Abstract
Purpose
Thickened liquids are frequently used as an intervention for dysphagia, but gaps persist in our understanding of variations in swallowing behavior based on incremental thickening of liquids. The goal of this study was to establish reference values for measures of bolus flow and swallowing physiology in healthy adults across the continuum from thin to extremely thick liquids.
Method
A sex-balanced sample of 38 healthy adults underwent videofluoroscopy and swallowed 20% weight-to-volume concentration barium prepared in thin and slightly, mildly, moderately, and extremely thick consistencies using a xanthan gum thickener. Participants took comfortable sips and swallowed without a cue; sip volume was measured based on presip and postsip cup weights. A standard operating procedure (the ASPEKT method: Analysis of Swallowing Physiology: Events, Kinematics and Timing) was used to analyze videofluoroscopy recordings.
Results
The results clarify that, for thin liquid sips (10–14 ml), a single swallow without clearing swallows is typical and is characterized by complete laryngeal vestibule closure, complete pharyngeal constriction, and minimal postswallow residue. Aspiration was not seen, and penetration was extremely rare. Bolus position at swallow onset was variable, extending as low as the pyriform sinuses in 37% of cases. With thicker liquids, no changes in event sequencing, laryngeal vestibule closure, pharyngeal constriction, or postswallow residue were seen. The odds of penetration were significantly reduced. A longer timing interval until onset of the hyoid burst movement was seen, with an associated higher bolus position at swallow onset. Other timing measures remained unaffected by changes in bolus consistency.
Conclusion
The results include new reference data for swallowing in healthy adults across the range from thin to extremely thick liquids.
Thickened liquids are frequently used in dysphagia management (Carnaby & Harenberg, 2013; Garcia, Chambers, & Molander, 2005). The goal of thickening liquids is to slow their flow, making them easier to control and providing a longer time window in which to achieve airway closure before the bolus arrives near the entrance to the larynx, thus reducing the risk of penetration–aspiration (Curran & Groher, 1990; Logemann et al., 2008). However, recent studies suggest that there may be a trade-off between safety and efficiency and that very thick liquids may be more likely to leave postswallow residue in the pharynx (Hind et al., 2012; Steele, Alsanei, et al., 2015). Although diet texture modification has become a cornerstone of dysphagia management, evidence to guide clinicians in choosing appropriate consistencies for clinical management is lacking (Newman, Vilardell, Clavé, & Speyer, 2016; Robbins et al., 2002; Steele, Alsanei, et al., 2015). In 2012, the International Dysphagia Diet Standardisation Initiative (IDDSI; http://www.iddsi.org) was launched with the goal of establishing standardized terminology and definitions for texture-modified foods and thickened liquids for use in dysphagia management (Cichero et al., 2017, 2013). A systematic review published by IDDSI shows significant gaps in our knowledge regarding the impact of viscosity on swallowing and concluded that there is a critical need for new studies that will explore the physiological and functional consequences of thickening liquids in both healthy and disordered populations (Steele, Alsanei, et al., 2015).
Given the almost ubiquitous use of texture modification as an intervention for dysphagia (Garcia et al., 2005), there are surprisingly few studies in the literature comparing videofluoroscopic measures of bolus flow and swallowing physiology across liquids of different consistency (Bisch, Logemann, Rademaker, Kahrilas, & Lazarus, 1994; Clavé et al., 2006; Lazarus et al., 1993; Lof & Robbins, 1990; Nicosia et al., 2000; Perlman, Vandaele, & Otterbacher, 1995). The majority of these studies compare only two levels of consistency at widely separated points on the flow continuum, for example, “thin liquid” versus “paste” barium. The results of these studies suggest that bolus flow measures (oral and pharyngeal transit times) are of longer duration with paste consistency stimuli (Lof & Robbins, 1990). On the other hand, kinematic measures, such as measures of hyoid movement distance or duration, appear to display minimal variation (Lof & Robbins, 1990; Perlman et al., 1995), although one recent study has identified consistency-dependent variations in hyoid velocity using nectar-thick versus thin barium (Nagy, Molfenter, Péladeau-Pigeon, Stokely, & Steele, 2015). To date, the most authoritative trial of starch-thickened liquids as an intervention for dysphagia reported immediate benefits of nectar- and spoon-thick liquid barium for patients who aspirated thin liquid but did not describe the differences in bolus flow or physiology associated with reduced aspiration (Logemann et al., 2008). A handful of nonradiographic studies have explored smaller increments of viscosity using nonbarium stimuli. These tend to report significant differences for comparisons between thin and extremely thick liquids but not for narrower contrasts (Chi-Fishman & Sonies, 2002; Steele, Bailey, & Molfenter, 2010; Steele, Molfenter, Péladeau-Pigeon, Polacco, & Yee, 2014; Steele & Van Lieshout, 2004). Despite a long-standing interest in rheology within the swallowing literature (Coster & Schwarz, 1987) and widespread use of thickened liquids in clinical practice, it is currently unknown whether incremental thickening of liquids is associated with incremental changes in bolus flow measures or other swallowing parameters.
Historically, the National Dysphagia Diet guidelines that were in use across North America regarding thickening proposed rheological boundaries between categories at 50, 350, and 1,750 mPa s, measured at a shear rate of 50 reciprocal seconds (American Dietetic Association, 2002). The authors of the National Dysphagia Diet acknowledged, however, that there was no empirical evidence to show behavioral or clinical changes at these boundaries (American Dietetic Association, 2002) and the use of 50 reciprocal seconds to represent shear rates in the mouth has been questioned by several authors (Cutler, Morris, & Taylor, 1983; Hanson, 2016; Ong, Steele, & Duizer, 2018a; Shama & Sherman, 1973). Furthermore, the introduction of gum-based thickeners (e.g., xanthan gum, guar gum, locust bean gum) and other thickening agents (e.g., methylcellulose, agar, carrageenan) instead of, or in addition to, modified corn starch has added complexity to this issue (Vickers et al., 2015). Clinicians may assume, incorrectly, that manufacturer instructions for the amount of thickener needed to achieve “nectar”- or “honey”-thick consistency are calibrated based on viscosity, when this is not the case. The viscosity of a gum-thickened liquid will be much lower than a starch-thickened liquid with flow that appears similar based on flow off a spoon or when pouring from a cup (Hanson, 2016; Ong et al., 2018b).
The IDDSI framework classifies liquids into five levels of thickness, along a continuum of gravity flow; the IDDSI flow test measures thickness based on the height of the residual fluid column in a standard 10-ml slip-tip syringe after 10 s of flow (Cichero et al., 2017; Hanson, 2016). In this article, we report the results of a study measuring swallowing behavior in healthy adults under the age of 60 years, swallowing thin-liquid barium (IDDSI Level 0: thin) and the same barium product thickened with a xanthan gum thickener to IDDSI Levels 1–4: Level 1, slightly thick; Level 2, mildly thick; Level 3, moderately thick; and Level 4, extremely thick.
The primary objective of this study was to establish new reference values for videofluoroscopic measures of bolus flow and swallowing physiology across the range from thin to extremely thick liquids in healthy swallowing and to determine which parameters vary as a function of liquid consistency while controlling for the influence of participant sex and variations in sip volume. This was an exploratory, observational study rather than a hypothesis-driven experiment. It is hoped that the reference measures arising from this study will have utility for helping clinicians to identify the presence, severity, and mechanisms of swallowing impairment in patient populations. The larger study protocol included the videofluoroscopy data for thin and xanthan gum–thickened liquids that are described in this article as well as other experiments to measure tongue pressure and swallowing behaviors using liquids thickened with both starch and xanthan gum–based thickening agents, which have been reported elsewhere (Steele et al., 2019). Future comparisons to liquids with similar gravity flow but prepared using different thickening agents or different barium products are also planned.
Method
Participants
A sample of 40 participants consented to participate (20 men, 20 women). The mean age of the participants was 34 years (range: 21–58 years). Participants reported no prior or current history of swallowing complaints; difficulties with motor speech, gastroesophageal, or neurological function; sinusitis; or taste disturbance. Individuals with a prior history of radiation or surgery to the speech or swallowing apparatus (other than routine dental surgery, tonsillectomy, or adenoidectomy) were excluded, along with pregnant women, individuals with Type 1 diabetes (due to possible sensory differences in this group), and individuals with known allergies to latex, food coloring, or dental glue (due to the possibility of exposure to these products during data collection). Scheduling difficulties resulted in one male participant withdrawing prior to the videofluoroscopy. Technical difficulties with the videofluoroscopy recording resulted in a loss of thin liquid data for one female participant and one male participant. The final data set comprised full data for 582 boluses: three boluses of thin and slightly, mildly, moderately, and extremely thick liquids for 38 participants (19 women, 19 men) and partial data (i.e., 12 boluses covering the slightly to extremely thick range) for the 39th participant (female).
Stimuli
Low-concentration barium stimuli (20% w/v; Dantas, Dodds, Massey, & Kern, 1989; Steele, Molfenter, Péladeau-Pigeon, & Stokely, 2013; Stokely, Molfenter, & Steele, 2014) were prepared using Bracco Diagnostics E-Z-PAQUE powdered barium sulfate (96% w/w), bottled water (Nestlé Pure Life), and a xanthan gum–based thickener (Nestlé Resource ThickenUp Clear). The stimuli were prepared in thin, slightly thick, mildly thick, moderately thick, and extremely thick consistencies according to standard recipes (Barbon & Steele, 2018). Details regarding the rheological, gravity flow, and perceptual characteristics of these stimuli have been reported elsewhere (Barbon & Steele, 2018; Ong, Steele, & Duizer, 2018b).
Videofluoroscopy
Videofluoroscopy was performed in lateral projection according to a standard protocol using pulsed fluoroscopy (30 pulses per second) and recorded on a KayPENTAX Digital Swallow Workstation at 30 frames per second. In order to maximize the ecological validity of the experiment in simulating swallowing behaviors that might occur outside an assessment context, we decided not to fix bolus volume but, rather, to provide participants with cups containing 40 ml of liquid and ask them to take a natural sip and then to derive measures of sip volume based on cup weights. Each participant was presented with a tray containing cups of stimuli, arranged in blocks of three cups containing the same stimulus (see Figure 1). For the thin, slightly thick, and mildly thick liquids, the participant was instructed to take a cup, take one comfortable sip, swallow when they were ready (i.e., without a command; Daniels, Schroeder, DeGeorge, Corey, & Rosenbek, 2007; Nagy et al., 2013), and return the cup to a tray on a table immediately in front of the participant. The moderately and extremely thick stimuli were taken by teaspoon, again without a command. After swallowing a single bolus from each cup in a block of three cups, a sip of water was taken to rinse. The blocks were presented in order of ascending thickness (i.e., thin followed by slightly thick and then mildly, moderately, and extremely thick).
Figure 1.
Stimulus array used in the current experiment. A 20% w/v barium sulfate suspension (thin) was mixed with a xanthan gum thickener to reach Levels 1–4 of the International Dysphagia Diet Standardisation Initiative framework.
