Abstract
Objective
To determine the effect of vitamin D3 on interferon-β (IFN-β) response and immune regulation in MS mononuclear cells (MNCs).
Methods
MNCs from 126 subjects, including therapy-naive patients with different forms of MS, plus patients with MS receiving IFN-β or glatiramer treatment, plus healthy controls were incubated in vitro with IFN-β-1b ± vitamin D3 (calcitriol). Activation of the IFN-β–induced transcription factor, p-Y-STAT1, and antiviral myxovirus A (MxA) protein was measured with flow cytometry and Western blots; serum proteins were measured with a customized 31-protein multiplex assay.
Results
Vitamin D enhanced in vitro IFN responses, as measured by induction of p-Y-STAT1 and MxA in MNCs, T cells, and monocytes. Vitamin D augmentation of IFN responses was seen in untreated and in IFN-β-1b–treated MS. The combination of vitamin D plus IFN-β reduced Th1 and Th17 cytokines, and increased Th2 responses, reversing the effect of IFN-β alone. Exacerbations and progression in untreated patients reduced the vitamin D enhancement of IFN responses. Vitamin D had less effect on IFN response in clinically stable glatiramer-treated than in IFN-β–treated patients.
Conclusion
Vitamin D enhances IFN-β induction of multiple proteins and also reverses the Th1/Th2 bias in MS seen with IFN-β alone. The combination of vitamin D and IFN-β has potential benefit in ameliorating MS.
Vitamin D in serum controls the onset and activity of MS, and vitamin D affects clinical responses to interferon-β (IFN-β) therapy. The mechanism is unknown.
Vitamin D serum levels correlate with onset of MS and with clinical responses to IFN-β.1 The chance of developing MS in far Northern and far Southern latitudes and in cloudy regions of France is linked to lack of sunlight and to low serum vitamin D levels.2 In established MS, exacerbations and disease progression are linked to low serum vitamin D levels.3 In the BENEFIT study of clinically isolated demyelinating syndrome, high vitamin D levels correlated with fewer second attacks, fewer active MRI lesions, less T2 lesion load, and less brain atrophy.4
When exogenous vitamin D is added to IFN-β-1b therapy, there is MRI and clinical benefit. For instance, in a randomized, double-blind trial, 20,000 U/wk of oral vitamin D3 added to IFN-β-1b therapy led to fewer active Gd+ MRI lesions, faster tandem walking speed than at baseline, and a trend for less disability.5 In the subgroup with prestudy activity during IFN-β therapy, those supplemented with vitamin D had fewer Gd-enhancing and new T2 lesions and less T2 lesion burden.6 In a mix of IFN-β–treated and untreated Tasmanian patients, only those on IFN-β therapy showed clinical benefit from high serum vitamin D levels,7 suggesting that there are complex dose/response relationships in vivo. For instance, blacks have lower serum vitamin D levels than whites,8 and there was less benefit from 2 forms of IFN-β-1a in blacks than whites.9
We hypothesized that combination of vitamin D with IFN-β would enhance IFN-regulated responses. We examined the mechanism of in vitro vitamin D effects on intracellular IFN signaling, antiviral responses, inflammatory cytokines, and neuroprotective proteins in immune cells from untreated and IFN-treated patients with MS and healthy controls (HCs). We demonstrate that vitamin D enhances IFN responses in activated MS immune cells and also provokes a marked shift from Th1 to Th2 responses.
Methods
Subjects
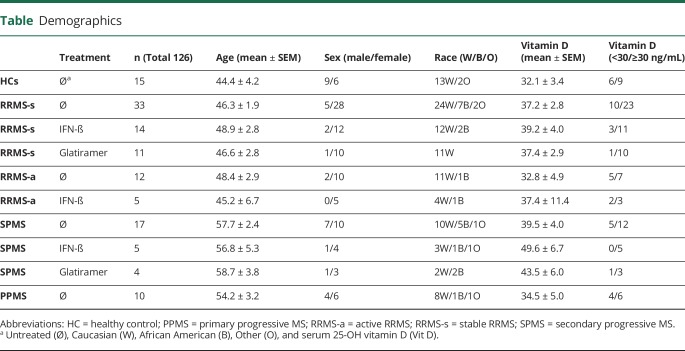
One hundred twenty-six subjects, 111 patients with MS, and 15 HCs were studied. Seventy-two therapy-naive patients with MS included 33 stable relapsing/remitting MS (RRMS-s), 12 active RRMS (RRMS-a), 17 secondary progressive MS (SPMS), and 10 primary progressive MS (PPMS). Thirty-nine treated patients with MS were receiving IFN-β (14 RRMS-s, 5 RRMS-a, and 5 SPMS) or glatiramer acetate (GA; 11 RRMS-s and 4 SPMS) (table).
Table.
Demographics
Data availability
All data from this study are included in the article, but anonymized data will be shared by request from any qualified investigator. It is not a clinical trial.
Standard protocol approvals, registrations, and patient consents
Written consent, approved by the University of Chicago institutional review board, was obtained from all subjects.
In vitro IFN response
Mononuclear cells (MNCs) were isolated on lympholyte-H density gradients (Cedarlane Laboratories, Burlington, Canada). In preliminary experiments from 33 sets of MNCs from HCs and patients with MS, IFN-β alone and vitamin D alone had minimal effects on protein secretion by resting cells, but induced robust production by in vitro–activated cells. The dose effect and kinetics of vitamin D (10–400 nM, over 0–48 hours) in these cells were tested and optimized with vitamin D preincubation using replicates at 0, 1, 2, 3, 6, 8, 12, 16, 18, 20, and 24 hours for activation of the p-Y-STAT1 transcription factor and also 0, 24, and 48 hours for induction of the antiviral myxovirus A (MxA) protein. U937 monocytoid and Jurkat T-cell lines exhibited similar responses.
Vitamin D elevated IFN-induced p-Y-STAT1 and MxA at all points, but did not prolong the amplitude after the peak. IFN and IFN plus Vit D both caused maximal rise at 30 minutes, and then, p-Y-STAT1 levels began to decline. Although the IFN + VitD levels were higher at all points, the shape of the kinetic curve was the same. HC and MS MNCs exhibited the same kinetics, with and without vitamin D. MxA protein was induced at 24 and increased at 48 hours before declining. We chose 30 minutes and 48 hours to compare differences between combination therapy and IFN alone.
We also compared media, 5 μg/mL concanavalin A (ConA) mitogen, or the combination of 5 ng/mL phorbol myristate acetate (PMA) and 1 μM ionomycin (Sigma-Aldrich). Cytokine induction was minimal with media alone. Cytokine levels were more variable with PMA/ionomycin than with ConA and exhibited less distinction between Th1, Th2, and other cytokine families than ConA. There were also no differences in kinetic response curves between IFN-β-1b (Bayer) and IFN-β-1a (Serono) at 160 U/mL for 0–2 hours (p-Y-STAT1) or 0–48 hours (antiviral MxA).
