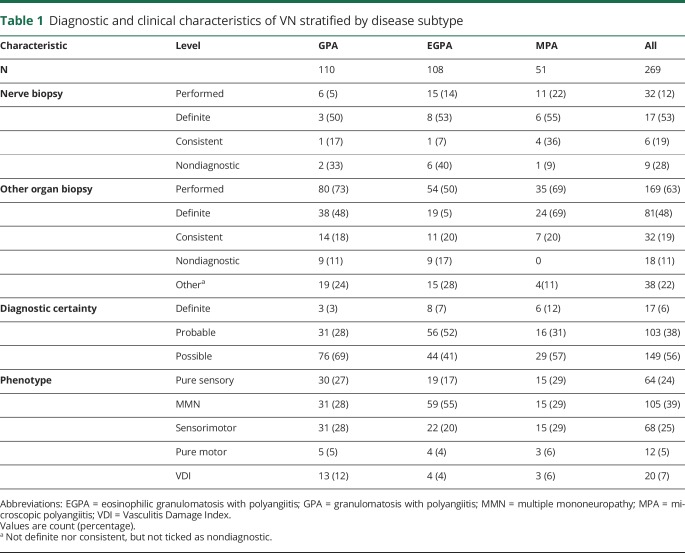
Clinical phenotype and biopsy data of patients with VN are summarized in table 1. VN was diagnosed in 28% (269/955) of patients and occurred more frequently in EGPA (65% of patients) than MPA (23%) and GPA (19%) (p < 0.001). Seven percent of patients had unspecified neuropathies extracted from the VDI (which was recorded at the 6-month follow-up visit) but not recorded in the initial CRF, suggesting that they evolved during the 6-month follow-up period.
Table 1.
Diagnostic and clinical characteristics of VN stratified by disease subtype
In 57% (153/269) of patients, peripheral neuropathy was confirmed by EMG/NCS. Among these patients, 47% (72/153) had a multiple mononeuropathy, 32% (49/153) a sensorimotor neuropathy, 5% (7/153) a motor neuropathy, and 16% (25/153) a sensory neuropathy.
Nerve biopsies were performed in 31/269 (12%) of patients and showed definite vasculitis in 55%. Detailed biopsy results were infrequently recorded. In EGPA, 5/9 nerve biopsies showing definite vasculitis or findings consistent with vasculitis also contained prominent eosinophilic infiltrates. Other organ biopsies were performed in 97/149 patients with possible VN (organ and number of patients biopsied: kidney: 50, ear, nose and throat: 21, skin: 19, lung: 14, temporal artery: 2, intestine: 2, bone marrow: 2, bronchus: 2; liver, brain, muscle, lymph node, spleen, subglottis, parotis: 1 each, respectively; of note, some patients had more than 1 biopsy performed). The biopsy findings were reported as definite in 49/97 (51%) and consistent with but not diagnostic of vasculitis in 19/97 (20%) of patients (combined 70%, table 1).
Differences between patients with and without VN
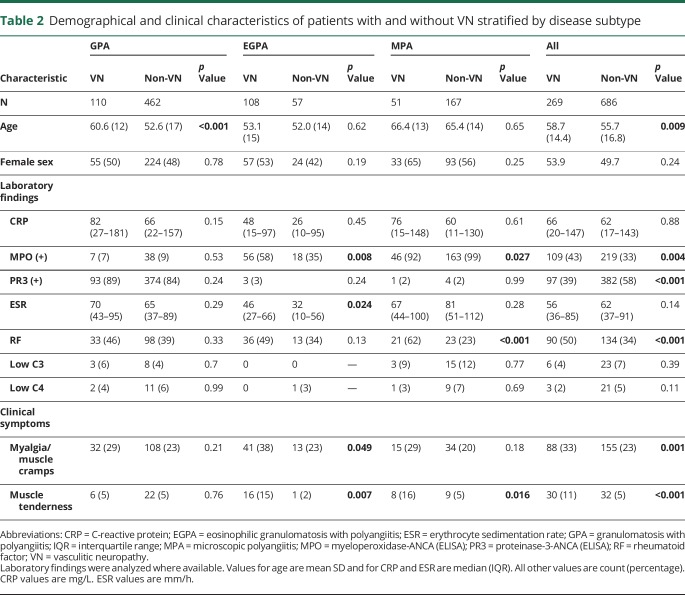
Demographical and clinical characteristics stratified by AAV disease subtype and VN involvement are summarized in table 2. Adjusting for age and positive testing for MPO- or PR3-ANCA antibodies, logistic regression analyses demonstrated that women were more likely to have VN compared with men in patients with EGPA (OR 2.4, 95% CI 1–5.0, p = 0.022).