Sip Volume
Presip and postsip cup weights were taken on an Ohaus digital balance (Model PA1502 analytical scale: capacity = 1.5 kg, readability = 0.01 g). Sip mass (grams) was derived from the cup weights (i.e., presip minus postsip) and multiplied by the specific gravity of each stimulus (grams per milliliter) to determine the sip volume (milliliters).
Rating Procedure
All videofluoroscopy ratings were performed in duplicate according to a standard operating procedure that was given the name ASPEKT method (Analysis of Swallowing Physiology: Events, Kinematics and Timing) and that has been partly described in previous publications (Waito, Steele, Péladeau-Pigeon, Genge, & Argov, 2018). Ratings were performed in the same physical location by all raters in order to control potentially influencing factors such as lighting and computer screen quality (Mayer, Rogus-Pulia, Peppler, & Thibeault, 2018). A full description of the ASPEKT method can be found in the Appendix (including operational definitions for all parameters and a description of the standard operating procedures followed when performing ratings for this study), but the steps are summarized below:
Each videofluoroscopy recording was spliced into bolus level clips and randomly assigned to two raters from a team of eight trained raters.
The number of swallows for each bolus was counted. Raters were trained that the following components must be present in order to consider a “swallow” to have occurred: (a) at least one of laryngeal elevation, hyoid excursion, and/or pharyngeal constriction and (b) upper esophageal sphincter opening (UESO). The term subswallows is used to refer to individual swallows when there is more than one swallow per bolus. Subswallows are further qualitatively classified as initial (the first subswallow in the series), piecemeal (a higher order subswallow, i.e., subswallows 2, 3, 4, … terminal, in which additional material is transported from the oral cavity), or clearing (a higher order subswallow of pharyngeal residue with no additional material added from the oral cavity) swallows.
Swallowing safety was rated on each and every subswallow using the 8-point Penetration–Aspiration Scale (PAS; Rosenbek, Robbins, Roecker, Coyle, & Wood, 1996). Raters were instructed that they should only score new material entering the airway on each subswallow. In the case of scores of 3, 5, 6, 7, or 8 (indicating airway invasion without ejection out of the laryngeal vestibule), the frame of bolus entry into the laryngeal vestibule is documented to enable subsequent evaluation of the timing of airway invasion relative to laryngeal vestibule closure (LVC). Additionally, the amount of material entering the airway was coded subjectively as 0 (none), 1 (trace), or 2 (more than trace). Bolus-level results were derived based on the highest PAS score seen across the series of subswallows for that bolus. Additionally, if a previous PAS event was noted to evolve across subsequent higher order swallows for the same bolus (i.e., worsen or recover to a higher position in the airway), this was documented in the rating comments.
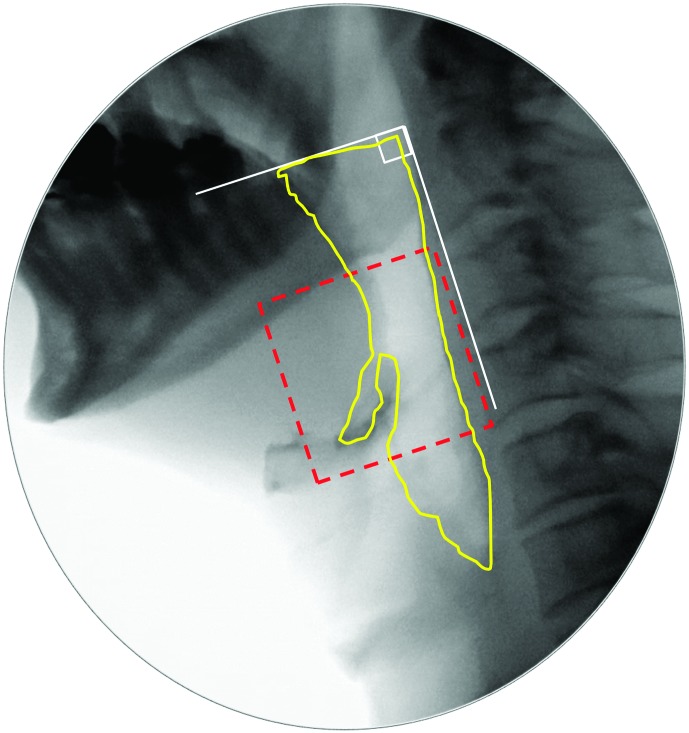
The temporal locations (i.e., frame numbers) of key bolus and gestural events in the swallow sequence were identified, with each event defined according to standard operational definitions. For the current article, nine events were tracked: bolus passing mandible (BPM), onset of the hyoid burst (HYB), LVC, UESO, maximum upper esophageal sphincter (UES) distension (UESMax), maximum pharyngeal constriction (MPC), UES closure (UESC), LVC offset (LVCOff), and “swallow rest” (i.e., the terminal frame of each swallow). Definitions for each of these events can be found in the Appendix. As part of this step, the completeness of LVC was also rated (complete or incomplete).
The location of the leading edge of the bolus was documented on the frames of HYB and LVC (Baijens et al., 2011; Martin-Harris et al., 2008). Definitions for these ordinal ratings can be found in the Appendix.
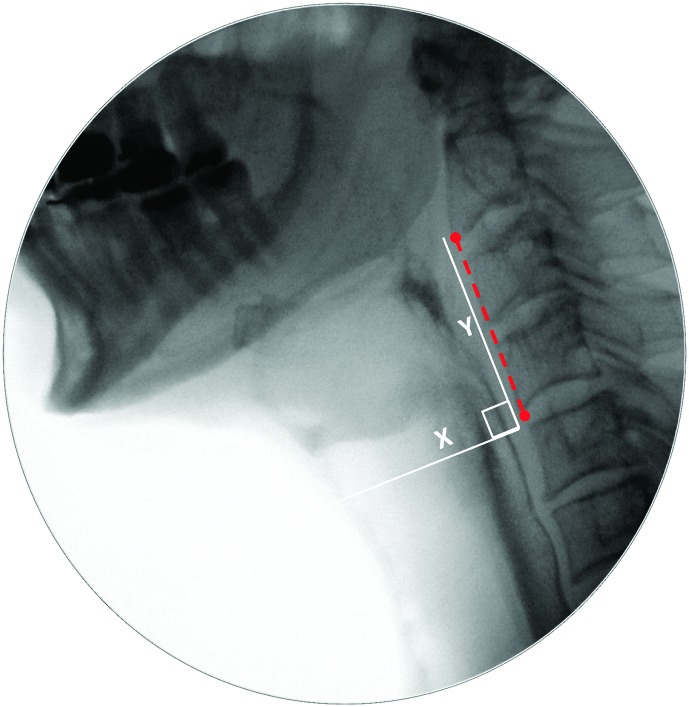
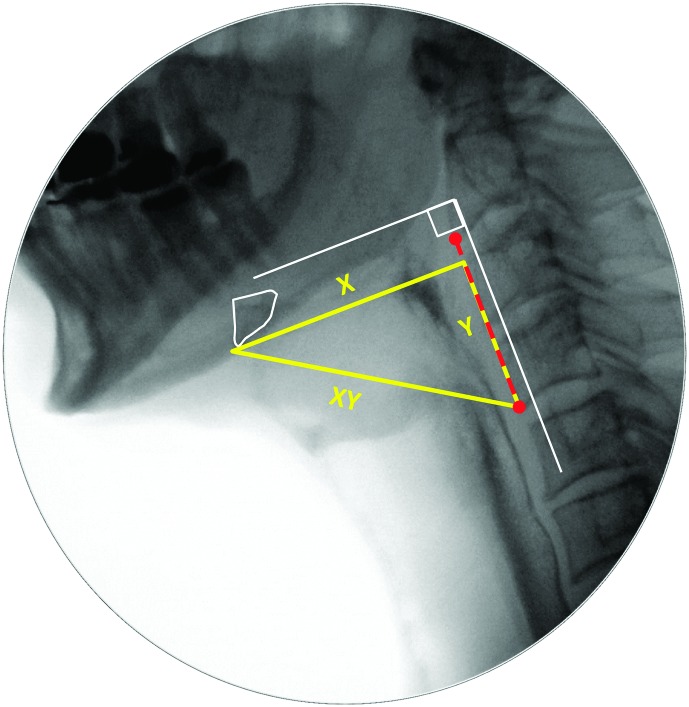
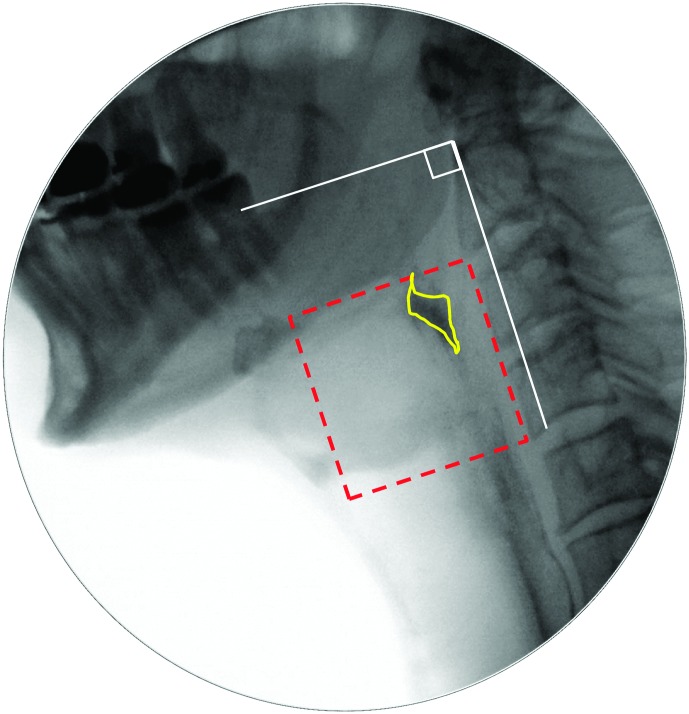
Pixel-based methods were used to trace the position of the hyoid (i.e., distance from the anterior inferior corner of C4), measured in the anterior (X) and superior (Y) planes relative to the length of a C2–C4 anatomical scalar (see example in the Appendix). The XY hypotenuse position (i.e., distance from the C4 origin) was derived using the Pythagorean theorem. This was completed on every frame starting from five frames prior to the frame chosen by raters as the frame of HYB until approximately five frames after the beginning of hyoid descent from its peak position. A MATLAB algorithm was then used to search through the series of hyoid position measures to confirm the frames of peak position in each plane (X, Y, and XY). In the case of a plateau in hyoid movement at its peak position, the first frame at peak position was used.