For all subsequent experiments, 4 × 106 MNCs/mL in 250 μL AIM-V medium (Life Technologies) were preincubated with 200 nM activated vitamin D3 (1,25 [OH]2 vitamin D3/calcitriol, Enzo Life Sciences) or media at 37°C in 5% CO2 for 12 hours and then activated for 2 days with ConA as a model of inflammation. We measured the peak p-Y-STAT1 activation at 30 minutes and MxA at 48 hours after ConA activation, using flow cytometry and Western blots.
Flow cytometry
Stimulated cells were fixed, permeabilized, and stained with monoclonal rabbit antibodies to phospho-STAT1 (Tyr701) (clone 58D6, Alexa Fluor 488 conjugate, Cell Signaling Technology, Danvers, MA) or monoclonal primary rat anti-MxA (Biogen, Cambridge, MA) followed by goat anti-rat immunoglobulin G (IgG) FITC (BioLegend), according to the manufacturer's protocols. To correct for fluorescence intensity variation between experiments, we used multiple internal controls. These included unstained background control, isotype control, medium control, and untreated sample control. Isotype control (Cell Signaling Technology) of rabbit IgG Alexa Fluor 488 was performed to correct for nonspecific binding of p-Y-STAT1 staining. We observed very minimal signal on isotype and unstained controls. Flow cytometry was performed on an LSRFortessa cell analyzer using FACSDiva software (Becton Dickenson, BD). Numbers of MNCs, lymphocytes, and monocytes, based on forward and side scatter, and median fluorescence intensity (MFI) of protein expression10 were analyzed with FlowJo software (BD).
After FlowJo software analysis (BD Biosciences), MFI raw values from each condition were exported to an Excel spreadsheet for calculating fold of change. We first normalized all samples by subtracting isotype controls. We then used the Vitamin D Stimulation Index (VDSI) to determine fold change in MFI of protein expression with a 4-component formula, [(IFN+VitD) − VitD alone]/(IFN alone − media)]. This index measures the additive effect of vitamin D plus IFN-β compared with IFN alone and corrects for differences in induction by IFN alone and for the minor effects of vitamin D alone or media alone.
Western blots
MNCs were lysed in Laemmli buffer plus protease inhibitors and 1 mM Na orthovanadate.11 Cell lysates were run on 8% separating/5% stacking gels and transferred to Immun-Blot polyvinylidene difluoride membranes for enhanced chemiluminescence analysis with luminol/enhancer solution and peroxide solution (Bio-Rad). Western blots were quantitated on a CHEMI Genius Bio Imaging System using GeneSnap Image Acquisition Software and analyzed on Gene tools (Syngene, Frederick, MD). Antibodies were primary rat anti-MxA (Biogen), p-STAT1 (Y701) rabbit MAb (Cell Signaling Technology), actin (I-19) goat polyclonal IgG, donkey anti-goat IgG-horseradish peroxidase (HRP), chicken anti-rat IgG-HRP, and goat anti-rabbit IgG-HRP (Santa Cruz Biotechnology). The relative fold of change between IFN alone and vitamin D added to IFN was calculated with the VDSI, below.
Gel densitometry (ChemiDoc XRS + system imager, Bio-Rad) is based on charge-coupled device high-resolution, high-sensitivity detection, followed by Gene Tools analysis (Syngene). This software automatically adjusts densitometry readouts and maintains the same ratio of MxA/actin regardless of the changing background or sharpness on image. Representative figures capture the intensity at one brightness level, but the raw data are imaged at a dynamic range of >4.0 orders of magnitude with a 65,535 pixel density (gray levels). During chemiluminescent exposure, care was taken to generate images that were not overexposed to prevent signal plateau/saturation and loss of dynamic range.
We found that Western blot experiments were actually more reproducible than other assays for this intracellular protein. We and other researchers find that Western blots of both p-Y-STAT1 and MxA are reliable markers of IFN responses and also show subtle differences in molecular weight due to posttranslational modifications that are missed with other techniques.12
Cytokine profiles
Supernatants from in vitro–activated MNCs with medium alone, vitamin D alone, IFN-β alone, and vitamin D added to IFN-β were measured with 31-cytokine customized multiplex (Procarta Immunoassay Kit, Affymetrix) in 40 patients. Targets were based on the MS literature and our preliminary experiments extracted from 280 candidates, including cytokines, chemokines, and neuroendocrine and neurotrophic proteins. Many of these induced proteins are detectable in vitro, unlike in serum, where most cytokines are at very low levels.13 Analytes with significant changes are listed in figure 6. Patient subtypes and HCs were randomized between plates. Assays were run according to the manufacturer's guidelines.
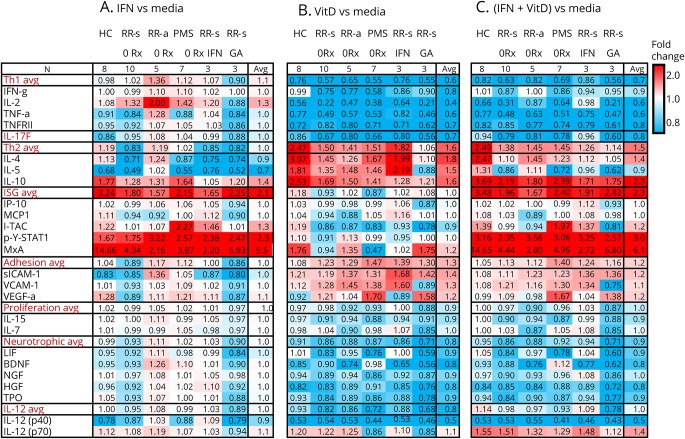
Figure 6. Multiplex of fold change of protein secretion by activated MNCs from HCs and different forms of MS.
(A) IFN-β alone vs media, expressed as ratio of IFN-β/media. (B) Vitamin D alone vs media. (C) IFN-β plus vitamin D vs media alone. Selected targets are grouped into Th1, Th17, and Th2 immune pathways and clusters of IFN-stimulated proteins (ISGs), adhesion molecules, and cytokines controlling homeostatic proliferation, neurotrophic factors, and IL-12 family. Values are fold change in the stimulation condition/media. Intensity of shading shows upregulation (red) or downregulation (blue). Target proteins include BDNF = brain-derived neurotrophic factor; HGF = hepatocyte growth factor; IFN-γ = interferon-γ; IL-2 = interleukin-2; TNF-α = tumor necrosis factor-α; TNFRII = TNF receptor type II; IL-17F, IL-4, IL-5, IL-10, IP-10 = IFN-induced protein-10 (CXCL10); MCP1 = macrophage chemotactic protein-1 (CCL2); I-TAC = IFN-inducible T-cell alpha chemoattractant (CXCL11); LIF = leukemia inhibitory factor; p-Y-STAT1 = phosphotyrosine-STAT1 transcription factor; MxA = myxovirus A; NGF = nerve growth factor; sICAM-1 = soluble intercellular adhesion molecule-1; TPO = thymopoietin; VCAM-1 = vascular cell adhesion molecule; VEGF-α = vascular endothelial growth factor-α, IL-15, IL-7, and IL-12 p40 and IL-12 p70 components.
