Hereditary sensory and autonomic neuropathy type 1 (HSAN-I)—an autosomal dominant, mainly sensory neuropathy—typically affects patients in their second and third decades, presenting with ulcerations and lancinating pain attacks.1 Diagnosis is heavily dependent on genetic analysis, focusing on 6 genes: serine palmitoyltransferase (SPT) long chain base subunits 1 and 2 (SPTLC1 and SPTLC2), atlastin 1 (ATL1) and 3 (ATL3), DNA methyltransferase 1, and Ras-related protein (RAB7). Most patients carry SPTLC1 mutations (OMIM*605712) that affect sphingolipids biosynthesis by modifying SPT substrate specificity to produce atypical neurotoxic deoxysphingoid bases (DSBs). Along with their accumulation in mutant cells, elevated DSBs were also found in plasma samples, thus becoming a candidate biomarker of disease.2
We identified a novel SPTLC1 missense mutation in a 22-year-old white male patient with early onset of sensory loss. Pathologic and familiar anamnesis was negative, except for isoniazid/pyridoxine chemoprophylaxis, not reaching neurotoxic doses. Sensory alterations were initially subtle with gradual loss of tactile and thermo-dolorific sensation on lower limbs, resulting in repetitive foot ulcerations that required surgical treatment and evolved into Pseudomonas osteomyelitis; of interest, the subject reported neither pain or positive sensory symptoms nor difficulties in motor performances throughout adolescence.
On examination, the patient had pronounced deep and—primarily—superficial sensory disturbances up to his knees after receiving anesthesia in his toes. Muscle strength was preserved; deep tendon reflexes were normal for upper extremities, while he had sluggish patellar reflexes and no ankle jerks. He also had pronated feet with hammer toes and signs of cutaneous dystrophy with nail alterations.
Nerve conduction studies disclosed an axonal, sensory-motor, length-dependent neuropathy (see supplemental data, links.lww.com/NXG/A186). No laser evoked potentials were detected after lower and upper limb stimulation. Autonomic functions were also evaluated with sympathetic skin reflex, Sudoscan, and cardiovascular testing, reporting no significant alteration.
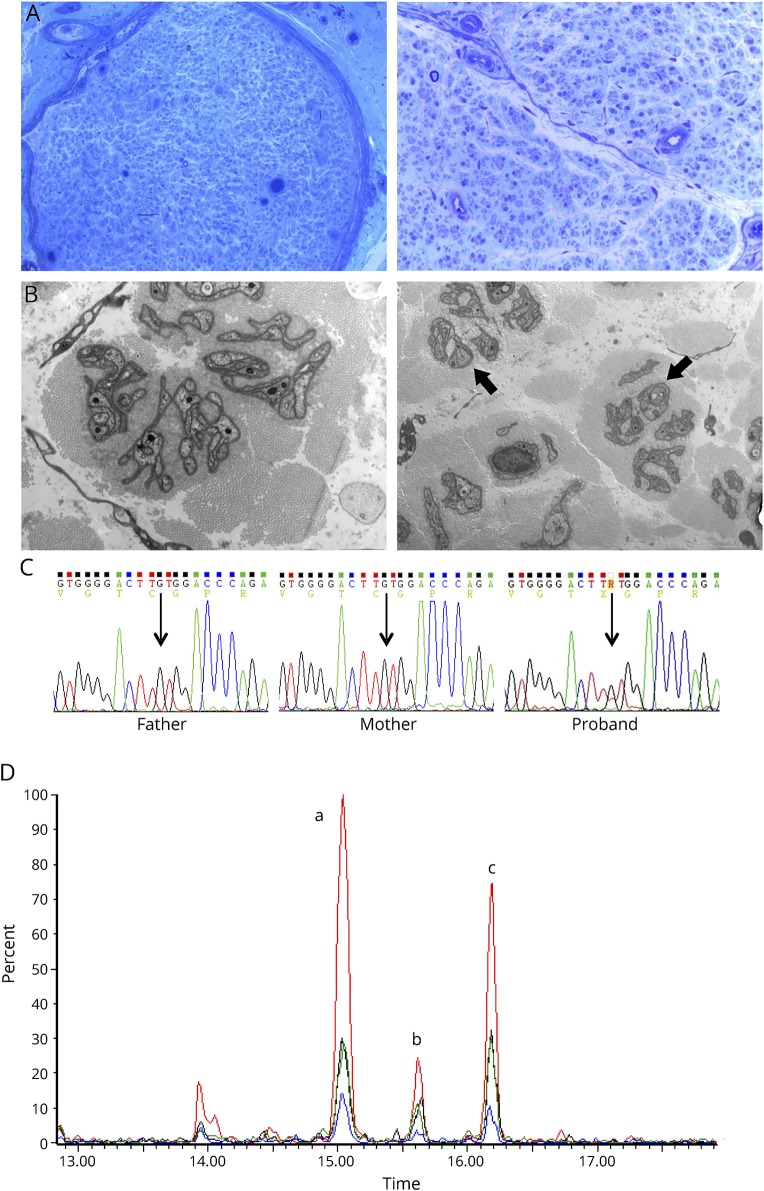
Sural nerve biopsy showed a chronic axonal process with almost complete loss of myelinated fibers and no evidence of active degeneration or clusters of regenerating fibers (figure, A).3 Ultrastructural study disclosed a severe loss of unmyelinated fibers, atrophic and denervated Schwann cell processes, and remarkable absence of collagen pockets (figure, B). Immunofluorescence microscopy of skin biopsy at distal calf also revealed rare intraepidermal nerve fibers.
Figure. Neuropathologic and biochemical correlates of p.Cys133Phe with sequencing details of this novel SPTLC1 mutation.