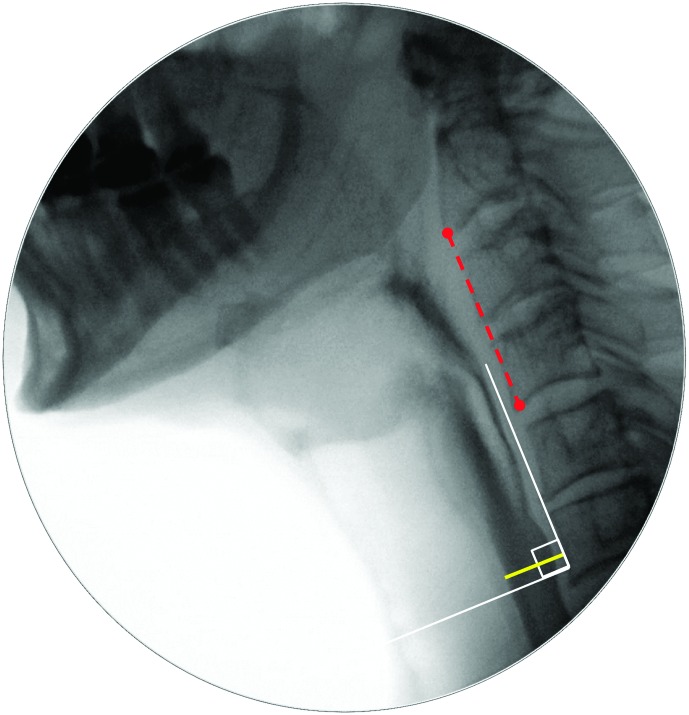
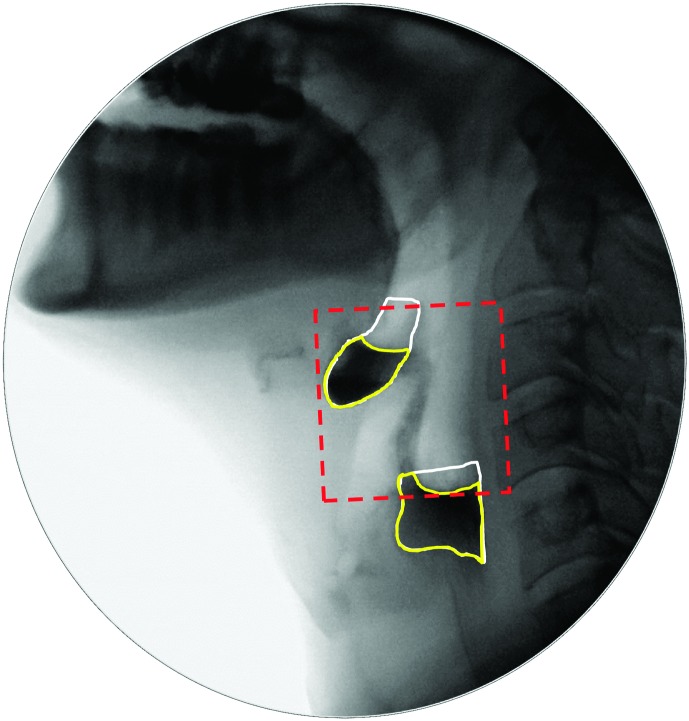
The diameter of UESO was traced (in pixels) on the frame of UESMax, relative to the length of an anatomical scalar (length of the cervical spine between the anterior inferior corners of the C2 and C4 vertebrae; Molfenter & Steele, 2014). An example can be found in the Appendix. Similarly, pharyngeal area was traced (in pixels) on the frames of MPC and swallow rest, relative to the squared length of a C2–C4 anatomical scalar. Examples can be found in the Appendix.
The area of any residue in the valleculae, in the pyriform sinuses, and/or elsewhere in the pharynx was traced (in pixels) on the frame of swallow rest, relative to the squared length of a C2–C4 anatomical scalar. An example can be found in the Appendix.
After the initial ratings were made independently, an Excel macro program was run to inspect them for agreement and identify cases that required discrepancy resolution. Interrater agreement for the initial ratings was strong for all types of measures; additional details can be found in the Appendix. Regardless of the high level of agreement demonstrated for all measures, strict criteria were used to handle discrepancies between raters. For example, any difference (of any magnitude) in ratings of the number of swallows per bolus, PAS scores, LVC (complete/incomplete), or bolus location at swallow onset was taken to a consensus meeting for resolution. Similarly, any difference > five frames between raters regarding the frame at which key events occurred was sent for resolution. Additional details regarding the thresholds used to identify measurements that required review and resolution can be found in the Appendix. Consensus meetings were attended by a minimum of three trained raters and involved review, repeat measurement, and debate regarding the discrepant ratings until consensus was achieved. Once discrepancies were resolved, the sequence of events was derived and timing intervals between events were calculated.
Statistical Analyses
Descriptive statistics for all variables were compiled in order to build a profile of healthy adult swallowing of liquids. Analyses were performed at the bolus level, using the highest (i.e., worst) PAS score and the highest number of swallows seen across all available subswallows for each bolus; the initial subswallow of each bolus was used for measures of sequence adherence, timing, and pixel-based measures. The descriptive statistics for timing measures will be reported in frames, given that the minimum temporal resolution of the rating process was in frames. For reference purposes, a table of timing measures will also be provided in milliseconds, using a conversion of 33 ms per frame.
Unless otherwise noted, an alpha criterion of .05 was used for all statistical comparisons. The effects of sex and bolus consistency on categorical or count data (i.e., PAS scores, event sequence adherence, and bolus location measures) were explored using frequencies (percentages), chi-square tests, and logistic regression. Linear mixed-model analyses of variance (ANOVAs) with compound symmetry structure were used to study variations in continuous parameter data (i.e., timing measures and pixel-based measures of structural movement, area, or residue). An iterative process was used, as detailed below:
Repeated-measures ANOVAs were run with a factor of sex to determine whether sex should be carried forward as a factor into the linear mixed models.
Pearson product–moment correlations were explored between sip volume and the dependent variables. Where correlations of r ≥ .3 were found, a decision was made to carry sip volume forward as a covariate into the linear mixed models.
The linear mixed models were run with a factor of consistency, a random effect of participant, and post hoc Sidak tests of pairwise comparisons. Sex and sip volume were included in the model where indicated based on the previous univariate explorations.
Significant main effects or interactions involving sip volume were further explored using scatter plots, Pearson correlations, and linear regression.
Effect sizes for significant pairwise comparisons involving sex or consistency were calculated using Cohen's d (Kotrlik & Williams, 2003).
Initial inspection of quantile–quantile plots for the continuous parameters revealed nonnormal distributions of residuals for sip volume, swallow reaction time, and MPC area. In these cases, a log transformation was applied prior to running the linear mixed models. For ease of interpretation and use as reference values in clinical settings, descriptive statistics and figures involving these parameters will be reported using nontransformed units. Pixel-based measures of residue were also found to have skewed distributions and were transformed to binary categorical measures; this will be described further below.
Results
Sip Volume
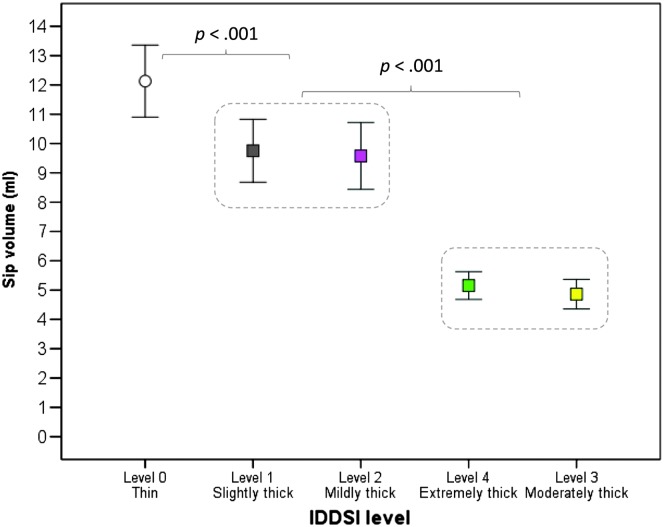
Average sip volume for the thin liquid stimulus was 12.12 ml (95% CI [10.71, 13.54]; 25th percentile, 8.12 ml; 75th percentile, 14.81 ml). One individual was a high outlier, with thin liquid sips consistently falling above 25 ml; these data points were removed and replaced with missing values prior to further analysis. Table 1 provides descriptive statistics for sip volume across the full range of stimuli that were studied. There were no significant differences in sip volume between male and female participants. A pattern of significantly smaller sip volumes was seen for thicker consistencies, as highlighted in Figure 2, F(4, 541.01) = 107.73, p < .001. The slightly and mildly thick liquids clustered together with significantly smaller sip volumes than the thin liquid (d ≥ 0.38, i.e., small). A further significant reduction in sip volume was seen for the comparison between the slightly/mildly thick liquids and the moderately/extremely thick liquids (d ≥ 0.98, i.e., large); this difference was expected, given that the moderately/extremely thick liquids were administered by a 5 ml–capacity teaspoon. Interestingly, sip volume for the extremely thick liquids was a little bit larger than that seen for the moderately thick liquids; this is thought to reflect the fact that the thicker consistency was able to retain its shape and mound on the spoon without overflowing, whereas the moderately thick liquid flowed over the boundaries of the spoon.
Table 1.
Descriptive statistics for sip volume (milliliters) by consistency, for thin and xanthan gum–thickened barium stimuli (20% w/v).
| Consistency | M | SD | 95% confidence interval |
|
|---|---|---|---|---|
| Lower bound | Upper bound | |||
| Thin | 12.13 | 6.68 | 10.90 | 13.36 |
| Slightly thick | 9.75 | 5.87 | 8.68 | 10.83 |
| Mildly thick | 9.58 | 6.23 | 8.44 | 10.72 |
| Moderately thick | 4.86 | 2.76 | 4.36 | 5.37 |
| Extremely thick | 5.15 | 2.59 | 4.68 | 5.63 |
Figure 2.
Sip volume by consistency, in descending order of volume. Sip volumes for slightly and mildly thick liquids were significantly smaller (p < .001) than those for thin liquids. A further significant reduction in sip volume was seen with moderately and extremely thick liquids, which were served by a teaspoon. IDDSI = International Dysphagia Diet Standardisation Initiative.
Number of Swallows per Bolus
Both the mode and median scores for the number of swallows per bolus had a value of 1, regardless of consistency. More than one swallow per bolus was seen 20% of the time. A chi-square test showed no differences in the frequency of multiple swallows per bolus by consistency.
Penetration–Aspiration
A maximum PAS score of 1 was seen across all boluses in the protocol, regardless of consistency, in 26 participants (i.e., 67%). Ten participants displayed transient penetration with ejection (i.e., a PAS score of 2) on a single consistency as follows: thin (n = 6, with two of these participants displaying a PAS score of 2 on the first thin bolus, two on the second thin bolus, one on the third thin bolus, and one on both the first and third thin boluses), slightly thick (n = 3, one on the first bolus, one on the second bolus, and one on the third bolus), and mildly thick (n = 1 on both the second and third mildly thick boluses). One further participant displayed a PAS score of 2 on a single bolus of two different consistencies (i.e., the third thin bolus and the first slightly thick bolus). The two remaining participants displayed more frequent occurrences of penetration. The first appeared to have a typical pattern of PAS scores of 2, seen on all thin and slightly thick boluses, on the second moderately thick bolus, and on the third extremely thick bolus. The final participant displayed penetration to the level of the true vocal folds without ejection (i.e., a PAS score of 5) on the first thin and first mildly thick bolus. All other boluses for these two participants had PAS scores of 1.