Serum vitamin D levels
Total 25-OH D, 25-OH D3, and 25-OH D2 were quantitated with liquid chromatography/mass spectroscopy (Quest Diagnostics, Schaumburg, IL). Total serum 25-OH D levels less than 20 ng/mL indicate vitamin D deficiency; 20–30 ng/mL suggest insufficiency; 30–100 ng/mL is considered optimal for healthy normal subjects.
Statistical analysis
Values between subject groups were compared with unpaired student t tests. Baseline vs later drug-induced values were analyzed with paired t tests in all conditions. Figures were generated using R package ggplot2. To compare protein expression after induction with vitamin D added to IFN vs IFN alone, we used the VDSI. It determines fold change in MFI with a 4-component formula, [(IFN+VitD) − VitD alone]/(IFN alone − media)]. This index measures the additive effect of vitamin D plus IFN-β compared with IFN alone, and corrects for differences in induction by IFN alone, and minor effects of vitamin D or media alone. Statistical significance was delineated as *p < 0.05 and **p < 0.01.
Results
Enhancing effects of vitamin D on intracellular IFN-β responses in MNCs
Untreated MS
IFN-β responses in vitro were first examined by measuring activation of the p-Y-STAT1 transcription factor and induction of the antivirus MxA protein in ConA-activated MNCs from 72 untreated patients with MS. IFN-β alone induced rapid phosphorylation of p-Y-STAT1, typically a 3-fold increase in p-Y-STAT1 levels compared with media alone (figure 1A). Twelve-hour pretreatment with vitamin D alone had only minimal effects on activation of p-Y-STAT (figure 1B). IFN-β plus vitamin D displayed dramatic induction, with a 10-fold increase in p-Y-STAT1 levels compared with IFN-β alone (p = 0.00002, paired t test).
Figure 1. Vitamin D enhances IFN-β–induced expression of p-Y-STAT1 and MxA in MNCs.
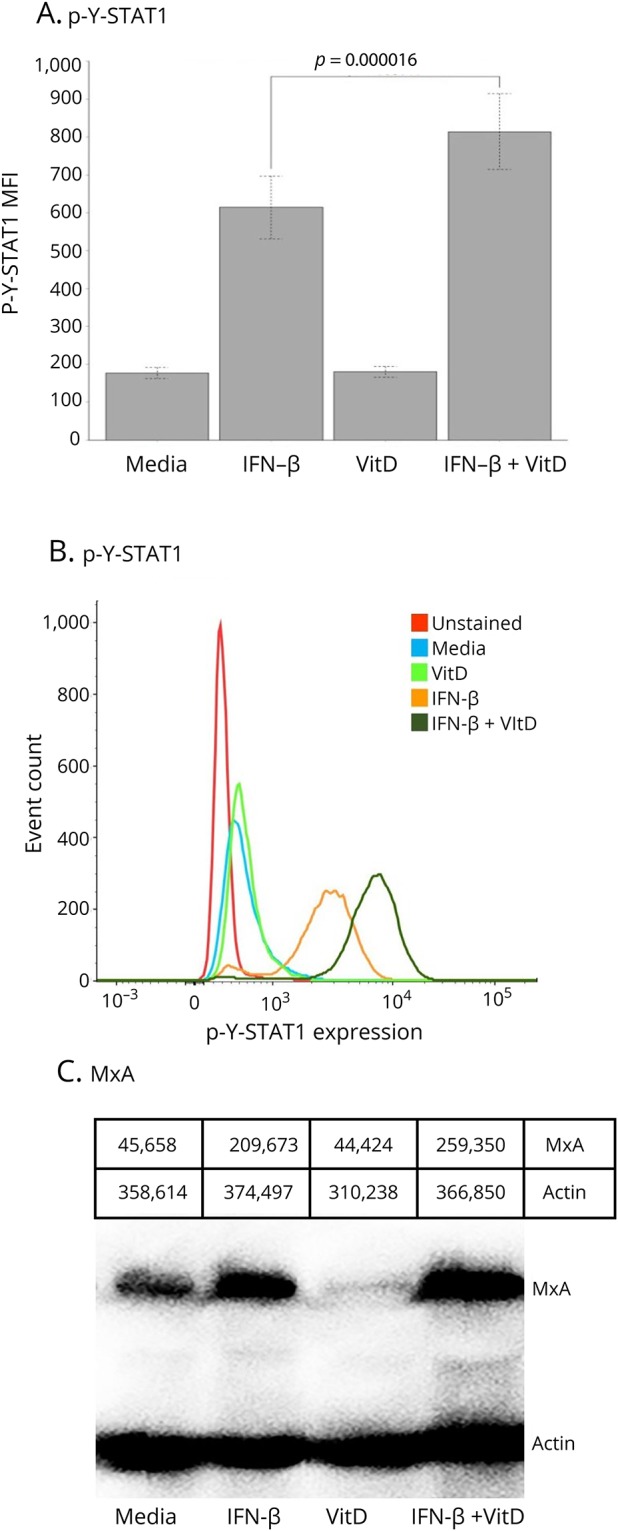
(A) In vitro vitamin D enhances IFN-β induction of p-Y-STAT1 in ConA-activated MNCs from 72 untreated patients with MS (p = 0.00002). (B) p-Y-STAT1 expression on flow cytometry histograms from a representative patient with RRMS-s. The media, IFN, VitD, and IFN+VitD curves are all stained with Alexa Fluor 488–labeled Mab to p-Y-STAT1. (C) MxA protein on Western blot of a representative patient with RRMS-s. ConA-activated MNCs were incubated in vitro with 160 U/mL IFN-β-1b for 30 minutes to 48 hours ± preincubation with 200 nM Vit D3 (calcitriol) for 12 hours. Gray-scale densities of each band are listed above figure. IFN-β = interferon-β; MxA = myxovirus protein.
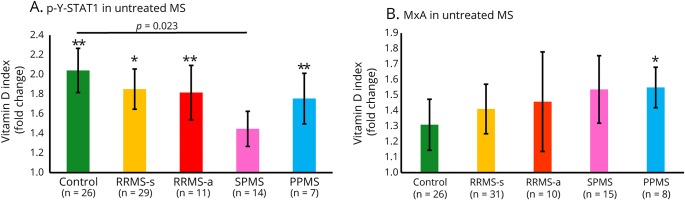
The effect of vitamin D on intracellular IFN-β response was compared between subgroups of untreated patients with different forms of MS. HCs were the best responders to in vitro vitamin D plus IFN-β and had the highest median elevation in p-Y-STAT1 (p < 0.01) (figure 2A). All patient groups except SPMS showed significant vitamin D enhancement of STAT1 activation. There was less induction in SPMS than in HC.
Figure 2. Vitamin D enhances intracellular IFN-β responses in therapy-naive MS and HCs.