(A) Sural nerve biopsy, semithin section: the biopsy showed a chronic axonal neuropathy pattern with remarkable loss of myelinated fibers without signs of degeneration or regeneration. Note that the few detectable fibers have a normal myelin sheath (original magnification: left picture 10×; right picture 40×). (B) Electron microscopy (7000×): ultrastructural study showed rare umyelinated fibers (indicated by arrows) with many denervated Schwann cell processes. Note the absence of collagen pockets. (C) Next-generation sequencing with an in-house panel targeted on 45 genes associated with hereditary, predominantly sensory neuropathies was performed on a PGM Ion Torrent machine (Thermo Fisher Scientific), disclosing a novel mutation in SPTLC1 that was absent in both healthy parents and the proband's brother. The mutation was further characterized by Sanger sequencing, demonstrating a heterozygous c.398G>T mutation in SPTLC1 exon 5 causing a p.Cys133Phe (alias p.C133F) substitution, as shown in this panel. In silico analysis predicted a pathogenic relevance of this substitution (PolyPhen-2: 1.0; SIFT score: 0.0; MutationTaster: “disease causing”) because it involves a phylogenetically conserved amino acid in a mutational hot spot. Indeed, most mutations associated with hereditary sensory and autonomic neuropathy type 1 phenotype are located at the monomer-monomer interface and cluster around the pyridoxal-5′-phosphate binding domain; thus, the substitution of a polar residue with a hydrophobic one at this level may affect enzyme's structure by changing substrate specificity, in analogy to previously described pathologic variants at the same Cys133 residue (Cys133Trp, Cys133Tyr, and Cys133Arg). (D) Plasma liquid chromatography–mass spectrometry untargeted lipidomic analysis: extracted chromatograms of fragment ion 268.30 m/z, diagnostic for the deoxysphingosine moiety. The 3 peaks correspond to 3 different eluting deoxyceramides. a: Cer(m18:0/22:0); b: Cer(m18:0/23:0); c: Cer(m18:0/24:0). The plot reports the proband subject (in red) vs 3 asymptomatic relatives (father: blue; mother: green; brother: black). PGM = Personal Genome Machine; SIFT = Sorting Intolerant From Tolerant.
Next-generation sequencing with an in-house panel targeted on 45 Charcot-Marie-Tooth disease (CMT) and sensory CMT-related genes disclosed a SPTLC1 mutation that was absent in both healthy parents and the proband's brother (wild-type allele). The heterozygous c.398G>T transversion caused a p.Cys133Phe substitution, not previously described in reference databases (Exome Aggregation Consortium, Exome Variant Server, and 1000 Genomes), nor in the Human Gene Mutation Database (figure, C). In silico analysis predicted its pathogenic relevance, as it involves a phylogenetically conserved amino acid in a mutational hot spot, and the substitution of a polar residue with a hydrophobic one may affect the enzyme structure by changing substrate specificity, in analogy to other previously described pathogenic variants at the same residue (Cys133Trp, Cys133Tyr, and Cys133Arg).