In terms of the amount of material observed to enter the airway, four of the 21 (19%) transient penetration events with ejection (i.e., PAS scores of 2) were coded as “more than trace” and the remainder was coded as “trace.” Of the two events of penetration without ejection (i.e., PAS scores of 5), one was coded as “trace” and the other was coded as “more than trace.” When each participant's maximum PAS score per consistency was entered into an ordinal logistic regression model, the risk of a PAS score of > 2 was significantly higher for thin liquids compared to all thicker consistencies (Wald χ2 = 25.01, df = 1, p < .001) and for thin and slightly thick liquids compared to mildly thick and thicker consistencies (Wald χ2 = 22.15, df = 1, p = .001). Relative risk statistics could not be computed for the two thickest consistencies due to the absence of any PAS scores of > 2 for the moderately and extremely thick liquids in this data set.
LVC
LVC was coded as complete on all boluses except three. Two of these events came from the same participant, who displayed partial LVC on the initial boluses of the thin and mildly thick liquids. The PAS score for both of these boluses was 5, indicating penetration to the level of the vocal folds without ejection. A single event of incomplete LVC was seen in a second participant on the third presentation of the slightly thick liquid; in this case, a PAS score of 1 was recorded. Due to the extremely low frequency of incomplete LVC in the data set, further analyses were not performed.
Timing Measures
Timing measures of possible interest were calculated based on the interval (i.e., difference in timing) between paired events. Inspection of these measures revealed a high degree of association between several parameters suggesting either tight temporal co-occurrence (i.e., differences with ≤ 2 frames duration, on average) or nonindependence (i.e., high Pearson product–moment correlations), as discussed below:
The interval from the frame of LVC until the frame of UESO had an average value of −1.4 frames or −46 ms (95% CI [−2.2, −0.6], i.e., −73 to −20 ms), indicating that the two events occurred in very close proximity. Inspection of the sequencing pattern between these two events showed that UESO occurred prior to or on the same frame as LVC in 78% of cases and lagged behind LVC in the remaining 21% of cases.
UESC and LVCOff also occurred in very tight proximity (average difference of 0.92 frames or 30 ms, 95% CI [0.56, 1.3], i.e., 18–43 ms). In the majority (i.e., 84.5%) of cases, LVCOff occurred simultaneously with or after UESC. In the 15.5% of cases where LVCOff preceded UESC, the latency until UESC had an average duration of 1.4 frames or 46 ms (95% CI [1.26, 1.53], i.e., 42–51 ms).
The interval between BPM and HYB was highly correlated with measures of BPM to UESO (r = .986) and BPM to UESC (r = .975).
UESO duration was significantly correlated with its subcomponent measures of UESO to MPC (r = .53) and MPC to UESC (r = .43).
Based on these observations, a parsimonious set of consecutive, nonredundant timing measures was chosen to represent key intervals related to the pharyngeal stage of swallowing, which are listed below:
1. Swallow Reaction Time (i.e., interval between BPM and HYB; Humbert et al., 2018): This parameter has also gone under a variety of different names in previous literature including “pharyngeal delay time” (Logemann et al., 2000; Logemann, Pauloski, Rademaker, & Kahrilas, 2002), “duration of stage transition” (Robbins, Hamilton, Lof, & Kempster, 1992), and “swallow response time” (Power et al., 2007);
2. interval between HYB and UESO;
3. UESO duration (i.e., interval between UESO and UESC).
For added interest, the temporal location of MPC was also explored as a percentage of the UESO duration interval.
In addition to these measures of pharyngeal event timing, two measures related to LVC were included, given the clinical importance of understanding the timing and duration of airway protection:
4. LVC Reaction Time (i.e., interval between HYB and LVC; Guedes et al., 2017; Humbert et al., 2018);
5. LVC duration (i.e., interval between LVC and LVCOff).
Table 2 contains descriptive statistics for these timing measures of interest, calculated in frames and derived in milliseconds. No significant effects of sex were identified for any of the timing measures. With respect to sip volume, a significant negative correlation (r = −.47) was found with the HYB to UESO interval, and a significant positive correlation (r = .34) was found with UESO duration. Sip volume was therefore included as a covariate in the linear mixed models for these parameters.
Table 2.
Descriptive statistics for swallow timing measures in frames and derived in milliseconds.
| Timing measure | Consistency | Event latency in frames |
Event latency in milliseconds (at 29.975 frames/s) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| M | SD | 95% confidence interval |
M | SD | 95% confidence interval |
||||
| Lower bound | Upper bound | Lower bound | Upper bound | ||||||
| Swallow Reaction Time (i.e., bolus passing the mandible to hyoid burst onset) | Thin | 3.25 | 5.32 | 2.06 | 4.44 | 109 | 177 | 69 | 148 |
| Slightly thick | 5.32 | 8.07 | 3.71 | 6.93 | 178 | 269 | 124 | 231 | |
| Mildly thick | 5.95 | 8.33 | 4.29 | 7.61 | 198 | 278 | 143 | 254 | |
| Moderately thick | 9.22 | 12.05 | 6.79 | 11.65 | 307 | 402 | 226 | 389 | |
| Extremely thick | 10.41 | 14.11 | 7.55 | 13.26 | 347 | 471 | 252 | 443 | |
| Hyoid burst onset to UES opening | Thin | 3.48 | 1.45 | 3.16 | 3.81 | 116 | 48 | 105 | 127 |
| Slightly thick | 3.70 | 1.82 | 3.33 | 4.06 | 123 | 61 | 111 | 135 | |
| Mildly thick | 3.83 | 1.74 | 3.48 | 4.18 | 128 | 58 | 116 | 139 | |
| Moderately thick | 4.55 | 1.65 | 4.21 | 4.88 | 152 | 55 | 141 | 163 | |
| Extremely thick | 4.65 | 1.52 | 4.34 | 4.95 | 155 | 51 | 145 | 165 | |
| UES opening duration | Thin | 13.72 | 1.88 | 13.30 | 14.14 | 458 | 63 | 444 | 472 |
| Slightly thick | 13.18 | 1.89 | 12.81 | 13.56 | 440 | 63 | 427 | 452 | |
| Mildly thick | 13.26 | 2.26 | 12.81 | 13.71 | 442 | 75 | 427 | 457 | |
| Moderately thick | 12.31 | 2.09 | 11.89 | 12.73 | 411 | 70 | 397 | 425 | |
| Extremely thick | 12.05 | 2.02 | 11.64 | 12.46 | 402 | 67 | 388 | 416 | |
| LVC Reaction Time (i.e., hyoid burst onset to LVC) | Thin | 5.38 | 3.00 | 4.71 | 6.05 | 179 | 100 | 157 | 202 |
| Slightly thick | 5.33 | 2.70 | 4.79 | 5.87 | 178 | 90 | 160 | 196 | |
| Mildly thick | 4.82 | 2.36 | 4.35 | 5.29 | 161 | 79 | 145 | 176 | |
| Moderately thick | 4.55 | 1.88 | 4.17 | 4.93 | 152 | 63 | 139 | 164 | |
| Extremely thick | 4.30 | 1.63 | 3.97 | 4.63 | 144 | 54 | 133 | 155 | |
| LVC duration | Thin | 13.06 | 3.24 | 12.34 | 13.79 | 436 | 108 | 412 | 460 |
| Slightly thick | 12.36 | 2.69 | 11.83 | 12.90 | 412 | 90 | 395 | 430 | |
| Mildly thick | 12.96 | 2.99 | 12.36 | 13.56 | 432 | 100 | 412 | 452 | |
| Moderately thick | 13.01 | 2.71 | 12.46 | 13.56 | 434 | 90 | 416 | 452 | |
| Extremely thick | 13.08 | 2.50 | 12.58 | 13.59 | 436 | 84 | 420 | 453 | |
Note. UES = upper esophageal sphincter; LVC = laryngeal vestibule closure.
Swallow Reaction Time
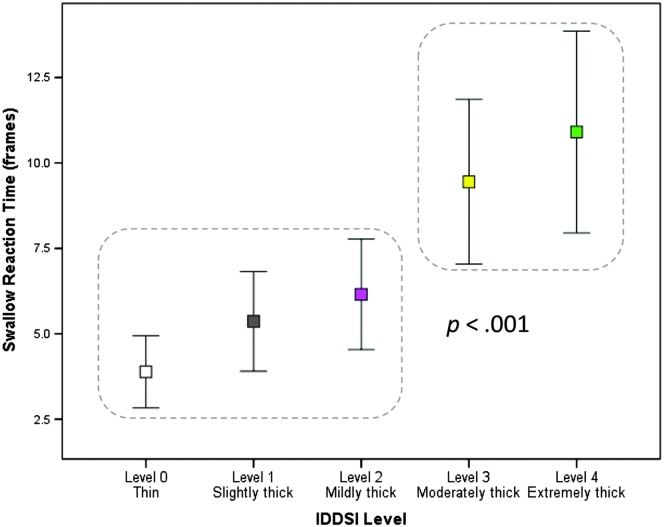
A significant main effect of consistency was found, F(4, 354.64) = 9.284, p < .001. As shown in Figure 3, significantly longer Swallow Reaction Times were seen for the moderately and extremely thick liquids compared to the thin, slightly thick, and mildly thick liquids (d ≥ 0.25, i.e., small).
Figure 3.
The effect of liquid consistency on swallow reaction time (i.e., the interval between the bolus passing the ramus of mandible and onset of the hyoid burst). Significantly longer swallow reaction times were seen with the moderately and extremely thick liquids (p < .001). IDDSI = International Dysphagia Diet Standardisation Initiative.
HYB to UESO
For the interval between onset of the HYB and UESO, there were no significant main effects or interactions involving consistency. Rather, a significant main effect of sip volume was seen, F(1, 470.58) = 52.04, p < .001, with shorter HYB to UESO intervals seen with larger sip volumes (r = −.47, R 2 = .21).
UESO Duration
A significant Consistency × Sip Volume interaction was found, F(4, 500.25) = 2.91, p = .021, along with a main effect of sip volume, F(1, 528.57) = 90.68, p < .001. Overall, a positive correlation between sip volume and UESO duration was seen (r = .34, R 2 = .12). The regression coefficient for the sip volume effect was highest for the extremely thick liquid (R 2 = .11), with R 2 values of .04, .09, .06, and .09 for the thin and slightly, mildly, and moderately thick liquids, respectively.