Table 2.
Demographical and clinical characteristics of patients with and without VN stratified by disease subtype
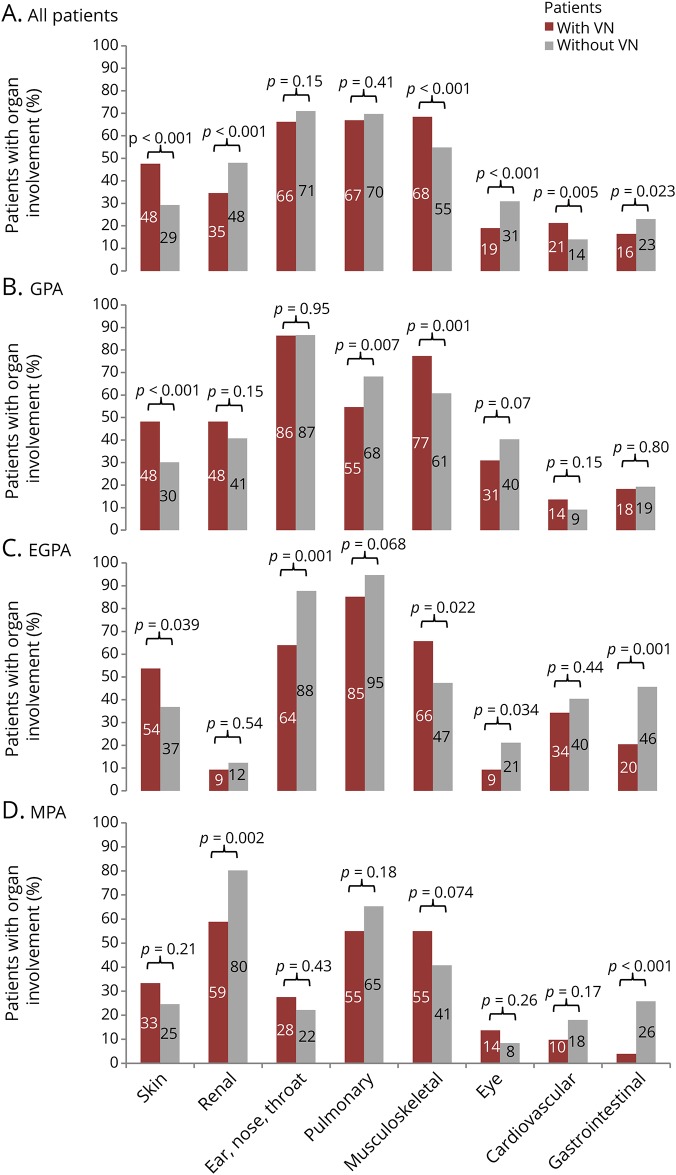
Differences in organ involvement between patients with and without VN are illustrated in figure 2. Patients with VN more often had skin (p < 0.001), musculoskeletal (p < 0.001), and cardiovascular (p = 0.005) involvement (figure 2). Within the musculoskeletal category, VN was associated with skeletal muscle symptoms including muscle weakness, myalgia, muscle cramps, and muscle tenderness (p < 0.001). In contrast, patients with VN were reported as having less renal (p < 0.001), eye (p < 0.001), and GI involvement (p = 0.023). VN was not associated with multiorgan (>5 organs) involvement (p = 0.567). Although cardiovascular involvement was more frequent in patients with VN in the overall AAV group, this difference was not seen in any of the disease subgroups (table 2).
Figure 2. Organ involvement of patients with and without VN by disease.
