Abstract
Purpose
Surgical interventions are routinely performed on children with osteogenesis imperfecta (OI) to stabilize long bones, often post fracture. We speculated that a combination of intramedullary reaming and intraosseous injection of recombinant bone morphogenetic protein-2 (BMP-2) could enhance periosteal ossification and ultimately cortical thickness and strength. This approach was conceptually tested in a preclinical model of genetic bone fragility.
Methods
Six experimental groups were tested including no treatment, intramedullary reaming, and reaming with 5 µg BMP-2 injection performed in the tibiae of both wild type (WT) and Col1a2G610C/+ (OI, Amish mutation) mice. Bone formation was examined at a two-week time point in ex vivo specimens by micro-computed tomography (microCT) analysis and histomorphometry with a dynamic bone label.
Results
MicroCT data illustrated increases in tibial cortical thickness with intramedullary reaming alone (Saline) and reaming plus BMP-2 injection (BMP-2) compared to no intervention controls. In the OI mice, the periosteal bone increase was not statistically significant with Saline but there was an increase of +192% (p = 0.053) with BMP-2 injection. Dynamic histomorphometry on calcein label was used to quantify new woven bone formation; while BMP-2 induced greater bone formation than Saline, the anabolic response was blunted overall in the OI groups.
Conclusions
These data indicate that targeting the intramedullary compartment via reaming and intraosseous BMP-2 delivery can lead to gains in cortical bone parameters. It is suggested that the next step is to validate safety and functional improvements in a clinical OI setting.
Keywords: BMP-2, osteogenesis imperfecta, sucrose acetate isobutyrate
Introduction
Osteogenesis imperfecta (OI) is a genetic disorder that is characterized by low bone mass and increased bone fragility.1Osteogenesis imperfecta is most commonly associated with mutations in genes encoding collagen type 1 (COL1A1 or COL1A2).2,3 This leads to disrupted collagen production, folding, and/or breakdown, resulting in worsened bone material properties, poor periosteal bone formation, and smaller bones with thinner cortices.4 Currently, OI treatment focuses on improving functional outcomes and reducing fracture risk.5,6 Physical therapy, rehabilitation and orthopaedic surgery are used in combination to improve patient quality of life. In terms of surgical approaches, the insertion of intramedullary rods can assist in straightening the femora and tibiae, encouraging standing and walking, as well as reducing risk of further fracture.6–8
Pharmacotherapy with systemic bisphosphonates has been widely adopted as a method for prophylactically reducing fracture rates in children. Although bisphosphonates do not increase bone quality, the decreases in bone resorption yield increased bone mass and strength.8,9 Nevertheless, concerns have arisen regarding the effects of systemic bisphosphonates on the growing skeleton. Bisphosphonate treatment has been shown to delay the healing of osteotomies in children with OI.10 In addition, bisphosphonate treatment results in cortical thickening primarily via retaining the endosteal surface rather than enhancement of periosteal bone formation.11 Based on the principles of bone biomechanics, increases in the overall diameter of a long bone (i.e. periosteal expansion) should yield proportionately greater bone strength than additional endosteal bone. Increasing bone on the outer surface of the cortex greatly increases the moment of inertia, which in turn decreases fracture risk.12,13
Thus, anabolic interventions may yield improved outcomes and reduced fracture risk in OI, and in some cases have the potential to be combined with bisphosphonate therapy. Recombinant human parathyroid hormone 1-34 (Teriparatide) has shown some anabolic effect in adults with OI,14,15 but use in children has been cautious due to concerns of promoting osteosarcoma.16 Alternatively, the administration of growth hormone in children with OI showed increased height velocity and bone density in some small studies.17,18 However, histomorphometry of bone biopsies showed increased bone turnover with growth hormone,18 which may be unwise to apply in OI where bone turnover is already elevated.19
In 2013, Seeherman et al reported that a single intraosseous injection of bone morphogenetic protein-2 (BMP-2) in a calcium phosphate matrix delivered into the femoral neck of non-human primates resulted in the formation of endosteal and periosteal bone.20 In order to maximize the sustained delivery of BMP-2, we employed a solution of sucrose acetate isobutyrate (SAIB), a biocompatible sugar-based ester previously demonstrated as an effective injectable carrier for BMP-2 that is superior to saline.21,22
Increasing periosteal bone formation via intraosseous BMP-2 injection was suggested as being potentially beneficial in a paediatric OI setting. Thus, this study aimed to deliver BMP-2 intraosseously in a knock-in mouse model of OI possessing a G610C mutation in the COL1A2 gene (Amish mutation).23 This represents a mild to moderate form of OI and it was conjectured to be a more clinically relevant model of the target patient population than the alternative moderate to severe models (e.g. the oim mouse line). In this study, animals received an intraosseous injection of BMP-2 in SAIB directly into the tibiae and were examined two weeks after intervention via radiographic and histological outcomes for periosteal bone formation.