Timing of MPC Relative to UESO
The timing of MPC, expressed as a percentage of the UESO duration interval, varied significantly by consistency, F(4, 507.28) = 18.46, p < .001. MPC occurred significantly earlier for the moderately and extremely thick liquids compared to the thin, slightly thick, and mildly thick liquids (d = 0.55, i.e., medium). Descriptive statistics are found in Table 3.
Table 3.
Descriptive statistics for the timing of maximum pharyngeal constriction, expressed as a percentage of upper esophageal sphincter opening duration.
| Consistency | M | SD | 95% confidence interval |
|
|---|---|---|---|---|
| Lower bound | Upper bound | |||
| Thin | 49.5 | 12.1 | 46.6 | 52.4 |
| Slightly thick | 48.8 | 11.0 | 46.1 | 51.5 |
| Mildly thick | 48.9 | 11.5 | 46.2 | 51.5 |
| Moderately thick | 44.3 | 10.1 | 41.5 | 47.1 |
| Extremely thick | 44.1 | 10.2 | 41.3 | 46.8 |
LVC Reaction Time and Duration
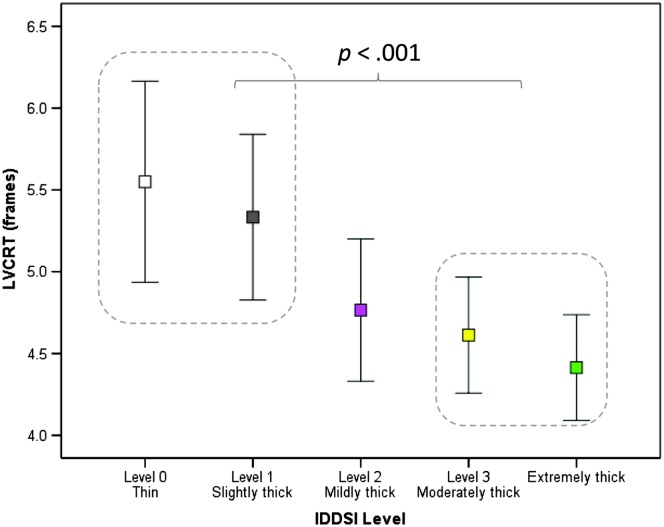
A significant main effect of consistency was found for LVC Reaction Time, F(4, 499.96) = 5.96, p < .001. As shown in Figure 4, closure of the laryngeal vestibule was achieved significantly faster with the moderately and extremely thick stimuli compared to the thin and slightly thick liquids (d = 0.33, i.e., small). Finally, no effect of consistency was seen for LVC duration.
Figure 4.
The effect of liquid consistency on laryngeal vestibule closure reaction time (i.e., the interval between onset of the hyoid burst and the frame of laryngeal vestibule closure). Significantly longer Laryngeal Vestibule Closure Reaction Times (p < .001) were seen with the moderately and extremely thick liquids compared to the thin and slightly thick stimuli. IDDSI = International Dysphagia Diet Standardisation Initiative; LVCRT = Laryngeal Vestibule Closure Reaction Time.
Event Sequence
The expected sequence to which paired event patterns were compared was BPM < HYB < UESO < UESMax < MPC < UESC. In contrast with previous literature (Herzberg, Lazarus, Steele, & Molfenter, 2018; Kendall, Leonard, & McKenzie, 2003; Kendall, McKenzie, Leonard, Gonçalves, & Walker, 2000; Molfenter, Leigh, & Steele, 2014), this sequence was selected because it emphasizes gestural rather than bolus events and focuses exclusively on pharyngeal and UES events rather than laryngeal events. As noted above, the timings of LVC onset and LVCOff were found to be tightly associated with UESO and UES closing, respectively. Table 4 illustrates the frequency (percentage) of adherence to these expected pairwise event sequences by consistency. Adherence to the expected paired event sequences exceeded 80% for all pairs, regardless of bolus consistency. For two event pairs (UESO < UESMax and UESMax < MPC), 100% adherence was observed. Chi-square statistics failed to find any significant differences in pairwise sequence adherence across the different consistencies.
Table 4.
Frequency (percentage) adherence to the expected sequence of pharyngeal phase events by consistency.
| Consistency | BPM < HYB | HYB < UESO | UESO < UESMax | UESMax < MPC | MPC < UESC | Overall sequence |
|---|---|---|---|---|---|---|
| Thin | 87.9 | 94.5 | 100.0 | 100.0 | 93.6 | 77.1 |
| Slightly thick | 87.6 | 95.6 | 100.0 | 100.0 | 97.4 | 80.5 |
| Mildly thick | 89.4 | 97.4 | 100.0 | 100.0 | 98.2 | 84.8 |
| Moderately thick | 82.6 | 100.0 | 100.0 | 100.0 | 99.1 | 81.7 |
| Extremely thick | 84.1 | 99.1 | 100.0 | 100.0 | 100.0 | 83.2 |
| Total | 86.3 | 97.3 | 100.0 | 100.0 | 97.7 | 81.5 |
Note. BPM = bolus passing mandible; HYB = hyoid burst onset; UESO = upper esophageal sphincter opening; UESMax = maximum diameter of upper esophageal sphincter distension; MPC = maximum pharyngeal constriction; UESC = upper esophageal sphincter closing.
Bolus Location Measures
Table 5 shows the frequency distribution of bolus location at swallow onset by consistency. As seen in the table, scores are distributed across the continuum, regardless of consistency. Significantly fewer moderately and extremely thick boluses were scored as having reached the pyriform sinuses compared to the thinner consistencies (χ2 = 44.21, df = 4, p < .001).
Table 5.
Frequencies (percentage) of ordinal ratings for the location of the leading edge of the bolus on the frames of hyoid burst and laryngeal vestibule closure by consistency.
| Parameter | Consistency | Ramus of mandible | Vallecular pit | Posterior laryngeal surface of epiglottis | Pyriform sinus | In UES | No appreciable swallow initiation |
|---|---|---|---|---|---|---|---|
| Bolus location at swallow onset (i.e., on the frame of hyoid burst onset) | Thin | 25.2 | 18.0 | 19.8 | 36.9 | 0.0 | 0.0 |
| Slightly thick | 26.1 | 20.0 | 26.1 | 27.8 | 0.0 | 0.0 | |
| Mildly thick | 27.4 | 14.5 | 28.2 | 29.9 | 0.0 | 0.0 | |
| Moderately thick | 34.2 | 24.6 | 28.9 | 12.3 | 0.0 | 0.0 | |
| Extremely thick | 27.9 | 34.2 | 32.4 | 5.4 | 0.0 | 0.0 | |
| Total | 28.2 | 22.2 | 27.1 | 22.5 | 0.0 | 0.0 | |
| Bolus location on the frame of laryngeal vestibule closure | Thin | 2.7 | 0.0 | 4.5 | 12.5 | 80.4 | 0.0 |
| Slightly thick | 4.3 | 0.0 | 0.9 | 15.4 | 79.5 | 0.0 | |
| Mildly thick | 1.7 | 0.0 | 1.7 | 23.9 | 72.6 | 0.0 | |
| Moderately thick | 0.9 | 0.9 | 3.4 | 35.9 | 59.0 | 0.0 | |
| Extremely thick | 0.0 | 0.0 | 6.1 | 33.0 | 60.9 | 0.0 | |
| Total | 1.4 | 0.4 | 3.2 | 27.1 | 67.9 | 0.0 |
Note. UES = upper esophageal sphincter.
Given the fact that the data showed no clear pattern with respect to a typical bolus location at swallow onset, the data were inspected to determine whether individual participants trended toward particular scores as a pattern. Consistent scoring across all three boluses of any given stimulus was not seen for any participant in the sample. A trend of similar scoring on two out of three boluses of the same stimulus was more common, as seen in 42% of cases overall. However, a chi-square test failed to find any significant difference in the frequency of a pattern of two out of three boluses at swallow onset by bolus consistency.
Table 5 shows the frequency distribution of bolus location at the time of LVC by stimulus. Here, fewer than 5% of boluses were scored as being at or above the ramus of mandible, in the valleculae, or at the posterior laryngeal surface of epiglottis, regardless of consistency. The majority (i.e., ≥ 72%) of the thin, slightly thick, and mildly thick boluses were scored as having already entered the UES on the frame of LVC. With the moderately and extremely thick liquids, a similar pattern was seen, but a larger proportion of these boluses (i.e., 33%–36%) were scored as being in the pyriform sinuses or higher at the frame of LVC (χ2 = 22.6, df = 4, p < .001).
Pixel-Based Measures
Hyoid Peak Position
Inspection of the distribution of hyoid peak position data revealed a small number of extreme high outliers (i.e., X position values > 2.00, Y position values > 1.7, XY position values > 2.5). These were removed and replaced with missing values prior to further analysis. The analysis of differences in hyoid peak position was performed separately for the three vectors of movement (X, Y, and XY); a Bonferroni corrected alpha criterion of p < .02 was used to adjust for expected autocorrelations between vectors. Sex was not included in the mixed-model ANOVAs due to the fact that anatomically normalized measures were used (Molfenter & Steele, 2014). Bivariate correlations showed no relationships above r = .1 between hyoid peak position measures and sip volume.
Descriptive statistics for hyoid peak position can be found in Table 6. No significant effects of consistency were found for peak XY position or peak X position. A significant effect of consistency was found for peak Y position, F(4, 489.17) = 3.662, p = .006. Inspection of the data did not reveal any systematic pattern of increased superior displacement for thicker liquids; rather, the consistency effect took the form of a single significant pairwise difference, with a higher peak position seen on the mildly thick compared to the slightly thick liquid (d = 0.21, i.e., small).
Table 6.
Descriptive statistics for hyoid peak position by consistency and plane of movement, measured as percentage of the C2–C4 reference scalar.
| Plane of movement | Consistency | M | SD | 95% confidence interval |
|
|---|---|---|---|---|---|
| Lower bound | Upper bound | ||||
| Horizontal (X) | Thin | 144 | 14 | 140 | 149 |
| Slightly thick | 142 | 15 | 138 | 147 | |
| Mildly thick | 143 | 15 | 139 | 148 | |
| Moderately thick | 142 | 15 | 137 | 146 | |
| Extremely thick | 142 | 15 | 138 | 147 | |
| Vertical (Y) | Thin | 91 | 23 | 84 | 98 |
| Slightly thick | 89 | 24 | 82 | 96 | |
| Mildly thick | 94 | 22 | 87 | 101 | |
| Moderately thick | 93 | 23 | 86 | 100 | |
| Extremely thick | 92 | 20 | 85 | 99 | |
| Hypotenuse (XY) | Thin | 170 | 16 | 165 | 175 |
| Slightly thick | 168 | 17 | 163 | 173 | |
| Mildly thick | 170 | 16 | 165 | 175 | |
| Moderately thick | 168 | 18 | 163 | 173 | |
| Extremely thick | 168 | 16 | 163 | 173 | |
UESMax
Table 7 contains descriptive statistics for anatomically normalized measures of UESMax, expressed as a percentage of the C2–C4 linear reference scalar. There were no significant sex effects on this parameter. A significant Sip Volume × Consistency interaction was found, F(4, 522.61) = 5.13, p < .001, as well as significant main effects of sip volume, F(1, 528.42) = 52.023, p < .001, and consistency, F(4, 517.31) = 8.95, p < .001. These effects took the form of wider UESO, in general, for larger sip volumes (R = .43, R 2 = .10) and on the thin liquid compared to the slightly and mildly thick liquids (d = 0.3, i.e., small). When explored by consistency, the highest correlations between UESO and sip volume were seen for the moderately and extremely thick liquids (R = .45, R 2 = .2 and R = .62, R 2 = .39, respectively).