(A) p-Y-STAT1, measured with flow cytometry, is generated in all groups, but there is less induction in SPMS than in HC. (B) MxA protein, measured with Western blots. The trend for enhanced responses in PPMS vs HC was not significant (p < 0.13, unpaired t test). ConA-activated MNCs were incubated in vitro with 160 U/mL IFN-β-1b for 30 minutes to 48 hours ± preincubation with 200 nM vitamin D3 (calcitriol) for 12 hours. Fold change of vitamin D plus IFN-β compared with IFN-β alone, determined using VDSI: [(IFN + VitD) − (VitD)]/[(IFN) − (no IFN)]; average with p values above SEM bar: *p < 0.05, **p < 0.01. Comparisons between groups use unpaired t tests, brackets. PPMS = primary progressive MS; RRMS-a = active relapsing/remitting MS; RRMS-s = stable RRMS; SPMS = secondary progressive MS.
For MxA protein, IFN-β alone showed greater induction than media or vitamin D alone in cells from therapy-naive patients with MS (figure 1C). IFN-β plus vitamin D induced much more MxA compared with IFN-β alone. Thus, vitamin D activates STAT1 and modifies the downstream response to IFN-β.
Vitamin D enhanced IFN-induced MxA production in all MS groups and in HCs (figure 2B). Therapy-naive patients with PPMS, with the highest median fold change in (p < 0.05, MxA), and patients with SPMS were the best responders to in vitro MxA induction by IFN-β plus vitamin D. The trend for enhanced responses in PPMS compared with HC was not significant. This is because the VDSI corrects for baseline MxA levels when it measures the incremental change from IFN-β alone vs the combination of IFN-β plus vitamin D. IFN-β alone induced far more MxA in HC (14.7-fold induction) than in all forms of MS (2- to 6-fold); RRMS-s had the highest level in untreated patients (described below; figure 6A). This low level of MxA parallels the subnormal response to type I IFN during active MS.12 Vitamin D corrected the inability of IFN-β alone to induce a robust response. The combination of vitamin D and IFN-β induced MxA more during relapses and progression than in HC.
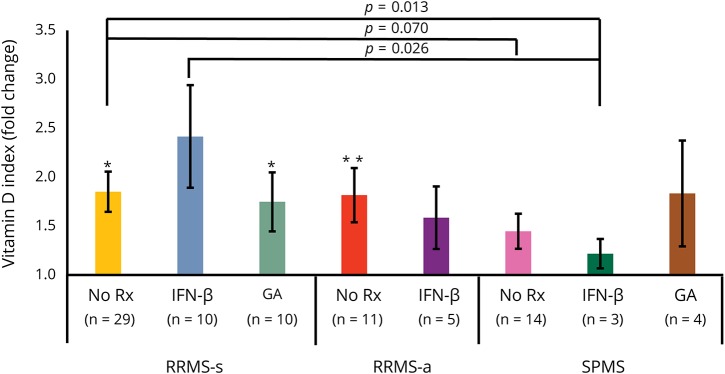
Treated MS
Vitamin D enhanced IFN-β induction of p-Y-STAT1 levels in MNCs from IFN-treated RRMS-s and RRMS-a, but had no effect in IFN-treated SPMS cells (figure 3). Responses in IFN-β-treated RRMS-s were greater than RRMS-a, and in turn greater than SPMS, paralleling our demonstration of IFN resistance in active and progressive MS.12 With glatiramer treatment, there was more activation of p-Y-STAT1 in RRMS-s and a trend for higher p-Y-STAT1 in a small number of SPMS (nonsignificant).
Figure 3. Vitamin D enhances IFN-β–induced p-Y-STAT1 expression in MNCs from patients with MS receiving MS therapies.
In all groups, there is enhanced induction of p-Y-STAT1, measured with flow cytometry. Methods and calculations as in figure 2. *p < 0.05, **p < 0.01. IFN-β = interferon-β; RRMS-a = active relapsing/remitting MS; RRMS-s = stable RRMS; SPMS = secondary progressive MS.
There was an age effect in IFN-treated patients with RRMS-s, but not in other groups. Younger patients with RRMS-s on IFN-β therapy (<45 years old) had better in vitro response to IFN-β plus vitamin D (3.5-fold induction) compared with older patients (1.5-fold induction) (p < 0.05). There were no sex or race effects on this response. MxA induction was similar to that seen with p-Y-STAT1 in treated patients (data not shown).
Thus, vitamin D improves in vitro responses to IFN-β in both untreated and in IFN-β-1b and GA-treated patients with MS. There is a lower response to IFN in MNCs from relapsing and progressive patients than in stable patients. This variability could be from the inflammatory milieu in MS or from differences in vitamin D serum levels.
Effect of low vs high serum levels of vitamin D
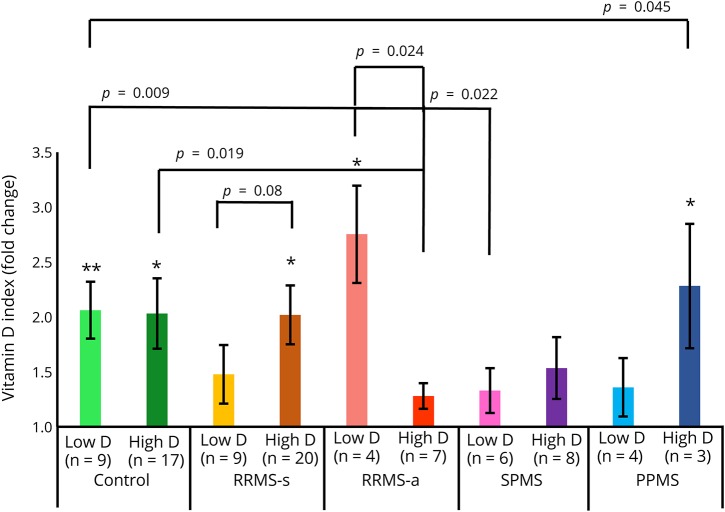
Subjects were divided into low (<30 ng/mL) and normal/high serum vitamin D (≥30 ng/mL) cohorts to compare their responses to in vitro IFN-β-1b plus vitamin D. In both low and high serum vitamin D groups, in vitro vitamin D enhanced activation of STAT1 by IFN-β (figure 4). HCs had good responses, regardless of serum vitamin D levels. In contrast, patients with RRMS-s, SPMS, and PPMS with high vitamin D were better responders than those with low vitamin D levels. This suggests that even on a background of high serum vitamin D levels, additional vitamin D enhances responses to IFN-β. Conversely, during exacerbations, untreated patients with RRMS-a with low serum vitamin D levels had even better in vitro induction of p-Y-STAT1 than those with high levels. This suggests that immune dysregulation and the characteristic low IFN-β responses during exacerbations would benefit from additional vitamin D.
Figure 4. Effect of high vs low serum vitamin D levels from therapy-naive patients with MS on IFN response in MNCs in vitro.