Methods
OI mouse model
Amish COL1A2G610C/+ OI knock-in mice were generated by Daley et al23 and sourced from Prof J Bateman (Murdoch Children’s Research Institute). Wildtype (WT) littermates served as unaffected controls. Mice were bred and housed in the specific pathogen-free transgenic facility on site. Mice were allowed ad libitum access to food and water. Institutional animal ethics approval was granted under protocol K315. Eight-week-old mice were used for this study.
Intraosseous BMP-2 injection procedure
Mice were anaesthetized using 35 mg/kg ketamine and 0.5 mg/kg xylazine with inhaled isoflurane used as necessary. The operative site was shaved and wiped with povidine-iodine solution. The right tibias of the experimental mice were initially reamed from the proximal end using a 27 G needle and flushed with saline, and then the tibial cavity was filled with a radiopaque dye (Ultravist 240; Bayer Health Care, NSW, Australia). The presence of this dye at the distal end of the medullary cavity was confirmed by intraoperative X-ray. Reamed tibias were then given intraosseous injections of either saline, or 5 µg BMP-2 in 20 µL 80:20 SAIB:ethanol and X-rayed once more. The absence of the contrast agent indicated that the cavity was now filled with treatment solutions. Mice were subcutaneously dosed with 10 mg/kg calcein (Sigma, NSW, Australia) at days –10 and –3 before cull. Mice were culled two weeks after surgery using a CO2 chamber.
Study design
Three experimental groups were tested for each genotype and animals were randomized into their treatment groups, with eight animals assigned to each group. The first group received no surgical interventions and was called the Nil treatment group. The remaining two groups underwent tibial reaming, and received intraosseous injections of either saline (Saline) or 80% sucrose acetate isobutyrate:ethanol (v/v) containing BMP-2 (BMP-2).
MicroCT analysis
After fixation in 4% paraformaldehyde, samples were stored in 70% ethanol before undergoing micro-computed tomography (microCT) scanning using a SkyScan 1174 compact microCT scanner (Kontich, Belgium). Samples were scanned in 70% ethanol, using a 0.5 mm aluminium filter, 50 kV X-ray tube voltage, and 800 µA tube electric current. Tibiae were scanned at a pixel resolution of 9.4 µm. The scanned images were reconstructed using NRecon (SkyScan) and analysed using CTAnalyser software (SkyScan). The analysis was focused on a region of interest (ROI) in the mid diaphysis of the tibia – a 1.2 mm region located 0.5 mm above the tibial-fibular junction (as shown Fig. 1a). New bone was defined as bone with a density of 0.3 g/cm3 – 0.9 g/cm3 (Fig. 1b) as calibrated by hydroxyapatite phantoms (SkyScan).
Fig. 1.
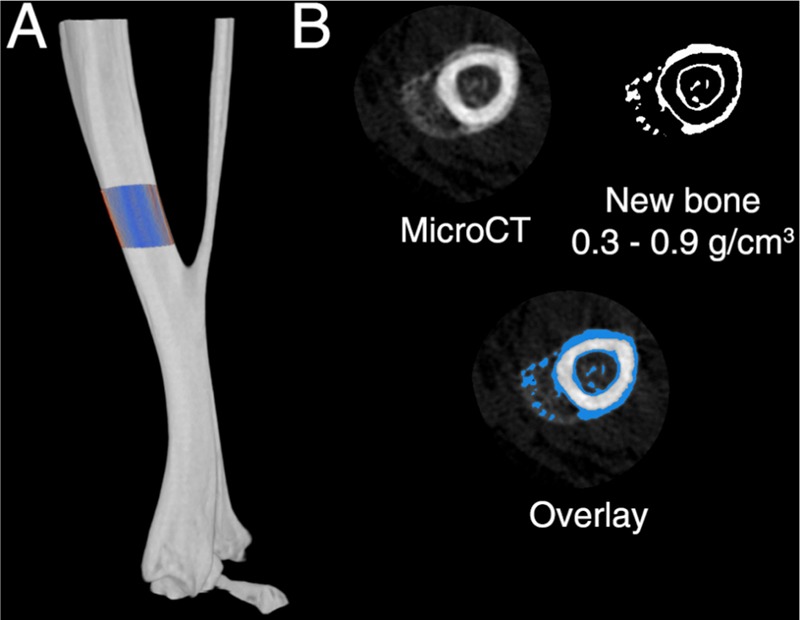
MicroCT analysis regions to assess new periosteal bone formation. (a) The region of interest (ROI) that was chosen, a 1.2 mm region located 0.5 mm above the tibial-fibular junction. (b) Within the ROI, a threshold of 0.3–0.9 g/mm3 bone density was used to define new bone. The overlay demonstrates the new bone threshold as compared to the raw microCT slice.