Table 7.
Descriptive statistics for maximum diameter of upper esophageal sphincter opening, expressed as percentage of the C2–C4 reference scalar.
| Consistency | M | SD | 95% confidence interval |
|
|---|---|---|---|---|
| Lower bound | Upper bound | |||
| Thin | 20.6 | 6.6 | 19.3 | 21.8 |
| Slightly thick | 18.7 | 5.8 | 17.7 | 19.8 |
| Mildly thick | 18.3 | 5.1 | 17.4 | 19.3 |
| Moderately thick | 15.6 | 5.3 | 14.7 | 16.6 |
| Extremely thick | 16.9 | 4.7 | 16.0 | 17.7 |
Pharyngeal Area at Rest
As shown in Table 8, the area of the pharynx at rest corresponded, on average, to 58% of the squared C2–C4 reference scalar in this sample of healthy adults (95% CI [53%, 64%]). Pharyngeal area was highly correlated with anatomical reference area (R = .764, R 2 = .583). Pharyngeal area at rest was significantly larger in male participants who had a mean value of 68% of the squared C2–C4 reference scalar compared to 49% in female participants, F(1, 35.80) = 24.75, p < .001 (d = 1.03, i.e., large). This finding is consistent with recently reported sex differences in acoustic pharyngometry measures of pharyngeal volume (Molfenter, Lenell, & Lazarus, 2018).
Table 8.
Descriptive statistics for measures of pharyngeal area at maximum constriction and at rest, expressed as percentage of the squared C2–C4 reference scalar.
| Parameter | Consistency | M | SD | 95% confidence interval |
|
|---|---|---|---|---|---|
| Lower bound | Upper bound | ||||
| Pharyngeal area at maximum constriction | Thin | 0.9 | 1.3 | 0.7 | 1.2 |
| Slightly thick | 1.0 | 1.5 | 0.7 | 1.3 | |
| Mildly thick | 1.5 | 1.7 | 1.2 | 1.8 | |
| Moderately thick | 0.8 | 1.0 | 0.6 | 1.0 | |
| Extremely thick | 0.6 | 1.1 | 0.4 | 0.8 | |
| Pharyngeal area at rest | 58 | 16 | 53 | 64 | |
Pharyngeal Constriction
Table 8 also includes descriptive statistics for the unobliterated area of the pharynx on the frame of MPC, expressed as a percentage of the squared C2–C4 reference scalar. The highest upper confidence interval boundary seen across the five consistencies was 1.8% of the squared C2–C4 reference scalar, suggesting that almost complete obliteration of the pharynx was the norm at the point of maximum constriction. There was no evidence of sex effects or of significant correlations between sip volume and pharyngeal constriction. A significant main effect of consistency was found, F(4, 204.28) = 8.21, p < .001. This took the effect of significantly less constriction (i.e., a larger traceable unobliterated area) for the slightly and mildly thick liquids compared to the moderately and extremely thick liquids (d ≥ 0.19, i.e., small).
Residue
As mentioned previously, the mode number of swallows seen per bolus was 1, regardless of consistency. Consequently, residue was measured on the swallow rest frame of the initial subswallow for each bolus by convention. Table 9 presents descriptive statistics for residue found in the valleculae, in the pyriform sinuses, and elsewhere in the pharynx and total residue by consistency. Three different conventions for measuring residue are included to facilitate the use of these data as reference values by clinicians. First, for the valleculae and pyriform sinuses, residue area is expressed as a percentage of the housing area (Pearson, Molfenter, Smith, & Steele, 2012). Second, to provide a common reference area across all residue locations, residue area is expressed as a percentage of the squared C2–C4 length reference scalar (Pearson et al., 2012). Finally, these two different ratios are combined in the Normalized Residue Ratio Scale measure for the valleculae and pyriform sites, as proposed by Pearson et al. (2012). Inspection of these data shows that the upper confidence interval boundaries for total pharyngeal residue area, expressed as a percentage of the squared C2–C4 reference scalar, was only 1.46%, seen on the mildly thick liquid. In other words, pharyngeal residue (in any location) was minimal in this sample of healthy adults, regardless of bolus consistency.
Table 9.
Descriptive statistics for postswallow residue.
| Location | Measurement approach | Metric | Thin | Slightly thick | Mildly thick | Moderately thick | Extremely thick |
|---|---|---|---|---|---|---|---|
| Valleculae | % full | M | 16 | 19 | 25 | 12 | 11 |
| SD | 31 | 33 | 39 | 26 | 26 | ||
| 95% CI lower bound | 10 | 13 | 18 | 7 | 6 | ||
| 95% CI upper bound | 22 | 26 | 33 | 17 | 16 | ||
| % of squared C2–C4 reference scalar | M | 0.38 | 0.59 | 0.63 | 0.31 | 0.33 | |
| SD | 0.83 | 1.22 | 1.02 | 0.67 | 0.78 | ||
| 95% CI lower bound | 0.21 | 0.35 | 0.43 | 0.17 | 0.17 | ||
| 95% CI upper bound | 0.54 | 0.82 | 0.83 | 0.44 | 0.48 | ||
| Normalized Residue Ratio Scale | M | 0.02 | 0.03 | 0.04 | 0.02 | 0.02 | |
| SD | 0.07 | 0.08 | 0.08 | 0.04 | 0.05 | ||
| 95% CI lower bound | 0.01 | 0.02 | 0.03 | 0.01 | 0.01 | ||
| 95% CI upper bound | 0.04 | 0.05 | 0.06 | 0.02 | 0.03 | ||
| Pyriform sinuses | % full | M | 1 | 3 | 4 | 2 | 2 |
| SD | 5 | 7 | 7 | 5 | 4 | ||
| 95% CI lower bound | 1 | 2 | 2 | 1 | 1 | ||
| 95% CI upper bound | 2 | 4 | 5 | 3 | 3 | ||
| % of squared C2–C4 reference scalar | M | 0.18 | 0.27 | 0.31 | 0.16 | 0.12 | |
| SD | 0.79 | 0.87 | 0.64 | 0.43 | 0.41 | ||
| 95% CI lower bound | 0.03 | 0.10 | 0.19 | 0.08 | 0.03 | ||
| 95% CI upper bound | 0.34 | 0.44 | 0.44 | 0.25 | 0.20 | ||
| Normalized Residue Ratio Scale | M | 0.00 | 0.01 | 0.00 | 0.00 | 0.00 | |
| SD | 0.02 | 0.02 | 0.02 | 0.01 | 0.01 | ||
| 95% CI lower bound | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | ||
| 95% CI upper bound | 0.01 | 0.01 | 0.01 | 0.00 | 0.00 | ||
| Elsewhere in the pharynx | % of squared C2–C4 reference scalar | M | 0.08 | 0.18 | 0.17 | 0.11 | 0.08 |
| SD | 0.31 | 0.49 | 0.41 | 0.35 | 0.35 | ||
| 95% CI lower bound | 0.02 | 0.08 | 0.10 | 0.04 | 0.01 | ||
| 95% CI upper bound | 0.14 | 0.27 | 0.25 | 0.18 | 0.15 | ||
| Total (all pharyngeal areas combined) | % of squared C2–C4 reference scalar | M | 0.68 | 1.04 | 1.15 | 0.57 | 0.55 |
| SD | 1.52 | 1.83 | 1.59 | 0.93 | 1.14 | ||
| 95% CI lower bound | 0.38 | 0.69 | 0.83 | 0.38 | 0.32 | ||
| 95% CI upper bound | 0.99 | 1.39 | 1.46 | 0.76 | 0.78 |
Note. CI = confidence interval.
The distributions of residue scores were heavily skewed, regardless of consistency or measurement method, with scores of 0 (no residue) being the most common. For this reason, parametric investigations of residue severity were not appropriate. The data were recoded into binary scores of residue less than or equal to or greater than the 95% confidence interval upper boundary (for thin), using the percentage of the squared C2–C4 reference scalar measures. Chi-square tests were used to explore the proportion of cases with residue above the upper confidence interval boundary by stimulus, beginning with the total residue area measurement. This revealed significantly higher frequencies (χ2 = 13.35, df = 4, p = .01) of above-threshold residue area (i.e., ≥ 20% of cases) for the slightly and mildly thick liquids (24% and 31% of cases, respectively). Further explorations of residue in the different locations revealed significantly higher frequencies (i.e., > 20% of cases) of above-threshold residue areas in the pyriform sinuses with the mildly thick consistency (24% of cases, χ2 = 11.28, df = 4, p = .024). No significant differences in the frequency of above-threshold residue were seen across consistency for the valleculae or for pharyngeal locations other than the valleculae and pyriform sinuses.
Discussion
This study allows us to characterize healthy swallowing of thin liquids in adults under the age of 60 years. In doing so, methodological constraints must be acknowledged. Several of the protocol design choices that were made in this study may differ from other studies of healthy swallowing in the literature. These include
the context of taking single, comfortably sized sips (or teaspoons-full) from a cup containing 40 ml of liquid (as opposed to delivering boluses of fixed volume);
the absence of verbal cues (Daniels et al., 2007; Nagy et al., 2013); and
use of a 20% w/v barium concentration (Dantas et al., 1989; Humbert et al., 2018; Steele et al., 2013; Stokely et al., 2014).
Additionally, image acquisition settings must be noted (Bonilha et al., 2013; Péladeau-Pigeon & Steele, 2013); studies in which timing measures are derived by counting frames in a videofluoroscopy recording are particularly vulnerable to differences in frame rate. Any one of these methodological choices has the potential to influence the resulting measures and to contribute to differences in study results versus previous literature involving controlled bolus volumes (e.g., Bisch et al., 1994; Clavé et al., 2008, 2006; Humbert et al., 2018; Jardine, Miles, & Allen, 2018; Kendall & Leonard, 2001; Kendall et al., 2003, 2000; Leonard & McKenzie, 2006; Martin-Harris, Brodsky, Michel, Lee, & Walters, 2007; Martin-Harris & Jones, 2008; Molfenter & Steele, 2012a), a cued swallow paradigm (Daniels et al., 2007; Nagy et al., 2013), higher barium concentrations (e.g., Guedes et al., 2017; Hind et al., 2012; Humbert et al., 2018; Jardine et al., 2018; Kendall & Leonard, 2001; Kendall et al., 2003, 2000; Leonard, Kendall, & McKenzie, 2004; Leonard & McKenzie, 2006; Martin-Harris et al., 2008, 2007), or different contrast media (Baijens et al., 2011; Clavé et al., 2008, 2006).