Vitamin D plus IFN-β–induced p-Y-STAT1 expression is quantitated with flow cytometry. The median vitamin D level for the entire cohort was 30; low < 30, high ≥ 30 ng/mL 25-OH vitamin D. Methods and calculations as in figure 2. *p < 0.05, **p < 0.01. RRMS-a = active relapsing/remitting MS; RRMS-s = stable RRMS; PPMS = primary progressive MS; SPMS = secondary progressive MS.
Vitamin D effects on responses to IFN-β in immune cell subsets
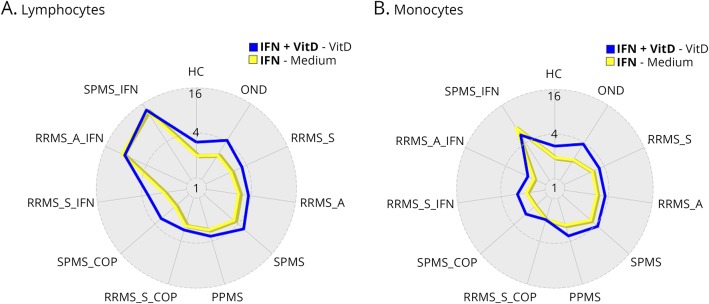
Vitamin D affected p-Y-STAT1 induction in lymphocytes and monocytes. Vitamin D plus IFN-β (figure 5, blue line) in most therapy-naive groups had a greater effect than IFN-β alone (yellow line) on activation of STAT1. However, there is little effect of vitamin D stimulation on lymphocytes from SPMS and RRMS-a (figure 5A), or on monocytes from SPMS (figure 5B), groups with known resistance to IFN-β stimulation.12 During IFN-β therapy compared with untreated MS, in vitro vitamin D forces induction of additional p-Y-STAT1 in RRMS-s and RRMS-a. This suggests that IFN-β therapy has reduced negative effects of inflammation or has primed immune cells for greater responses to IFN.14
Figure 5. Vitamin D and IFN-β effects on p-Y-STAT1 induction in lymphocytes (A) and monocytes (B).
Vitamin D plus IFN-β (blue line) in most therapy-naive groups had a greater effect than IFN-β alone (yellow line) on activation of STAT1 in ConA-activated lymphocytes and monocytes. Values in radar plot represent median fold change in log2 scale expression of p-Y-STAT1 measured by flow cytometry. Blue line: (IFN+ VitD) − (Vit D); yellow: (IFN)-(media alone). IFN-β = interferon-β; RRMS-a = active relapsing/remitting MS; RRMS-s = stable RRMS; SPMS = secondary progressive MS.
There were high correlations in expression of p-Y-STAT1 between monocytes and lymphocytes in all groups, after induction by IFN alone (r = 0.82, p = 3.4 × 10−12) and by IFN + VitD (r = 0.91, p = 2.4 × 10−17), and also in the fold of change (r = 0.68, p = 2.6 × 10−3).
Multiplex analysis of vitamin D effects on immune and neuroprotective factors in MS MNCs
Th1, Th17, and Th2 cytokines
A 31-analyte multiplex assay was used to investigate how the vitamin D plus IFN-β combination modulates immune and nonimmune responses. We targeted inflammatory and noninflammatory cytokines, IFN-stimulated proteins, neurotrophic factors, and cell proliferation and adhesion molecules in 40 subjects (figure 6).
IFN-β alone tended to induce Th1 cytokines. Ratios of IFN/media are in figure 6A, and corresponding difference values in pg/mL are shown in figure e-1A (links.lww.com/NXI/A151). IL-2 was elevated in vitro in untreated and IFN-treated MS MNCs, especially during exacerbations, but not in cells from glatiramer-treated patients. IFN-γ was slightly increased in MNCs from untreated RRMS-a and progressive MS, but not in glatiramer-treated RRMS-s or HC. IFN-β alone inhibited Th2 cytokines (IL-4 in all groups except RRMS-a and HC; IL-5 in all groups except RRMS-a). IL-10 was an exception, with induction by IFN-β in all groups except RRMS-s on IFN therapy (figure 6A). This may be due to differential effects on IL-10 induction among immune subsets. We and others find that IFN-β induces IL-10 in T cells, but inhibits production in monocytes.15,16 During attacks, in untreated and IFN-β-treated MS, in vitro IFN-β elevated many Th1 and some Th2 cytokines. Thus, the Th1/Th2 ratio remained elevated during the “cytokine storm” that characterizes exacerbations.
Vitamin D alone, in contrast to IFN-β alone, markedly reduced Th1 and Th17 cytokines for all untreated groups and for IFN-β- and glatiramer-treated MS groups and HC. These cytokines included IL-2, IFN-γ, TNF-α, and TNFRII, which is secreted by activated T cells, and IL-17F. Vitamin D, again in contrast to IFN-β, increased Th2 cytokine expression, including IL-4, IL-5, and IL-10 (figure 6B and figure e-1B, links.lww.com/NXI/A151). GA-treated patients were the exception, with no induction of Th2 cytokines compared with the media control, but Th2 cytokine levels were higher than levels seen with IFN-β alone.
Vitamin D added to IFN-β maintained the shift from Th1 to Th2 caused by vitamin D alone, and dramatically reversed the Th1-inducing effect of IFN-β alone, in therapy-naive and IFN-treated MS (figure 6C and figure e-1C, links.lww.com/NXI/A151). Again, the exception was in GA treated, where there was minimal induction. Overall, the change was close to that seen with vitamin D alone. IFN-β plus vitamin D markedly reduced IL-17F in MNCs from HCs and all patients with MS, including those on glatiramer therapy.
Prototypical IFN-stimulated proteins
Induction of p-Y-STAT1 was enhanced 3-fold by vitamin D added to IFN-β in MNCs from HC and groups of therapy-naive and treated MS (figure 6C). MxA was induced in all groups, but much more in HCs than in any MS group (figure 6C). This reflects the low IFN-induced MxA protein levels in MS compared with HC reported by Feng et al.12 IFN-β and vitamin D, alone and in combination, had only modest effects on chemokine levels in this system.
Adhesion and neurotrophic factors
Vitamin D and IFN-β together tended to stimulate secretion of adhesion molecules intercellular adhesion molecule-1 and vascular cell adhesion molecule-1, as well as vascular endothelial growth factor-α (figure 6C and figure e-1C, links.lww.com/NXI/A151). Although IFN-β alone caused slight induction of neurotrophic factors (leukemia inhibitory factor, brain-derived neurotrophic factor, nerve growth factor [NGF], hepatocyte growth factor, and thymopoietin), vitamin D plus IFN-β had minimal or inhibitory effects. These neurotrophic factors are predominantly products of the nervous system or other tissues, and serum levels may differ from the modest ability of cultured MNCs to produce these factors. For instance, IFN-β has direct neuroprotective effects by inducing NGF in neurons and astrocytes,17 but we did not see induction in MNCs.