Confocal imaging of calcein dosing
Tibiae were bisected in the coronal plane using a diamond saw and the proximal ROI chosen for microCT analysis. Fine grit sandpaper was used to create a flat surface and the bone cross section was imaged directly on a Leica TCS SP5 confocal microscope (Wetzlar, Germany). Calcein signal was detected using the 488 nm laser line and 500–560 nm emission bands. Image capture settings were retained for all samples. The bone surface was imaged using a 10 × objective using the Tile Scan (4–9 image fields) and Z-stack functions (2–4 slices), followed by automated stitching of the tiled region and application of a maximum intensity projection via the LAS-AF software to recreate the two-dimensional bone surface.
The raw confocal images were globally manipulated using Adobe Photoshop CS6 using only linear functions to maximize contrast in batch processing mode using identical settings for each image. For analysis of the periosteal surface, the endosteum and medullary canal were removed from the raw image, large speckles and imaging artefacts were removed using a dust and scratches filter, and the image was converted to greyscale. Images were inverted and imported into ImageJ for fluorescence quantification using a greyscale threshold value of 230.
Statistics
Statistical analyses were conducted using GraphPad Prism (Version 7; GraphPad Software, CA, USA). D’Agostino and Pearson normality tests were used to determine whether datasets were normally distributed. One-way ANOVA with multiple comparisons (Tukey’s) was used to compare each genotype. Statistical significance was set at α < 0.05. The right (treated) tibia of each animal was treated as a unit of analysis. All graphs are presented as mean values, with error bars representing standard deviation.
Results
Radiographic analysis of BMP-2-induced bone formation
Two weeks after intraosseous injection into the tibiae, X-rays of whole tibiae yielded no considerable changes on radiographs, barring small protrusions of new bone at or adjacent to the injection site in some BMP-2-treated specimens (Fig. 2). Group sizes were n = 8 except for the OI/BMP-2 group, which had n = 7 animals because one animal was culled humanely due to complications from anaesthetics during surgery.
Fig. 2.
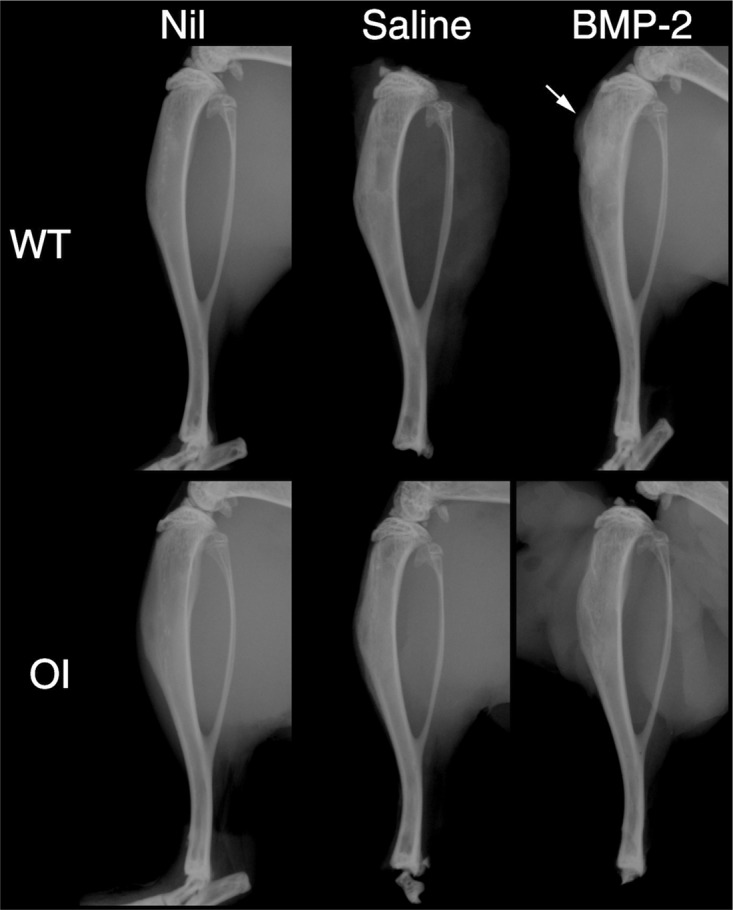
Representative X-rays of tibia in osteogenesis imperfecta (OI) or wild type (WT) mice receiving either no treatment (Nil) or tibial reaming and either saline injections (Saline) or sucrose acetate isobutyrate (SAIB) containing bone morphogenetic protein-2 (BMP-2). Arrow indicates the presence of bone formation near the surgical site.