When designing a study involving videofluoroscopy, it is important to balance the need to limit radiation exposure against the goal of collecting a sufficient number of repetitions of each swallowing task in order to account for within-participant variability across boluses. The decision to allow participants to take comfortable sips allowed us to collect three repetitions of each task while limiting the duration of the complete study. Sip volumes for the thin liquid stimulus averaged 12 ml with an interquartile range of 6.69 ml. These volumes fall in a similar range to those reported in other studies in which a natural sip size paradigm has been employed with thin liquid barium stimuli, both in healthy individuals (Steele et al., 2019) and in samples of patients referred for videofluoroscopy (Steele, Peladeau-Pigeon, Tam, Zohouri-Haghian, & Mukhurjee, 2015). Given that the literature suggests that some physiological parameters of swallowing vary in relation to bolus volume (see Molfenter & Steele, 2012b, for a review), caution is warranted when comparing the results of this study to those in which different volumes have been tested (e.g., Bisch et al., 1994; Clavé et al., 2008, 2006; Humbert et al., 2018; Jardine et al., 2018; Kendall & Leonard, 2001; Kendall et al., 2003, 2000; Leonard & McKenzie, 2006; Martin-Harris et al., 2007; Martin-Harris & Jones, 2008; Molfenter & Steele, 2012a).
Notwithstanding these caveats, this study suggests that healthy swallowing of low-concentration thin liquid barium can be characterized as the following:
Comfortable sip volumes typically fall in the range of 10–14 ml, consistent with previous studies (Bennett, Van Lieshout, Pelletier, & Steele, 2009; Steele, Péladeau-Pigeon, et al., 2015, 2019).
A typical sip is swallowed completely in a single swallow.
The location of the leading edge of the bolus at the point of HYB is highly variable both within and across individuals; the bolus has reached the vallecular space or lower in the majority of cases, consistent with evidence in previous studies (Humbert et al., 2018; Linden, Tippett, Johnston, Siebens, & French, 1989; Martin-Harris et al., 2007; Stephen, Taves, Smith, & Martin, 2005).
LVC is complete, and PAS scores of > 1 are unusual, consistent with previous reports (Daggett, Logemann, Rademaker, & Pauloski, 2006; Humbert et al., 2018). Particular individuals may tend toward PAS scores of 2 as a pattern.
The sequence of pharyngeal events is stable and proceeds in the majority of cases as follows: BPM < HYB < UESO < UESMax < MPC < UESC. The subsequences of UESO < UESMax and UESMax < UESC have previously been described as obligatory sequences in the literature (Herzberg et al., 2018; Kendall et al., 2003; Molfenter et al., 2014).
Closure of the laryngeal vestibule is closely timed with UESO, consistent with previous reports (Herzberg et al., 2018; Jardine et al., 2018; Kendall et al., 2003; Molfenter et al., 2014; Molfenter & Steele, 2012a).
Peak hyoid position, measured in the X, Y, and XY planes as distance from the anterior inferior corner of the C4 vertebrae, is at least 140% (X), 80% (Y), and 165% (XY) of the length of the C2–C4 reference scalar. These values are slightly larger than those previously reported for thin liquid boluses with a mean volume of 8 ml (Molfenter & Steele, 2014; Nagy, Molfenter, Péladeau-Pigeon, Stokely, & Steele, 2014).
MPC occurs after the point of maximum UESO and is typically complete, leaving no traceable, unobliterated space on a lateral-view videofluoroscopic image. This finding is consistent with recent descriptions of swallowing in a healthy young cohort by Jardine et al. (2018).
Finally, postswallow pharyngeal residue is minimal (i.e., < 1% of the squared C2–C4 reference scalar); this finding is also consistent with the recent study by Jardine et al.
With these characteristics established, it is possible to explore systematic variations in swallowing according to task or in specific populations, such as healthy seniors or individuals referred for swallowing evaluation. The primary aim of the current study was to measure bolus flow and swallowing physiology across the range from thin to extremely thick liquids to illustrate the influence of thicker bolus consistencies on swallowing in healthy adults. Again, methodological constraints must be acknowledged; foremost among these are the decision to use natural sip sizes rather than fixed bolus volumes, the fact that the blocks of stimulus consistency were delivered in a fixed order of increasing thickness (such that order effects cannot be ruled out), and the decision to use an array of xanthan gum–thickened liquids. The results of this study should not be generalized to liquids thickened with different thickeners until evidence to support such extrapolation has been established, controlling for differences in viscosity or gravity flow across liquids thickened with different products. Acknowledging this limitation, this study suggests that the following patterns may be expected with thickened liquids in healthy adults under the age of 60 years:
Smaller sip volumes are seen for slightly and mildly thick liquids compared to thin liquids.
Even smaller sip volumes are seen with teaspoon administration of moderately and extremely thick liquids.
Multiple swallows per bolus are not typical for thicker liquids.
Slower bolus flow is seen with moderately and extremely thick liquids, leading to longer Swallow Reaction Time measures and a trend toward a higher bolus location at swallow onset.
LVC is complete.
LVC Reaction Time is shorter with moderately and extremely thick liquids; in combination with the longer Swallow Reaction Times mentioned above, this suggests a later HYB movement and faster achievement of LVC once the HYB begins.
The risk of PAS scores of > 2 is lower than with thin liquids.
There is no difference in sequencing of pharyngeal or laryngeal vestibule events compared to the sequences seen with thin liquids.
No differences in timing measures of HYB to UESO or of UESO duration should be expected with thicker liquids (rather, differences in these timing measures are better explained by bolus volume).
There are no differences in peak hyoid position with thicker liquids.
A reduction in UESMax opening is seen for slightly and mildly thick liquids compared to thin liquids.
MPC remains complete and is unaffected by liquid consistency.
Postswallow residue remains extremely rare, but a small increase in the frequency of residue (with an upper boundary of 1% of the squared C2–C4 reference scalar) is seen with slightly and mildly thick liquids.
The current data do not provide clear evidence of boundaries along the consistency continuum where specific changes in swallowing behavior can be expected. As a rule, the effects of slightly and mildly thick liquids clustered together compared to thin liquids, and the effects of moderately and extremely thick liquids clustered together compared to the thinner consistencies. Of course, it remains to be determined whether incremental thickening has a similar or different pattern of effect in people with dysphagia. Evidence from the current study regarding the particular parameters or mechanisms that display responsiveness to thickening may serve to inform treatment decisions and future research hypotheses.
We have decided to use frames as the primary unit of measure for reporting timing measures in this study (see Table 2). Although several previous studies in the literature have reported timing measures in milliseconds, it must be recognized that these measures are typically derived from frame counts or from time code generators that display time in milliseconds on the capture monitor. As such, the minimum resolution of time for videofluoroscopic studies of swallowing behavior is defined by the image acquisition rate of the videofluoroscopy machine and recording system. In Table 2, we have provided the frame-based timing measures into milliseconds, noting that these measures were derived using a calculation of 29.975 frames/s. A further issue related to the units used for reporting timing measures arises in the calculation of descriptive statistics across a group of participants or across task repetitions. In Table 2, we have included two decimal places when reporting timing measures in frames; however, it must be recognized that there is no interpretable meaning to fractions of a frame. A poignant illustration of this issue arises when considering the typical latencies between events that occur very close together in time. As noted in the Results section, some events were found to typically occur within one frame of each other, and in these cases, redundancy was reduced by choosing one of the two events as the event of record for sequencing or latency measures. The interval from the frame of LVC until the frame of UESO is an example, for which the average time difference across the thin liquid boluses in our data set was calculated as −1.4 frames (i.e., −46 ms), with 95% CI [−2.2, −0.6] (i.e., −73 to −20 ms). Closer inspection of these data shows that UESO and LVC occurred simultaneously on 9% of the thin boluses in this data set. In the majority of cases (i.e., 69%), UESO preceded LVC with the mean time difference being 3.8 frames (i.e., 125 ms). This detail helps to explain the corresponding finding that the head of the bolus was noted to already be in the UES on the frame of LVC in 80.4% of cases, as shown in Table 5. The reverse sequence, with LVC occurring prior to UESO, was seen in 21% of cases, with an average time difference of 3.2 frames (i.e., 106 ms). Measures of central tendency for latency measures that are very small must be recognized to reflect a distribution that falls on either side of the simultaneous midline, and here, the inclusion of decimal places in frame-based timing measure reporting points to trends in the frequency distribution of event sequencing.
A limitation of the current data set is the fact that differences in sip volume were seen across the different consistencies, making it difficult to differentiate the effects of sip size from consistency. Although some of the statistical results favor one explanation over another, caution should be exercised regarding these findings, and it should be noted that both characteristics of the stimuli in this study represent regions or point estimates along continua rather than a representative sample of points along a broad distribution. The consistency–sip volume confound occurred as a result of the protocol decision to allow participants to take comfortable sips and the necessity of using a spoon to deliver the thicker consistencies. Future studies in which sip size is controlled and experimentally manipulated will be needed to tease these two factors apart.
Conclusions
In this study, we provide a comprehensive description of the characteristics of swallowing in healthy adults, under the age of 60 years, across the consistency range from thin to extremely thick liquids. These data represent new reference values, to which data using different stimuli or collected in different populations can be compared. By adhering strictly to a standard operating procedure and obtaining all measures in duplicate from independent raters, we have been able to achieve high levels of agreement for all parameters. In cases where ratings disagreed, these were resolved using a consensus review. The resulting statistics include confidence intervals, which should help clinicians to discern specific pathophysiological mechanisms that contribute to impaired swallowing safety or efficiency during clinical videofluoroscopy examinations.
Acknowledgments
Funding for this study was provided through an RO1 grant from the National Institute on Deafness and Other Communication Disorders (Grant DC011020) to the first author. The authors gratefully acknowledge input from Ben Hanson, Lisa Duizer, David James, Julie Cichero, and Peter Lam regarding study design and stimulus development.
Author Contributions: Catriona Steele was the principal investigator for the project and was responsible for project design, statistical analysis, and article writing. Melanie Peladeau-Pigeon, Carly Barbon, Brittany Guida, Ashwini Namasivayam-MacDonald, Weslania Nascimento, Sana Smaoui, Melanie Tapson, Teresa Valenzano, Ashley Waito, and Talia Wolkin were responsible for participant recruitment, swallowing data collection, signal processing, videofluoroscopy rating, and article editing.