Multifunctional cytokines
Vitamin D plus IFN-β induced secretion of IL-12(p70), but inhibited secretion of IL-12(p40). IL-12, a monocyte product, induces Th1 cells, predominantly through the actions of the IL-12 p70 heterodimer that consists of p35 and p40 subunits. The IL-12p40 homodimer, however, blocks IL-12 p70 binding to its receptor. These data would suggest that the marked fall in IL-12 p70 and rise in IL-12 p40 would be detrimental in MS. However, there is no therapeutic benefit in MS of anti-IL-12p40 monoclonal antibody (binding IL-12 p40 homodimer, IL-12 p70 heterodimer, and the p40 subunit in IL-12/23). The lack of efficacy with targeted therapy against IL-12 may arise from the effects on shared subunits of multiple IL-12 family cytokines.
Vitamin D plus IFN-β slightly reduced cytokines that augment homeostatic proliferation (IL-7, IL-15) in most HCs and patients with MS. Reduction of IL-7 may be beneficial in MS. IL-7 induces Th1 cells, and antibodies to IL-7 inhibit experimental autoimmune/allergic encephalomyelitis.18 IL-15 RNA expression is highly dysregulated in untreated MS.19 IL-15 induces proliferation of cytolytic CD8 cells,20 which can sever axons in vitro,21 yet IL-15 also blunts the function of CD8 cytolytic T-cell.19 Reduction of IL-15 by IFN-β plus vitamin D potentially counterbalances cytolytic CD8 T-cell function and promotes in vivo and in vitro IFN-β-driven enhancement of regulatory CD8 T-cell function in MS seen in vitro and in vivo.22
Discussion
Vitamin D enhances responses to IFN-β by increasing activation of p-Y-STAT1. It also reverses or enhances downstream effects of IFN-β alone. Augmentation of signaling by vitamin D occurs in cells from untreated MS (figures 1 and 2) and from patients on IFN-β and glatiramer therapy (figure 3).
Vitamin D alone downregulates proinflammatory Th1 and Th17 cytokines and increases anti-inflammatory Th2 cytokines (figure 6B and figure e-1b, links.lww.com/NXI/A151). IFN-β alone, conversely, clearly increases Th1 and Th17 production and decreases IL-4 and IL-5 secretion. The immune-enhancing effect of IFN-β is not surprising, as type I IFNs are potent antiviral agents, activate cytolytic T cells, and induce Th1 cells in vitro and in vivo.23,24
Addition of vitamin D to IFN-β reversed some of the effects of IFN-β alone. The combination downregulated inflammatory Th1 and Th17 cytokines, but upregulated serum levels of anti-inflammatory Th2 cytokines (IL-4, IL-5, and IL-10), and also induced the MxA antiviral protein and adhesion molecules (figure 6C and figure e-1C, links.lww.com/NXI/A151). Vitamin D modifies IFN responses in both lymphocytes and in monocytes (figure 5). Thus, vitamin D, alone or combined with IFN-β therapy, shifts immunity in MS from a proclivity for Th1 and Th17 cytokine production to a Th2 profile, in addition to increasing regulatory CD4 T-cell numbers.25
Relevant to the high monocyte:T-cell ratio in MS plaques,26 IFN-β alone induces IL-10 production by activated T cells, but reduces IL-10 in monocytes.15 Vitamin D, as well as prostaglandins and cyclic adenosine monophosphate agonists, induces IL-10 in monocytes15 and is likely to be complementary to IFN therapy. High levels of serum vitamin D predicted stronger in vitro vitamin D plus IFN-β responses (figure 4). Combining vitamin D with IFNs to reverse the Th1/Th2 effect could have therapeutic benefit in MS and other autoimmune diseases.
Our in vitro data showing that vitamin D enhances IFN-β effects in most forms of MS (figure 4) provide an explanation for clinical observations. High serum vitamin D is linked to preventing the onset of MS, and enhancing the benefit of IFN-β therapy on MS relapses, accumulation of disability, and MRI lesions.4 Oral vitamin D supplementation in a prospective trial prevented a second episode of demyelination after optic neuritis.27 Vitamin D supplementation during IFN-β therapy had MRI and clinical benefits.5–7
Serum 25-OH vitamin D levels in MS were much lower before widespread vitamin D supplementation. Vitamin D levels averaged 12 ng/mL in Finnish women in 1983, but rose to 28 by 2009.28 Serum vitamin D levels were 17–22 ng/mL in nonsupplementing Eastern Europe, but 26–30 in North America in 2004 in the BEYOND study of double-dose IFN-β-1b.29 Vitamin D levels in our patients with MS are now above 30 ng/mL (table). The effect of enhanced IFN-β signaling by vitamin D in untreated and IFN-treated patients is stronger in RRMS-s than in SPMS, a form of MS with intrinsic low IFN responses.11,12 Low serum vitamin D levels and cohorts with more clinically advanced MS may have reduced the efficacy of IFN-β in early pivotal trials.
During exacerbations in untreated MS, vitamin D enhanced IFN-β effects to a degree intermediate between relapsing and progressive forms of MS (figure 3). Enhancement was greater when serum vitamin D levels were low (figure 4). This augmentation suggests that adequate serum vitamin D would help overcome the transient IFN resistance seen during exacerbations.11,12 Variability in response to vitamin D plus IFN-β is highest in untreated stable MS, suggesting that some patients with “stable” MS are in a danger zone with greater likelihood of having attacks.
During glatiramer treatment, in vitro vitamin D plus IFN-β enhanced p-Y-STAT1 activation in RRMS-s and SPMS and in HC (figure 3). The combination reduced IL-17 and caused a shift from a Th1 to a Th2 profile, more than in IFN-β-treated patients. Nonetheless, higher serum vitamin D levels have less clinical benefit during glatiramer treatment than during IFN-β therapy.29 Vitamin D and glatiramer both bias immunity toward Th2 responses,30–32 so adding vitamin D to glatiramer may not have much additional effect. The Th2 shift may also provide a basis for the benefits of high serum vitamin D levels on natalizumab therapy.33
Vitamin D has an opposite effect on Th1 vs Th2 family cytokines compared with IFN-β. This indicates that there are molecular effects in addition to activation of p-Y-STAT1. Vitamin D affects inflammatory responses, oxidative stress, cell adhesion, cellular growth, and DNA repair in multiple cell types. Three percent of all genes are controlled in some way by vitamin D.34 Vitamin D regulates 229 genes in immortalized B cells and additional genes in other tissues.35 The vitamin D receptor binds 2,776 genomic sites; these are overrepresented for active transcription and enhancement of gene expression. Some of these are genes identified in MS genome-wide association studies, and many are related to IFN signaling (JAK1, Tyk2, STAT3, IRF8, and RGS1).36 In the BENEFIT study of IFN-β-1b in clinically isolated syndromes, 63 vitamin D–regulated genes affected Gd+ MRI lesions; 62 of these were also regulated by IFN-β.37 The molecular basis for these interactions is under investigation.