MicroCT reconstructions revealed an increase in cortical thickness in Saline and BMP-2-treated tibia, which appeared to be largely periosteal (Fig. 3a). This was subsequently confirmed by quantification of new (low-density) bone in the WT mice with Saline (+178%; p = 0.0216) and BMP-2 treatment (+185%; p = 0.0123) (Fig. 3b). In the OI mice, there was an increase in periosteal bone in the Saline-treated (+128%; p = 0.7092) and BMP-2-treated (+192%; p = 0.0529) groups.
Fig. 3.
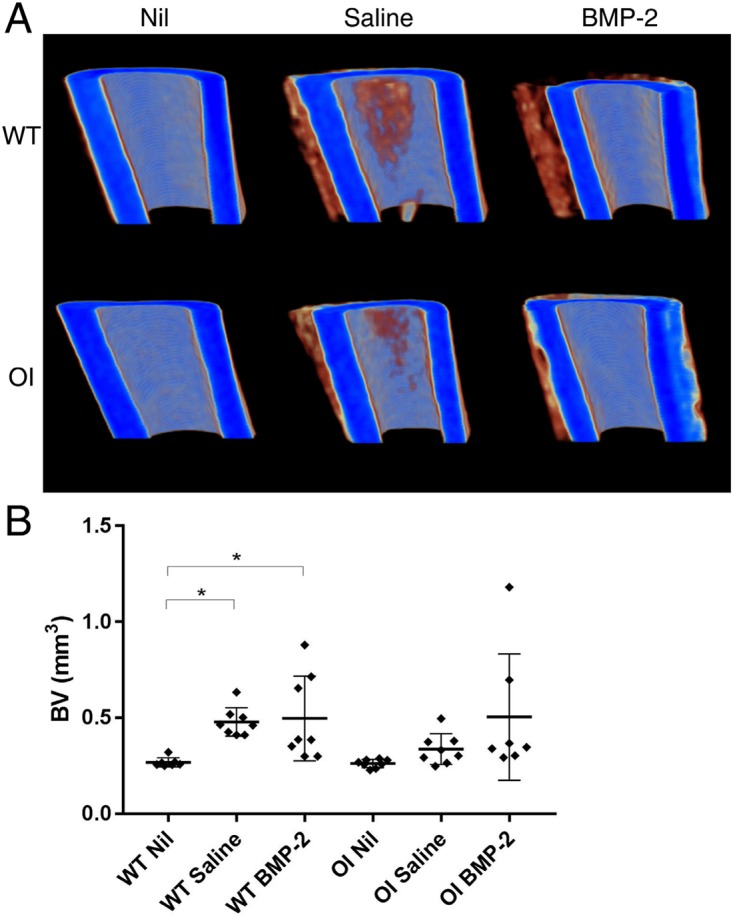
(a) MicroCT reconstructions of representative samples taken from the upper region of interest (ROI) of tibia. (b) Periosteal bone formed in the tibial ROI following intraosseous injection with either saline (Saline) or sucrose acetate isobutyrate (SAIB) containing bone morphogenetic protein-2 (BMP-2) as measured by microCT. *p < 0.05; **p < 0.01.
Calcein quantification reveals new periosteal bone formation in the tibia
Calcein was administered subcutaneously at two time points one week apart towards the end of the study to allow measurement of new bone formation. Analysis of traditional measures such as the mineral apposition rate via dual labelling was deemed impractical due to the nature of the response to reaming and BMP-2 delivery, which resulted in callus-like woven bone formation on the surfaces of the bone cortex (Fig. 4a). Consequently, the total calcein-labelled area was systematically quantified in both the periosteal region or endosteal and medullary regions. In the periosteal region, there was more fluorescent label detected when comparing the WT Nil treatment to the Saline (+2328%, p = 0.0636) and significantly more in the BMP-2 group (+3262%, p = 0.0079) (Fig. 4b). This trend was also seen in the OI mice, which showed an increase in the Saline-treated group (+1420%, p = 0.2936) and a significant increase in the BMP-2-treated group (+2452%, p = 0.0071) compared to the Nil group.
Fig. 4.