Comparison of the frequency of organ involvement between AAV patients with and without VN in the overall DCVAS cohort (A), and in patients with GPA (B), EGPA (C), and MPA (D). Numbers displayed above the bars are percentages of patients with organ involvement. EGPA = eosinophilic granulomatosis with polyangiitis; GPA = granulomatosis with polyangiitis, MPA = microscopic polyangiitis; VN = vasculitic neuropathy.
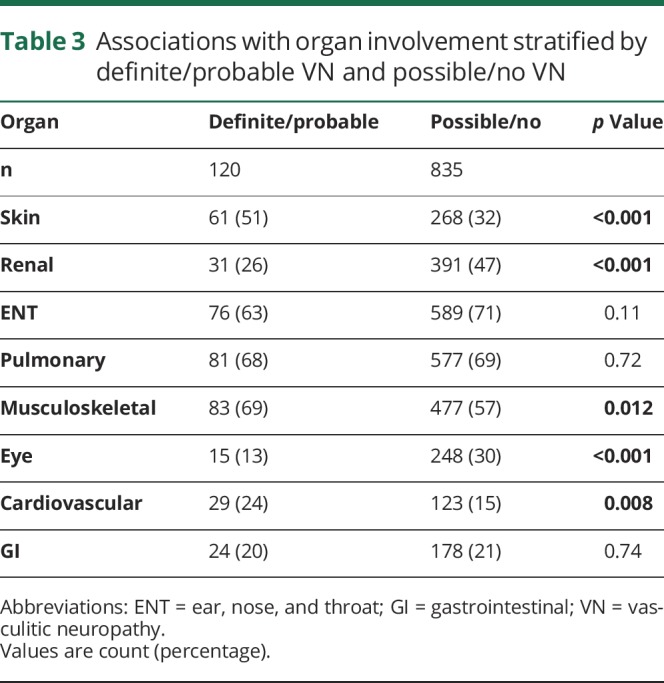
When stratifying the patients into 2 categories by VN certainty (definite/probable VN and possible VN/no VN), the association with MPO-ANCA positivity (probable/definite VN: 50% vs none/possible VN: 34%, p = 0.001) remained unchanged. Further results of VN associations with organ involvement stratified by these 2 categories are shown in table 3.
Table 3.
Associations with organ involvement stratified by definite/probable VN and possible/no VN
Laboratory values detailed in table 2 did not differ between the 2 groups. CSF analyses were infrequently performed and demonstrated no significant differences between patients with and without VN: CSF pleocytosis was present in 2/25 patients with VN and in 1/12 patient without VN (p = 0.999), and elevated protein was found in 5/26 patients with VN and 2/12 patients without VN (p = 0.999), whereas oligoclonal bands were recorded in 2/15 patients with VN and 0/10 patients without VN (p = 0.500). Three of the patients with VN with abnormal CSF had CNS manifestations. One of the 2 patients with pleocytosis (who also had elevated protein) had a TIA and headache. Two additional patients with elevated protein had CNS involvement, one with pachymeningitis and the other with confusion and headache. The 2 patients with VN with positive oligoclonal bands were not reported to have CNS involvement.
Discussion
Within the DCVAS study, which is the largest prospectively studied patient population with AAV to date, we found that the PNS was frequently involved in AAV: the prevalence was 65% in EGPA, 23% in MPA, and 19% in GPA. Thus, de novo neurologic symptoms in the context of a systemic disease are important clues to the diagnosis of AAV.
Whereas the frequency of VN in GPA and EGPA in the DCVAS cohort confirmed previous reports, the prevalence of VN in MPA (23%) was lower than reported in most retrospective analyses.1 However, VN prevalence estimates from previously published smaller series in MPA have been highly variable, ranging from 7% to 58%.1 The strength of the DCVAS study in comparison to previous reports is its large multinational scale and prospectively studied patient population, with the prevalence of peripheral nerve vasculitis being one of the study objectives. The inclusion of nearly 1,000 patients with AAV is likely to make the prevalence reported here a better estimate of the true prevalence rate.