The mutation functional impact was also addressed with untargeted lipidomic analysis (see supplemental data, links.lww.com/NXG/A186), detecting abnormally high levels of 3 deoxyceramides (22:0, 23:0, and 24:0) in the proband's plasma samples, compared with asymptomatic relatives (mother, father, and brother; figure, D). The aberrant m18:0 deoxysphingosine species were identified by their diagnostic MS/MS fragment ion (280.30 m/z,4), thus reinforcing the hypothesis of SPT incorporating alanine into sphingolipids. On the other hand, we did not observe any significant proof of glycine use as an alternative substrate, as there were no corresponding m17:0 sphingoid bases (fragment ion 253.27 m/z).
In conclusion, we identified a novel SPTLC1 mutation associated with early-onset, fairly typical HSAN1, despite the absence of pain attacks. Besides neurophysiologic and pathologic data, genetic interpretation and biochemical evidence strengthen the hypothesis of p.Cys133Phe pathogenicity, reflecting the mutant enzyme's ability to incorporate alanine instead of serine to generate atypical DSB. The pathologic mechanisms of their toxicity are still unanswered,5 but it is intriguing to note that high DSB levels have also been detected in patients with diabetic and paclitaxel induced neuropathy, suggesting a possible common neurotoxic effect to explain the clinical similarities of these neuropathies. Furthermore, DSB plasma measures could become a new diagnostic and prognostic marker, all the more so since HSAN1 might be a potentially treatable neuropathy through modulation of deoxysphingolipid formation by amino acid availability. In particular, l-serine supplementation could reduce disease severity and progression by limiting DSB accumulation (as already shown in mouse models6 and a recent clinical trial7), thus urging early diagnosis of HSAN1 from a genetic and biochemical point of view, especially for patients with atypical mutations.
Appendix. Authors
Study funding
No targeted funding reported.
Disclosure
Disclosures available: Neurology.org/NG.
References
- 1.Auer-Grumbach M, De Jonghe P, Verhoeven K, et al. Autosomal dominant inherited neuropathies with prominent sensory loss and mutilations: a review. Arch Neurol 2003;60:329–334. [DOI] [PubMed] [Google Scholar]
- 2.Houlden H, Bull K, Murphy SM, et al. Hereditary sensory and autonomic neuropathy type 1: correlation of severity and plasma atypical deoxy-sphyngoid bases. J Neurol Neurosurg Psychiatry 2012;83(suppl 2):1042. [Google Scholar]
- 3.Houlden H, King R, Blake J, et al. Clinical, pathological and genetic characterization of hereditary sensory and autonomic neuropathy type 1 (HSAN I). Brain 2006;129:411–425. [DOI] [PubMed] [Google Scholar]
- 4.Zitomer NC, Mitchell T, Voss KA, et al. Ceramide synthase inhibition by fumonisin B1 causes accumulation of 1-deoxysphinganine. A novel category of bioactive 1-deoxysphingoid bases and 1-deoxydihydroceramides biosynthesized by mammalian cell lines and animals. J Biol Chem 2009;284:4786–4795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reilly MM, Greensmith L, Wilson ER, et al. Hereditary sensory neuropathy type 1-associated deoxysphingolipids cause neurotoxicity, acute calcium handling abnormalities and mitochondrial dysfunction in vitro. Neurobiol Dis 2018;117:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garofalo K, Penno A, Schmidt BP, et al. Oral l -serine supplementation reduces production of neurotoxic deoxysphingolipids in mice and humans with hereditary sensory autonomic neuropathy type 1. J Clin Invest 2011;121:14–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fridman V, Suriyanarayanan S, Novak P, et al. Randomized trial of l-serine in patients with hereditary sensory and autonomic neuropathy type 1. Neurology 2019;92:e359–e370. [DOI] [PMC free article] [PubMed] [Google Scholar]