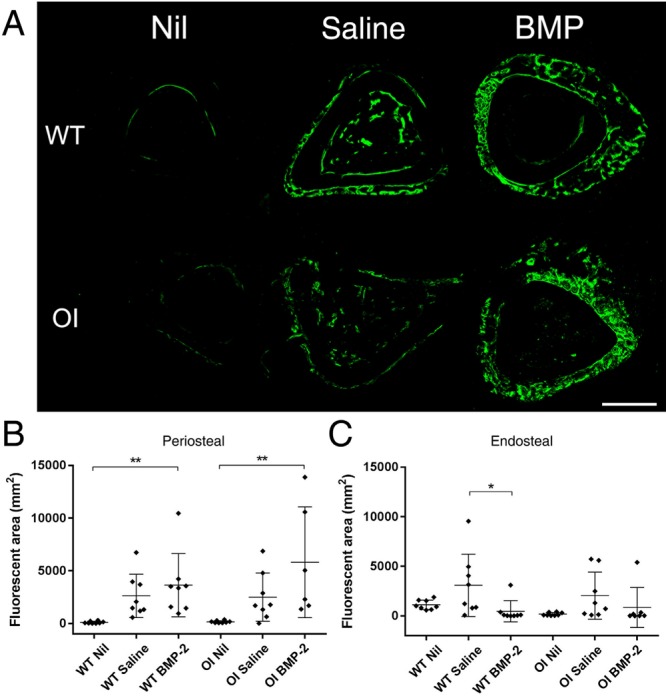
(a) Representative confocal images showing calcein labelling on a tibial cross-section highlighting the increase in signal seen in the treatment groups. (b) Significantly more fluorescent calcein was detected on the periosteal surface of tibiae treated with either Saline or bone morphogenetic protein-2 (BMP-2), than Nil. (c) An increase in calcein signal is seen on the endosteal surface and medullary canal in the Saline treated group compared to the Nil and BMP-2 treated groups. Confocal imaging was used to capture calcein signal on cross-sections of tibiae and was quantified in ImageJ as fluorescent area. *p < 0.05; **p < 0.01.
When the endosteal and medullary compartment was analysed, a different trend was seen (Fig. 4c). The highest level of calcein signal was detected in the Saline-treated groups of both genotypes, which showed non-significant increases of +175% (p = 0.1297) and +1004% (p = 0.1203) for WT and OI respectively when compared to Nil treatment. In the WT mice, the BMP-2 group showed only 15% of the calcein signal of the Saline-treated group (p = 0.0346). In the OI mice, the BMP-2 group had only 42% of the calcein signal observed in the Saline group (p = 0.4231).
Discussion
Consistent with the prior literature,20 intramedullary reaming resulted in an increase in new bone formation, as measured by microCT and calcein labelling. Clinically, the reaming of long bones is a method that can be used alongside the implantation of an intramedullary nail for fixation of diaphyseal fractures. There has been some controversy in the literature regarding the contribution of reaming to intramedullary fixation, as it has been associated with thermal necrosis,24 fat embolism,25,26 and pulmonary complications.27 However, reaming and intramedullary nail exchange has been proposed as a technique for the treatment of aseptic non-union fractures.28–31
These studies have revealed increases in periosteal bone formation associated with reaming. This is consistent with prior preclinical reaming studies, even in the absence of intramedullary nailing.32,33 The mechanism of new periosteal rather than simply endosteal bone formation is unclear. It is possible that reaming creates a generalized inflammatory response affecting the periosteum, due to release of cancellation bone fragments,34 multipotent progenitor cells,35,36 and growth factors37 from within the medullary canal. Alternatively, in addition to causing injury to the endosteal surface and blood supply, reaming can greatly increase periosteal blood flow.38,39 The increased periosteal bone formation associated with reaming may be due to marrow products moving to the periosteal surface40 or the increased blood flow increasing the contact of progenitor cells.
A possible counter-hypothesis is that reaming may result in biophysical strain sensed by the osteocytes, which is translated into biochemical signals. Osteocytes constitute 90–95% of the cellular component of mature bone, and are characterized by highly interconnected dendritic processes that span the bone via canaliculi.41 Osteocytes have critical roles in regulating bone remodelling and maintaining the bone matrix, and they do this by transforming chemical and mechanical stimuli into cell signals that control osteoblast and osteoclast activity.42 Osteocytes can sense mechanical signals directly as forces through the bone matrix43,44 and reaming can result in extensive increases in mechanical strain on the bone. In addition, the injection of either saline or SAIB/BMP-2 may increase the fluid pressure in the lacunar canaliculi. The periosteal response may be mediated by osteocytes signalling to the osteoblasts to increase bone volume on the outer surface, in an attempt to improve the bone’s resistance to strain.
This study has a number of limitations, the most prominent being the variability of the data. This may be in part due to the variances of individual mouse bones in terms of cortical bone, as well as surgical variability in terms of the reaming procedure and the precise dosing of BMP-2 by intramedullary injection. While every endeavour was made to keep these as consistent as possible, a larger animal model (e.g. rat) would be an alternative that could yield more consistent outcomes. Subsequent studies could also take advantage of newer, higher-resolution microCT scanning systems and/or live microCT imaging to produce dynamic in vivo histomorphometry data to segregate differences in periosteal and endosteal bone formation.