The association of VN with cardiovascular involvement in this study is a novel finding. Possible genetic, epigenetic, and environmental factors leading to this potential association remain to be determined. Further studies are warranted to explain this unexpected finding.
In contrast to a previous large study on the associations of VN with other organ manifestations, we did not find an association of VN with multiorgan involvement (p = 0.567). Our finding that VN occurred less commonly when organs associated with increased mortality (except from cardiac involvement) were affected is in accordance with that study. In particular renal, pulmonary (GPA only) and GI tissues were less frequently affected in patients with VN.4
We found an increased rate of musculoskeletal involvement, particularly myalgias and weakness in patients with VN (68%) compared with those without VN (55%). Muscle involvement in VN is often considered to be subclinical, but our data suggest that it may lead to clinical manifestations in the form of myalgias, muscle tenderness, or weakness in some patients. Alternatively, musculoskeletal pain and weakness might ensue from the VN itself. Our study confirmed the association of VN with skin and musculoskeletal involvement demonstrated in previous investigations.9–12
In line with previous studies, VN in EGPA in the DCVAS showed a strong association to the ANCA-positive EGPA subgroup,2,13 which has been associated with renal and skin involvement. However, it remains unclear whether these 2 EGPA phenotypes based on ANCA status are 2 distinct entities or rather part of the same spectrum. It has been speculated that ANCA positivity might be a surrogate for a more diffuse, widespread vasculitic involvement, which would be supported by the association of peripheral nerve affection with the ANCA-positive group found in our study.2,14,15
CSF findings were infrequently recorded and predominantly negative in the absence of CNS involvement. Studies of CSF abnormalities in systemic VN are sparse and, unlike the current investigation, uncontrolled. In the only larger series, which reported on 40 patients with MPA with VN, elevated CSF protein levels occurred in 34% of patients,16 which was in accordance with findings in the DCVAS. The DCVAS data corroborate the Peripheral Nerve Society Guideline recommendation against routinely performing a lumbar puncture in patients with suspected VN without proximal or CNS involvement.17
Another finding in our study was the low proportion of patients with AAV undergoing nerve biopsy (12%), and more than half of patients (56%) were classified as having “possible VN”. Our data suggest that the majority of patients with systemic VN in routine clinical practice are not biopsied and hence evade ascertainment in biopsy-based VN series.18 In line with previous reports, 53% of nerve biopsies in our study demonstrated histologic evidence of vasculitis, supporting the value of nerve biopsy in case of diagnostic uncertainty.1,9 Furthermore, patients with a new onset peripheral neuropathy in the setting of an active AAV confirmed by either ANCA positivity or another organ biopsy have a high pretest probability for a positive result on nerve biopsy. However, if nerve biopsy is generally avoided to establish the vasculitic origin of a concomitant neuropathy in a patient with an AAV confirmed by non-PNS histology or ANCA positivity,17 implementation of less certain clinical diagnostic criteria for VN is mandated, such as the Brighton or Peripheral Nerve Society consensus definitions.
A major limitation of our study was the absence of a predefined algorithm adhering to published guidelines providing guidance to the study physicians on assessment for VN. The DCVAS study was not designed to assess the characteristics of PNS or other organ manifestations but to develop diagnostic and classification criteria.
However, investigators were advised to record neuropathic signs and symptoms only if they emerged concurrent with the vasculitic illness and were judged to be related to the AAV, excluding preexisting polyneuropathies. We therefore believe that the vast majority of neuropathies in this study had a vasculitic origin. The recording of neuropathy phenotypic characteristics was nonuniform, precluding a reliable tabulation of sensorimotor vs sensory and anatomic patterns.
Despite the efforts to balance ethnicity through global recruitment, there is a predominance of patients of Caucasian origin (∼69%) in this study, precluding a generalization of the results to other ethnicities. Furthermore, differences in the sensitivity of the assays used for the measurement of ANCA at the participating centers might have affected the diagnostic accuracy and biased the results. However, diagnosis of AAV in the DCVAS study was not exclusively based on ANCA positivity but rather on the clinical syndrome and additional paraclinical findings.