There were several limitations of this study. There were only modest numbers of patients with relapses. Nonetheless, responses to IFN-β in these patients were intermediate between stable RRMS and progressive MS, paralleling our studies of serum IFN levels and IFN signaling.11
Second, cells were activated in vitro because resting MS MNCs generated only low levels of cytokines. Dysregulated immunity is a hallmark of MS and reflects CNS inflammation. Activation of a small number of cells can provoke significant autoimmune disease, and shifts in levels of cytokines can have significant effects on immunity.38,39 Specific brain antigens (myelin basic protein, myelin-oligodendrocyte glycoprotein, and proteolipid protein), tetanus, and viral antigens have been used to activate MS cells. They are not the causative agents in MS, but do induce weak cytokine responses in MS and in some HCs. As a model of acute and chronic low-level inflammation in MS, ConA was used to activate MNCs. This mannose-binding lectin broadly activates monocytes and CD4 and CD8 T cells, including the CD8+ CD28− regulatory population that is subnormal in MS.40,41 This subset is not stimulated by commonly used anti-CD3 plus anti-CD28 antibodies, and ConA is thus a more appropriate activator for MS immune cells.
IFN-β is infrequently used in PPMS because it only minimally slows the worsening ambulatory dysfunction. Nonetheless, patients with PPMS had strong responses to IFN-β-1b plus vitamin D induction relative to IFN-β-1b alone (figure 2). It is possible that vitamin D could potentiate benefits of IFN-β in PMS, such as reducing breakthrough relapses and maintaining cognition.42
In conclusion, vitamin D induces a shift from Th1 to Th2 immunity in activated MNCs. When combined with IFN-β, it enhances activation of STAT1, a transcription factor that controls IFN signaling, and also reverses most of the Th1-inducing effects seen with IFN-β alone. This provides an explanation for benefits of vitamin D in preventing and ameliorating different forms of MS before and during IFN-β therapy. Vitamin D is an inexpensive and safe addition to amplify the therapeutic benefit of IFN-β.
Glossary
- BDNF
brain-derived neurotrophic factor
- ConA
concanavalin A
- EAE
experimental autoimmune/allergic encephalomyelitis
- GA
glatiramer acetate
- HC
healthy control
- HGF
hepatocyte growth factor
- IFN-β
interferon-β
- LIF
leukemia inhibitory factor
- MFI
median fluorescence intensity
- MNC
mononuclear cell
- MxA
myxovirus A
- NGF
nerve growth factor
- PMA
phorbol myristate acetate
- PMS
progressive MS
- PPMS
primary progressive MS
- RRMS
relapsing/remitting MS
- RRMS-a
active RRMS
- RRMS-s
stable RRMS
- SPMS
secondary progressive MS
- TPO
thymopoietin
- VDR
vitamin D receptor
- VDSI
Vitamin D Stimulation Index
Author contributions
X. Feng: data acquisition, drafting/revising the manuscript, study concept or design, analysis or interpretation of data, statistical analysis, study supervision, and approval of data. Z. Wang: data acquisition, drafting/revising the manuscript, analysis or interpretation of data, and statistical analysis. Q. Howlett-Prieto: data acquisition and statistical analysis. N. Einhorn: data acquisition and drafting/revising the manuscript. S. Causevic: data acquisition, study concept or design, analysis or interpretation of data, statistical analysis, and study supervision. A. Reder: data acquisition, drafting/revising the manuscript, study concept or design, analysis or interpretation of data, statistical analysis, study supervision, and obtaining funding.
Study funding
This study was funded by unrestricted grant support from Bayer Healthcare Pharmaceuticals and the US National Multiple Sclerosis Society (NMSS), RG4509A.
Disclosure
X. Feng has received unrestricted grant support from Bayer, Biogen, Roche-Genentech, Merck-Serono, Novartis, and Teva. Z. Wang, Q. Howlett-Prieto, N. Einhorn, and S. Causevic report no disclosures. A.T. Reder has received unrestricted grant support from Bayer, Biogen, Roche-Genentech, Merck-Serono, Novartis, and Teva and is a consultant for Bayer, Biogen, Roche-Genentech, Merck-Serono, and Novartis. Go to Neurology.org/NN for full disclosures.
References
- 1.Sintzel MB, Rametta M, Reder AT. Vitamin D and multiple sclerosis: a comprehensive review. Neurol Ther 2018;7:59–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Orton SM, Wald L, Confavreux C, et al. Association of UV radiation with multiple sclerosis prevalence and sex ratio in France. Neurology 2011;76:425–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Munger KL, Levin LI, Hollis BW, Howard NS, Ascherio A. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA 2006;296:2832–2838. [DOI] [PubMed] [Google Scholar]
- 4.Ascherio A, Munger KL, White R, et al. Vitamin D as an early predictor of multiple sclerosis activity and progression. JAMA Neurol 2014;71:306–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soilu-Hänninen M, Aivo J, Lindström BM, et al. A randomised, double blind, placebo controlled trial with vitamin D3 as an add on treatment to interferon β-1b in patients with multiple sclerosis. J Neurol Neurosurg Psychiatry 2012;83:565–571. [DOI] [PubMed] [Google Scholar]
- 6.Aivo J, Lindsröm BM, Soilu-Hänninen M. A randomised, double-blind, placebo-controlled trial with vitamin D3 in MS: subgroup analysis of patients with baseline disease activity despite interferon treatment. Mult Scler Int 2012;2012:802796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stewart N, Simpson S, van der Mei I, et al. Interferon-β and serum 25-hydroxyvitamin D interact to modulate relapse risk in MS. Neurology 2012;79:254–260. [DOI] [PubMed] [Google Scholar]
- 8.Nwosu BU, Maranda L, Berry R, et al. The vitamin D status of prison inmates. PLoS One 2014;9:e90623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cree BAC, Al-Sabbagh A, Bennett R, Goodin D. Response to interferon beta-1a treatment in African American multiple sclerosis patients. Arch Neurol 2005;62:1681–1683. [DOI] [PubMed] [Google Scholar]
- 10.Reder AT, Antel JP, Oger J, McFarland TA, Rosenkoetter M, Arnason BGW. Low T8 antigen density on lymphocytes in active multiple sclerosis. Ann Neurol 1984;16:242–249. [DOI] [PubMed] [Google Scholar]
- 11.Feng X, Han D, Kilaru BK, Franek BS, Niewold TB, Reder AT. Inhibition of interferon-beta responses in multiple sclerosis immune cells associated with high-dose statins. Arch Neurol 2012;69:1303–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feng X, Petraglia AL, Chen M, Byskosh PV, Boos MD, Reder AT. Low expression of interferon-stimulated genes in active multiple sclerosis is linked to subnormal phosphorylation of STAT1. J Neuroimmunol 2002;129:205–215. [DOI] [PubMed] [Google Scholar]