Another limitation of this study is that SAIB alone was not used as a vehicle control, and while SAIB is highly biocompatible it may have independent biological effects. The doses of BMP-2 used were consistent with the production of abundant new bone in prior reports, including in combination with the SAIB delivery system.21,22 Moreover, differences between the groups were seen via histological and microCT analysis. In the BMP-2-treated groups, the new bone appeared more like woven bone or an un-remodelled hard callus. Thus, it is possible that the mechanisms behind the additional bone formation may differ between the groups. Based on histological images, reaming resulted in increased appositional bone growth, while BMP-2 may produce new endochondral bone parosteal to the original cortex.
An important caveat about all drug therapies for OI that influence bone metabolism, both anabolic and anti-resorptive, is that they lead to increases in bone volume without necessarily improving bone quality. Regardless of quality, increases in bone volume nevertheless lead to increases in overall bone strength and expected reductions in fracture risk. The relationship between the increased bone and mechanical strength need to still be mechanically investigated both at a macro level through bending tests, and at a micro level through indentation tests as areas for future study.
Conclusion
This study indicates that the process of reaming, with or without the addition of BMP-2, can lead to additional bone formation in both WT mice and a genetic mouse model of OI. This stimulation of new bone is reproducible and anticipated to increase long bone strength, and thus and may have important translational implications for surgical intervention for brittle bone fractures.
Open access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (CC BY-NC 4.0) licence (https://creativecommons.org/licenses/by-nc/4.0/) which permits non-commercial use, reproduction and distribution of the work without further permission provided the original work is attributed.
Compliance with ethical standards
Funding statement
Project funding was provided by the Sticks and Stones Foundation (Australia). TLC was supported by funding from an Australian Postgraduate Award from the Australian Research Council. Neither funding bodies had any roles in the design of the study and collection, analysis, and interpretation of data or in writing the manuscript.
Ethical statement
Ethical approval: Ethical approval was granted prior to commencement of this study by the Children’s Hospital at Westmead/Children’s Medical Research Institute Animal Ethics Committee, protocol K315. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Informed consent: Informed consent was not required.
ICMJE Conflict of interest statement
DGL is a consultant for Orthopediatrics and reports grants and non-financial support from Novartis Pharma, grants from N8 Medical, grants from Celgene, grants from Amgen Inc, outside the submitted work.
AS reports grants and non-financial support from Novartis Pharma, grants from N8 Medical, grants from Celgene, grants and non-financial support from Amgen Inc, outside the submitted work.
TLC report grants from the Sticks and Stones Foundation and grants from the Australian Research Council during the conduct of the study.
LCC reports no conflict of interest.
Acknowledgements
Kathy Mikulec-Langton and Lauren Peacock provided technical assistance with animal surgeries and monitoring. Confocal microscopy was performed at the Westmead Scientific Platforms, which are supported by the Westmead Research Hub, the Cancer Institute New South Wales, the National Health and Medical Research Council and the Ian Potter Foundation.
Author Contributions
TLC: Acquisition, analysis and interpretation of the data, drafting and revision of the manuscript.
LCC: Acquisition of the confocal images and drafting and revision of the manuscript.
AS: Conception and substantial drafting of the work.
DGL: Conception and substantial drafting of the work.
All authors have read and approved the manuscript.