In conclusion, the presence of such clinical features as MPO-ANCA positivity, diagnosis of EGPA, and skin, musculoskeletal or cardiovascular involvement may be used to identify patients at risk of neuropathy in those with a new diagnosis of AAV. The VN criteria developed for this study might be useful in future epidemiologic studies in systemic vasculitis lead by non-neurologists to improve the level of diagnostic certainty when nerve biopsy is not performed or inconclusive. Future studies of AAV-associated VN would also benefit from a more detailed compilation of the clinical characteristics of the neuropathy. In a patient with a clinical phenotype typical of VN, the value of other organ biopsies demonstrating active vasculitis to increase the diagnostic certainty of VN warrants further investigation and might inform future diagnostic guidelines for VN in systemic vasculitis.
Acknowledgment
The authors thank all participating patients and investigators for their contribution to this study. They thank the University of Oxford, the American College of Rheumatology, the European League Against Rheumatism (EULAR) and the Vasculitis Foundation for funding the DCVAS study, and the Clinical Trial Unit Basel, especially Silke Purschke for logistical support. Dr. Bischof was supported by the Freiwillige Akademische Gesellschaft, Switzerland, and the Gottfried and Julia Bangerter-Rhyner Foundation, Switzerland.
Glossary
- AAV
ANCA-associated vasculitis
- ANCA
antineutrophil cytoplasmic antibody
- CRF
case report form
- DCVAS
Diagnostic and Classification criteria for primary systemic VASculitis
- EGPA
eosinophilic granulomatosis with polyangiitis
- GI
gastrointestinal
- GPA
granulomatosis with polyangiitis
- MPA
microscopic polyangiitis
- MPO
myeloperoxidase
- NCS
nerve conduction study
- PNS
peripheral nervous system
- PR3
proteinase-3
- VDI
Vasculitis Damage Index
- VN
vasculitic neuropathy
Appendix. Authors
Study funding
No targeted funding reported.
Disclosure
A. Bischof and V.K. Jaeger report no disclosures. R.D.M. Hadden has received personal and departmental payments from CSL Behring and Grifols. R.A. Luqmani reports grants and honoraria from Celgene, Chemocentryx, Grunenthal, GSK, Medimmune, and Roche. A.-K. Pröbstel reports no disclosures. P.A. Merkel reports receiving consulting fees from AbbVie, Biogen, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, ChemoCentryx, Genentech/Roche, Genzyme/Sanofi, GlaxoSmithKline, InflaRx, Insmed, and Kiniksa and research support from AstraZeneca/Medimmune, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, ChemoCentryx, Genentech/Roche, GlaxoSmithKline, Kypha, and Terumo BCT. R. Suppiah received the Rose Hellaby Medical Scholarship, which funded his work on the DCVAS study at inception; he has received speaker fees from Roche, AbbVie, and Pfizer and travel support for international conferences from AbbVie and Pfizer, all unrelated to the submitted work. A. Craven reports no disclosures. M.P. Collins has received consulting fees from Medpace, Inc. and CSL Behring for work unrelated to vasculitis. T. Daikeler reports no disclosures. Go to Neurology.org/NN for full disclosures.