- 13.Pachner AR, DiSano K, Royce DB. Clinical utility of a molecular signature in inflammatory demyelinating disease. Neurol Neuroimmunol Neuroinflamm 2018;6:e520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feng X, Wang Z, Causevic S, et al. Vitamin D enhances interferon-beta response in multiple sclerosis. Neurology 2016;86:P3.103. [Google Scholar]
- 15.Feng X, Yau D, Holbrook C, Reder AT. Type I interferons inhibit interleukin-10 production in activated human monocytes and stimulate IL-10 in T cells: implications for Th1-mediated diseases. J Interferon Cytokine Res 2002;22:311–319. [DOI] [PubMed] [Google Scholar]
- 16.Rudick RA, Ransohoff RM. Cytokine secretion by multiple sclerosis monocytes. Relationship to disease activity. Arch Neurol 1992;49:265–270. [DOI] [PubMed] [Google Scholar]
- 17.Boutros T, Croze E, Yong VW. Interferon-β is a potent promoter of nerve growth factor production by astrocytes. J Neurochem 1997;69:939–946. [DOI] [PubMed] [Google Scholar]
- 18.Lee LF, Axtell R, Tu GH, et al. IL-7 promotes T(H)1 development and serum IL-7 predicts clinical response to interferon-β in multiple sclerosis. Sci Transl Med 2011;3:93ra68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamaguchi KD, Ruderman DL, Croze E, et al. IFN-beta-regulated genes show abnormal expression in therapy-naïve relapsing-remitting MS mononuclear cells: gene expression analysis employing all reported protein-protein interactions. J Neuroimmunol 2008;195:116–120. [DOI] [PubMed] [Google Scholar]
- 20.Correale J, Villa A. Isolation and characterization of CD8+ regulatory T cells in multiple sclerosis. J Neuroimmunol 2008;195:121–134. [DOI] [PubMed] [Google Scholar]
- 21.Denic A, Wootla B, Rodriguez M. CD8(+) T cells in multiple sclerosis. Expert Opin Ther Targets 2013;17:1053–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Noronha A, Toscas A, Jensen MA. Contrasting effects of alpha, beta, and gamma interferons on nonspecific suppressor function in multiple sclerosis. Ann Neurol 1992;31:103–106. [DOI] [PubMed] [Google Scholar]
- 23.Byskosh PV, Reder AT. Interferon beta-1b effects on cytokine mRNA in peripheral mononuclear cells in multiple sclerosis. Mult Scler 1996;1:262–269. [DOI] [PubMed] [Google Scholar]
- 24.Tough DF. Modulation of T-cell function by type I interferon. Immunol Cell Biol 2012;90:492–497. [DOI] [PubMed] [Google Scholar]
- 25.Royal W, Mia Y, Li H, Naunton K. Peripheral blood regulatory T cell measurements correlate with serum vitamin D levels in patients with multiple sclerosis. J Neuroimmunol 2009;213:135–141. [DOI] [PubMed] [Google Scholar]
- 26.Lassmann H, Suchanek G, Ozawa K. Histopathology and the blood-cerebrospinal fluid barrier in multiple sclerosis. Ann Neurol 1994;36:S42–S46. [DOI] [PubMed] [Google Scholar]
- 27.Derakhshandi H, Etemadifar M, Feizi A, et al. Preventive effect of vitamin D3 supplementation on conversion of optic neuritis to clinically definite multiple sclerosis: a double blind, randomized, placebo-controlled pilot clinical trial. Acta Neurol Belg 2013;113:257–263. [DOI] [PubMed] [Google Scholar]
- 28.Munger KL, Hongell K, Åivo J, Soilu-Hänninen M, Surcel H-M, Ascherio A. 25-hydroxyvitamin D deficiency and risk of MS among women in the Finnish Maternity Cohort. Neurology 2017;89:1578–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fitzgerald KC, Munger KL, Köchert K, et al. Association of vitamin D levels with multiple sclerosis activity and progression in patients receiving interferon beta-1b. JAMA Neurol 2015;72:1458–1465. [DOI] [PubMed] [Google Scholar]
- 30.Gröber U, Spitz J, Reichrath J, Kisters K, Holick MF. Vitamin D: update 2013: from rickets prophylaxis to general preventive healthcare. Dermatoendocrinol 2013;5:331–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dhib-Jalbut S, Chen M, Said A, Zhan M, Johnson KP, Martin R. Glatiramer acetate-reactive peripheral blood mononuclear cells respond to multiple myelin antigens with a Th2-biased phenotype. J Neuroimmunol 2003;140:163–171. [DOI] [PubMed] [Google Scholar]
- 32.Valenzuela RM, Kaufman M, Balashov KE, Ito K, Buyske S, Dhib-Jalbut S. Predictive cytokine biomarkers of clinical response to glatiramer acetate therapy in multiple sclerosis. J Neuroimmunol 2016;300:59–65. [DOI] [PubMed] [Google Scholar]
- 33.Scott TF, Hackett CT, Dworek DC, Schramke CJ. Low vitamin D level is associated with higher relapse rate in natalizumab treated MS patients. J Neurol Sci 2013;330:27–31. [DOI] [PubMed] [Google Scholar]
- 34.Bouillon R, Carmeliet G, Verlinden L, et al. Vitamin D and human health: lessons from vitamin D receptor null mice. Endocr Rev 2008;29:726–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ramagopalan SV, Hanwell HE, Giovannoni G, et al. Vitamin D-dependent rickets, HLA-DRB1, and the risk of multiple sclerosis. Arch Neurol 2010;67:1034–1035. [DOI] [PubMed] [Google Scholar]
- 36.Berlanga-Taylor AJ, Disanto G, Ebers GC, Ramagopalan SV. Vitamin D-gene interactions in multiple sclerosis. J Neurol Sci 2011;311:32–36. [DOI] [PubMed] [Google Scholar]
- 37.Munger KL, Köchert K, Simon KC, et al. Molecular mechanism underlying the impact of vitamin D on disease activity of MS. Ann Clin Transl Neurol 2014;1:605–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Genç K, Dona DL, Reder AT. Increased CD80(+) B cells in active multiple sclerosis and reversal by interferon beta-1b therapy. J Clin Invest 1997;99:2664–2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Germain RN. The art of the probable: system control in the adaptive immune system. Science 2001;293:240–245. [DOI] [PubMed] [Google Scholar]
- 40.Antel JP, Arnason BG, Medof ME. Suppressor cell function in multiple sclerosis: correlation with clinical disease activity. Ann Neurol 1979;5:338–342. [DOI] [PubMed] [Google Scholar]
- 41.Karaszewski JW, Reder AT, Anlar B, Kim WC, Arnason BG. Increased lymphocyte beta-adrenergic receptor density in progressive multiple sclerosis is specific for the CD8+, CD28− suppressor cell. Ann Neurol 1991;30:42–47. [DOI] [PubMed] [Google Scholar]
- 42.Lacy M, Hauser M, Pliskin N, Assuras S, Valentine MO, Reder A. The effects of long-term interferon-beta-1b treatment on cognitive functioning in multiple sclerosis: a 16-year longitudinal study. Mult Scler 2013;19:1765–1772. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data from this study are included in the article, but anonymized data will be shared by request from any qualified investigator. It is not a clinical trial.