References
- 1.Glorieux FH. Osteogenesis imperfecta. Best Pract Res Clin Rheumatol 2008;22:85–100. [DOI] [PubMed] [Google Scholar]
- 2.Byers PH, Wallis GA, Willing MC. Osteogenesis imperfecta: translation of mutation to phenotype. J Med Genet 1991;28:433–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marini JC, Forlino A, Cabral WA, et al. Consortium for osteogenesis imperfecta mutations in the helical domain of type I collagen: regions rich in lethal mutations align with collagen binding sites for integrins and proteoglycans. Hum Mutat 2007;28:209–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rauch F, Travers R, Parfitt AM, Glorieux FH. Static and dynamic bone histomorphometry in children with osteogenesis imperfecta. Bone 2000;26:581–589. [DOI] [PubMed] [Google Scholar]
- 5.Engelbert RHH, Pruijs HEH, Beemer FA, Helders PJM. Osteogenesis imperfecta in childhood: treatment strategies. Arch Phys Med Rehabil 1998;79:1590–1594. [DOI] [PubMed] [Google Scholar]
- 6.Zeitlin L, Fassier F, Glorieux FH. Modern approach to children with osteogenesis imperfecta. J Pediatr Orthop B 2003;12:77–87. [DOI] [PubMed] [Google Scholar]
- 7.Wilkinson JM, Scott BW, Clarke AM, Bell MJ. Surgical stabilisation of the lower limb in osteogenesis imperfecta using the Sheffield Telescopic Intramedullary Rod System. J Bone Joint Surg Br 1998;80:999–1004. [DOI] [PubMed] [Google Scholar]
- 8.Rauch F, Glorieux FH. Osteogenesis imperfecta. Lancet 2004;363:1377–1385. [DOI] [PubMed] [Google Scholar]
- 9.Cheung MS, Glorieux FH. Osteogenesis imperfecta: update on presentation and management. Rev Endocr Metab Disord 2008;9:153–160. [DOI] [PubMed] [Google Scholar]
- 10.Munns CFJ, Rauch F, Zeitlin L, Fassier F, Glorieux FH. Delayed osteotomy but not fracture healing in pediatric osteogenesis imperfecta patients receiving pamidronate. J Bone Miner Res 2004;19:1779–1786. [DOI] [PubMed] [Google Scholar]
- 11.Rauch F, Travers R, Munns C, Glorieux FH. Sclerotic metaphyseal lines in a child treated with pamidronate: histomorphometric analysis. J Bone Miner Res 2004;19:1191–1193. [DOI] [PubMed] [Google Scholar]
- 12.Milgrom C, Giladi M, Simkin A, et al. The area moment of inertia of the tibia: a risk factor for stress fractures. J Biomech 1989;22:1243–1248. [DOI] [PubMed] [Google Scholar]
- 13.Wergedal JE, Veskovic K, Hellan M, et al. Patients with Van Buchem disease, an osteosclerotic genetic disease, have elevated bone formation markers, higher bone density, and greater derived polar moment of inertia than normal. J Clin Endocrinol Metab 2003;88:5778–5783. [DOI] [PubMed] [Google Scholar]
- 14.Gatti D, Rossini M, Viapiana O, et al. Teriparatide treatment in adult patients with osteogenesis imperfecta type I. Calcif Tissue Int 2013;93:448–452. [DOI] [PubMed] [Google Scholar]
- 15.Orwoll ES, Shapiro J, Veith S, et al. Evaluation of teriparatide treatment in adults with osteogenesis imperfecta. J Clin Invest 2014;124:491–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vahle JL, Sato M, Long GG, et al. Skeletal changes in rats given daily subcutaneous injections of recombinant human parathyroid hormone (1-34) for 2 years and relevance to human safety. Toxicol Pathol 2002;30:312–321. [DOI] [PubMed] [Google Scholar]
- 17.Antoniazzi F, Bertoldo F, Mottes M, et al. Growth hormone treatment in osteogenesis imperfecta with quantitative defect of type I collagen synthesis. J Pediatr 1996;129:432–439. [DOI] [PubMed] [Google Scholar]
- 18.Marini JC, Hopkins E, Glorieux FH, et al. Positive linear growth and bone responses to growth hormone treatment in children with types III and IV osteogenesis imperfecta: high predictive value of the carboxyterminal propeptide of type I procollagen. J Bone Miner Res 2003;18:237–243. [DOI] [PubMed] [Google Scholar]
- 19.Baron R, Gertner JM, Lang R, Vignery A. Increased bone turnover with decreased bone formation by osteoblasts in children with osteogenesis imperfecta tarda. Pediatr Res 1983;17:204–207. [DOI] [PubMed] [Google Scholar]
- 20.Seeherman HJ, Li XJ, Smith E, Parkington J, Li R, Wozney JM. Intraosseous injection of rhBMP-2/calcium phosphate matrix improves bone structure and strength in the proximal aspect of the femur in chronic ovariectomized nonhuman primates. J Bone Joint Surg Am 2013;95:36–47. [DOI] [PubMed] [Google Scholar]