References
- 1.Collins MP, Arnold WD, Kissel JT. The neuropathies of vasculitis. Neurol Clin 2013;31:557–595. [DOI] [PubMed] [Google Scholar]
- 2.Comarmond C, Pagnoux C, Khellaf M, et al. Eosinophilic granulomatosis with polyangiitis (Churg-Strauss): clinical characteristics and long-term followup of the 383 patients enrolled in the French Vasculitis Study Group cohort. Arthritis Rheum 2013;65:270–281. [DOI] [PubMed] [Google Scholar]
- 3.Moosig F, Bremer JP, Hellmich B, et al. A vasculitis centre based management strategy leads to improved outcome in eosinophilic granulomatosis and polyangiitis (Churg-Strauss, EGPA): monocentric experiences in 150 patients. Ann Rheum Dis 2013;72:1011–1017. [DOI] [PubMed] [Google Scholar]
- 4.Suppiah R, Hadden RD, Batra R, et al. Peripheral neuropathy in ANCA-associated vasculitis: outcomes from the European Vasculitis Study Group trials. Rheumatology (Oxford) 2011;50:2214–2222. [DOI] [PubMed] [Google Scholar]
- 5.Craven A, Robson J, Ponte C, et al. ACR/EULAR-endorsed study to develop Diagnostic and Classification Criteria for Vasculitis (DCVAS). Clin Exp Nephrol 2013;17:619–621. [DOI] [PubMed] [Google Scholar]
- 6.Hadden RDM, Collins MP, Živković SA, et al. Vasculitic peripheral neuropathy: case definition and guidelines for collection, analysis, and presentation of immunisation safety data. Vaccine 2017;35:1567–1578. [DOI] [PubMed] [Google Scholar]
- 7.Exley AR, Bacon PA, Luqmani RA, et al. Development and initial validation of the Vasculitis Damage Index for the standardized clinical assessment of damage in the systemic vasculitides. Arthritis Rheum 1997;40:371–380. [DOI] [PubMed] [Google Scholar]
- 8.Mukhtyar C, Lee R, Brown D, et al. Modification and validation of the Birmingham vasculitis activity score (version 3). Ann Rheum Dis 2009;68:1827–1832. [DOI] [PubMed] [Google Scholar]
- 9.Collins MP, Mendell JR, Periquet MI, et al. Superficial peroneal nerve/peroneus brevis muscle biopsy in vasculitic neuropathy. Neurology 2000;55:636–643. [DOI] [PubMed] [Google Scholar]
- 10.Üçeyler N, Braunsdorf S, Kunze E, et al. Cellular infiltrates in skin and sural nerve of patients with polyneuropathies. Muscle Nerve 2017;55:884–893. [DOI] [PubMed] [Google Scholar]
- 11.Vrancken AF, Gathier CS, Cats EA, Notermans NC, Collins MP. The additional yield of combined nerve/muscle biopsy in vasculitic neuropathy. Eur J Neurol 2011;18:49–58. [DOI] [PubMed] [Google Scholar]
- 12.Vital C, Vital A, Canron MH, et al. Combined nerve and muscle biopsy in the diagnosis of vasculitic neuropathy. A 16-year retrospective study of 202 cases. J Peripher Nerv Syst 2006;11:20–29. [DOI] [PubMed] [Google Scholar]
- 13.Sokolowska BM, Szczeklik WK, Wludarczyk AA, et al. ANCA-positive and ANCA-negative phenotypes of eosinophilic granulomatosis with polyangiitis (EGPA): outcome and long-term follow-up of 50 patients from a single Polish center. Clin Exp Rheumatol 2014;32:S41–S47. [PubMed] [Google Scholar]
- 14.Kallenberg CG. Churg-Strauss syndrome: just one disease entity? Arthritis Rheum 2005;52:2589–2593. [DOI] [PubMed] [Google Scholar]
- 15.Pagnoux C. Churg-Strauss syndrome: evolving concepts. Discov Med 2010;9:243–252. [PubMed] [Google Scholar]
- 16.Sugiura M, Koike H, Iijima M, et al. Clinicopathologic features of nonsystemic vasculitic neuropathy and microscopic polyangiitis-associated neuropathy: a comparative study. J Neurol Sci 2006;241:31–37. [DOI] [PubMed] [Google Scholar]
- 17.Collins MP, Dyck PJ, Gronseth GS, et al. Peripheral Nerve Society Guideline on the classification, diagnosis, investigation, and immunosuppressive therapy of non-systemic vasculitic neuropathy: executive summary. J Peripher Nerv Syst 2010;15:176–184. [DOI] [PubMed] [Google Scholar]
- 18.Ng PS, Dyck PJ, Laughlin RS, Thapa P, Pinto MV, Dyck PJB. Lumbosacral radiculoplexus neuropathy: incidence and the association with diabetes mellitus. Neurology 2019;92:e1188–e1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Raw data were not acquired as part of a clinical trial. Data from the DCVAS study used for analysis of this study are available from the corresponding author (T.D.) after consultation with the DCVAS steering committee on reasonable request. The data are not publicly available because of ethical restrictions.