- 21.Cheng TL, Murphy CM, Cantrill LC, et al. Local delivery of recombinant human bone morphogenetic proteins and bisphosphonate via sucrose acetate isobutyrate can prevent femoral head collapse in Legg-Calve-Perthes disease: a pilot study in pigs. Int Orthop 2014;38:1527–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheng TL, Valtchev P, Murphy CM, et al. A sugar-based phase-transitioning delivery system for bone tissue engineering. Eur Cell Mater 2013;26:208–221. [DOI] [PubMed] [Google Scholar]
- 23.Daley E, Streeten EA, Sorkin JD, et al. Variable bone fragility associated with an Amish COL1A2 variant and a knock-in mouse model. J Bone Miner Res 2010;25:247–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leunig M, Hertel R. Thermal necrosis after tibial reaming for intramedullary nail fixation: a report of three cases. J Bone Joint Surg Br 1996;78:584–587. [PubMed] [Google Scholar]
- 25.Giannoudis PV, Tzioupis C, Pape H-C. Fat embolism: the reaming controversy. Injury 2006;37:S50–S58. [DOI] [PubMed] [Google Scholar]
- 26.Mellor A, Soni N. Fat embolism. Anaesthesia 2001;56:145–154. [DOI] [PubMed] [Google Scholar]
- 27.Anwar IA, Battistella FD, Neiman R, Olson SA, Chapman MW, Moehring HD. Femur fractures and lung complications: a prospective randomized study of reaming. Clin Orthop Relat Res 2004;422:71–76. [PubMed] [Google Scholar]
- 28.Sledge SL, Johnson KD, Henley MB, Watson JT. Intramedullary nailing with reaming to treat non-union of the tibia. J Bone Joint Surg Am 1989;71:1004–1019. [PubMed] [Google Scholar]
- 29.Hak DJ, Lee SS, Goulet JA. Success of exchange reamed intramedullary nailing for femoral shaft nonunion or delayed union. J Orthop Trauma 2000;14:178–182. [DOI] [PubMed] [Google Scholar]
- 30.Furlong AJ, Giannoudis PV, DeBoer P, Matthews SJ, MacDonald DA, Smith RM. Exchange nailing for femoral shaft aseptic non-union. Injury 1999;30:245–249. [DOI] [PubMed] [Google Scholar]
- 31.Zelle BA, Gruen GS, Klatt B, Haemmerle MJ, Rosenblum WJ, Prayson MJ. Exchange reamed nailing for aseptic nonunion of the tibia. J Trauma 2004;57:1053–1059. [DOI] [PubMed] [Google Scholar]
- 32.Brueton RN, Brookes M. Intramedullary reaming and callus formation Gahr RH, Hein W, Seidel H, eds. Dynamische Osteosynthese. Berlin Heidelberg: Springer, 1995:7–17. [Google Scholar]
- 33.Danckwardt-Lillieström G, Grevsten S, Johansson H, Olerud S. Periosteal bone formation on medullary evacuation: a bone formation model. Ups J Med Sci 1972;77:57–61. [DOI] [PubMed] [Google Scholar]
- 34.Frölke JP, Bakker FC, Patka P, Haarman HJ. Reaming debris in osteotomized sheep tibiae. J Trauma 2001;50:65–69. [DOI] [PubMed] [Google Scholar]
- 35.Wenisch S, Trinkaus K, Hild A, et al. Human reaming debris: a source of multipotent stem cells. Bone 2005;36:74–83. [DOI] [PubMed] [Google Scholar]
- 36.Hoegel F, Mueller CA, Peter R, Pfister U, Suedkamp NP. Bone debris: dead matter or vital osteoblasts. J Trauma 2004;56:363–367. [DOI] [PubMed] [Google Scholar]
- 37.Schmidmaier G, Herrmann S, Green J, et al. Quantitative assessment of growth factors in reaming aspirate, iliac crest, and platelet preparation. Bone 2006;39:1156–1163. [DOI] [PubMed] [Google Scholar]
- 38.Reichert IL, McCarthy ID, Hughes SP. The acute vascular response to intramedullary reaming: microsphere estimation of blood flow in the intact ovine tibia. J Bone Joint Surg Br 1995;77:490–493. [PubMed] [Google Scholar]
- 39.Greksa F, Tóth K, Boros M, Szabó A. Periosteal microvascular reorganization after tibial reaming and intramedullary nailing in rats. J Orthop Sci 2012;17:477–483. [DOI] [PubMed] [Google Scholar]
- 40.Danckwardt-Lillieström G. Reaming of the medullary cavity and its effect on diaphyseal bone: a fluorochromic, microangiographic and histologic study on the rabbit tibia and dog femur. Acta Orthop Scand Suppl 1969;128:1–153. [DOI] [PubMed] [Google Scholar]
- 41.Schaffler MB, Kennedy OD. Osteocyte signaling in bone. Curr Osteoporos Rep 2012;10:118–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Knothe Tate ML. ‘Whither flows the fluid in bone?’ An osteocyte’s perspective. J Biomech 2003;36:1409–1424. [DOI] [PubMed] [Google Scholar]
- 43.Burr DB, Schaffler MB, Yang KH, et al. The effects of altered strain environments on bone tissue kinetics. Bone 1989;10:215–221. [DOI] [PubMed] [Google Scholar]
- 44.Harter LV, Hruska KA, Duncan RL. Human osteoblast-like cells respond to mechanical strain with increased bone matrix protein production independent of hormonal regulation. Endocrinology 1995;136:528–535. [DOI] [PubMed] [Google Scholar]