Appendix
Additional Details Regarding the ASPEKT Videofluoroscopy Rating Method (Analysis of Swallowing Physiology: Events, Kinematics and Timing) Used for the Study “Reference Values for Healthy Swallowing Across the Range From Thin to Extremely Thick Liquids”
The ASPEKT method has been developed in the Swallowing Rehabilitation Research Laboratory at the Toronto Rehabilitation Institute, University Health Network, as a standard operating procedure for objective rating of videofluoroscopies (videofluoroscopic swallowing study [VFSS]) for research and involves the following steps:
1. VFSS Recording Review and Clipping
Prior to rating each VFSS recording, each full-length recording is reviewed to identify the time codes associated with onset/offset of the x-ray for each sip or bolus contained in the recording. The boundaries identified are then used to splice the original full-length recording into smaller video clips, each containing the swallows associated with a single bolus. These boundaries are entered into a spreadsheet, which is then passed to MATLAB for video clipping. The spliced bolus-level video clips, with no audio track, are labeled with a random file number, with the master key retained in a file on the lab research server. Bolus level video clips are generated in sets of 150, which are referred to as batches of videos.
2. Video Rating Assignment
Each bolus level video clip is randomly assigned to two raters. Raters have previously completed a training program and demonstrated competency in all required rating procedures.
3. Computer Setup and Software
Raters are provided a computer with Windows 7 (or later) operating system and two monitors (one for video viewing and another for data entry). The 64-bit ImageJ software (National Institutes of Health, https://imagej.nih.gov) is used to review each bolus-level video clip. ImageJ allows the users to view the videos in real time as well as frame by frame (forward frame advancement as well as backward, if this is helpful). Raters are given the freedom to review the video clips as many times as they wish. Raters are instructed to avoid ImageJ contrast adjustment and enhancement tools.
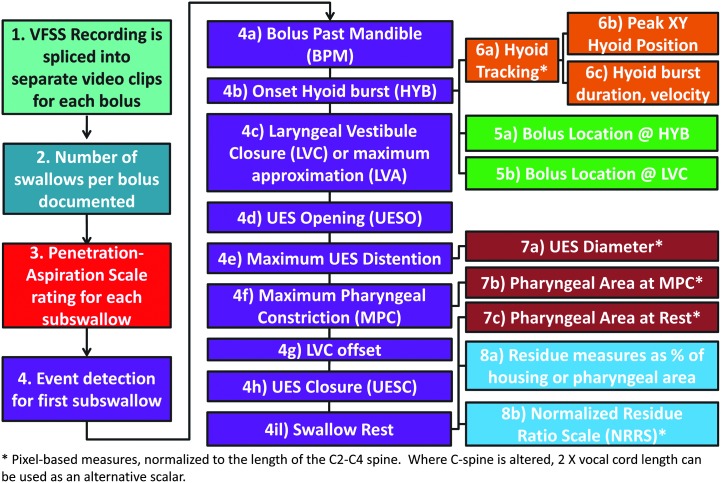
4. Overview of the Parameters Rated
Figure A1, below, provides an overview of the different parameters that can be collected using the ASPEKT method.
Figure A1.
Overview of the ASPEKT rating method.
5. Counting the Number of Swallows for Each Bolus (ASPEKT Method Step 2)
a. The rater is asked to review the entire bolus-level video clip and to identify the number of swallow(s). The following components must be present in order to consider a “swallow” to have occurred: (a) at least one of laryngeal elevation, hyoid excursion, and/or pharyngeal constriction and (b) upper esophageal sphincter opening (UESO). The term subswallows is used to refer to individual swallows when there is more than one swallow per bolus.
b. Subswallows are further qualitatively classified as initial (the first subswallow in the series), piecemeal (a higher order subswallow in which additional material is transported from the oral cavity), or clearing (a higher order swallow of pharyngeal residue with no additional material added from the oral cavity) swallows.
6. Rating Penetration–Aspiration (ASPEKT Method Step 3)
a. Swallow Level Results
Airway invasion is rated for each and every subswallow using the 8-point Penetration–Aspiration Scale (PAS; Rosenbek, Robbins, Roecker, Coyle, & Wood, 1996):
1 = Material does not enter the airway.
2 = Material enters the airway, remains above the vocal folds, and is ejected from the airway.
3 = Material enters the airway, remains above the vocal folds, and is not ejected from the airway.
4 = Material enters the airway, contacts the vocal folds, and is ejected from the airway.
5 = Material enters the airway, contacts the vocal folds, and is not ejected from the airway.
6 = Material enters the airway, passes below the vocal folds, and is ejected into the larynx or out of the airway.
7 = Material enters the airway, passes below the vocal folds, and is not ejected from the trachea despite effort.
8 = Material enters the airway and passes below the vocal folds, and no effort is made to eject.
Raters are instructed that they should only score new material entering the airway on each subswallow. In the case of scores of 3, 5, 6, 7, or 8 (indicating airway invasion without ejection out of the laryngeal vestibule), the frame of bolus entry into the laryngeal vestibule is documented to enable subsequent evaluation of the timing of airway invasion relative to laryngeal vestibule closure (LVC).
b. Amount of Material Entering the Airway
The amount of material entering the airway is coded subjectively as 0 (none), 1 (trace), or 2 (more than trace).
c. Bolus-Level Summary Results for Penetration and Aspiration
Bolus-level results are derived based on the highest PAS score seen across the series of subswallows for that bolus. In addition, if a previous PAS event is noted to evolve across subsequent, higher order subswallows for the same bolus (i.e., worsen or recover to a higher position in the airway), this is noted in the rating comments.
7. Event Timing (ASPEKT Method Step 4)
In order to facilitate the calculation of timing measures, raters are asked to record the frame numbers at which a series of key events occurs. The list of events is chosen on a study-by-study basis, and there is room for additional events to be added to the master list in the future. For the current study, the following operational definitions were used to define the events of interest:
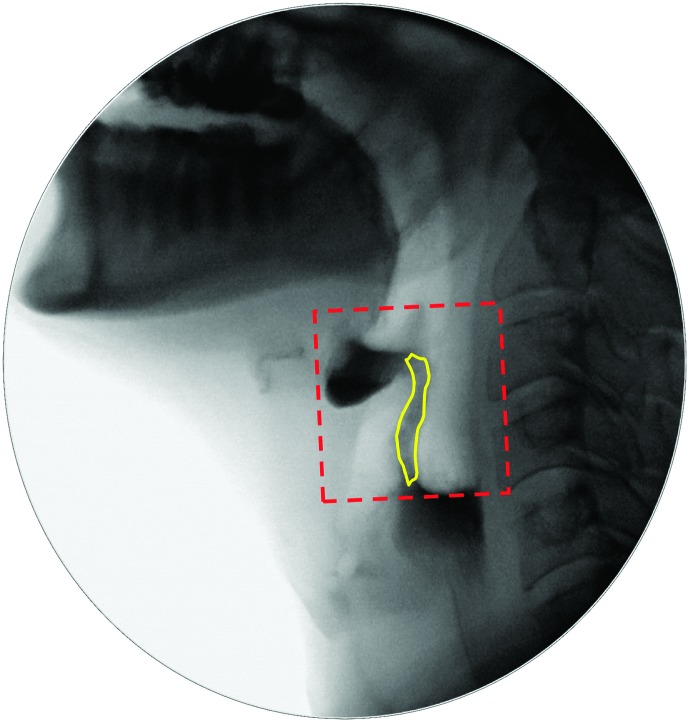
a. Bolus passing mandible (BPM): the first frame where the leading edge of the bolus touches or crosses the shadow of the ramus of mandible. In cases where the bolus was considered to have escaped prematurely from the mouth into the pharynx, the first frame showing bolus material at or below the ramus of mandible was counted as the BPM frame. When a double mandible shadow was seen on the lateral view image, the lower edge of the more superior ramus was used as the landmark.
b. Onset of the hyoid burst (HYB): the first anterior–superior “jump” of the hyoid that is associated with a swallow. This event has previously been referred to using the terminology onset of maximal hyoid excursion or onset of the pharyngeal response (Robbins, Hamilton, Lof, & Kempster, 1992).
c. LVC: the first frame showing contact between the arytenoid process and the inferior surface of the epiglottis. In cases where there is no contact, the frame of maximum approximation of the arytenoid process to the inferior surface of the epiglottis is used, and the term laryngeal vestibule approximation is used instead of LVC.
d. UESO: the first frame where the leading edge of the bolus (or, in rare cases, air) passes through the upper esophageal sphincter (UES). The UES is a narrow segment or region that typically lies between C4 and C6; the narrowest opening seen between C4 and C6 during a swallow is marked as the location of the sphincter (Leonard, Kendall, & McKenzie, 2004). In addition, recognizing that the UES moves superiorly during the swallow (Kahrilas, Logemann, Lin, & Ergun, 1992), the narrowest portion may be located above C4. The superior boundary of the tracheal air column can be used as a guide to decide where the location of the UES is during pharyngeal shortening. The specific location chosen for measurement is judged subjectively by the rater, and subsequent interrater agreement comparisons serve to flag cases where the chosen location may differ across raters and require review.
e. Maximum UES distension (UESMax): the frame where the UESO has the widest width (i.e., diameter), judged perpendicular to the cervical spine on a lateral-view fluoroscopy image.
f. Maximum pharyngeal constriction (MPC): the earliest frame showing maximum obliteration of the space in the pharynx. This event must occur before the upper pharynx begins to relax and before the tracheal air column begins to descend.
g. UES closure (UESC): the first frame where the UES achieves closure behind the bolus tail. This does not require closure of the entire UES segment, simply closure at a single point along the segment.
h. LVCOff: the first frame where there is visible opening (white space) of the laryngeal vestibule. This requires some separation of the tissues or of the arytenoids from the inferior surface of the epiglottis, but complete opening is not required. The leaf of the epiglottis may still be in a downward position. This event cannot be identified in cases of incomplete LVC.
i. Swallow rest: the terminal event of each swallow, identified as the first frame showing the pyriform sinuses at their lowest position, relative to the spine, prior to any hyoid burst or laryngeal elevation for a subsequent subswallow. For the terminal subswallow, this event is further defined as occurring within 30 frames (approximately 1 s) of UESC, prior to any nonswallow events such as coughing, talking, or UES reopening.
8. Judging the Completeness of LVC (ASPEKT Method Step 4c)
The frame of LVC (or laryngeal vestibule approximation) is reviewed, and the rater judges whether closure is complete (or incomplete). A rating of “complete” requires a seal between the epiglottis and the arytenoids, leaving no visible airspace.
9. Ordinal Ratings of Bolus Location on Key Event Frames (ASPEKT Method Step 5)
The location of the leading edge of the bolus is recorded on key frames during the pharyngeal swallow. For this study, bolus location was tracked on the frames of HYB and LVC.
a. Bolus location at swallow onset (i.e., on the frame of HYB)
On the frame of HYB, the scoring convention recommended in the MBSImp was used (Martin-Harris et al., 2008): A score of 0 was assigned when the leading edge of the bolus head was in the region of the posterior angle of the ramus and back of the tongue, a score of 1 was assigned when the bolus head had reached the pit of the valleculae, a score of 2 was given when the bolus head was at the posterior laryngeal surface of the epiglottis, a score of 3 was given when the bolus head was in the pyriform sinus (i.e., inferior to the arytenoids), and a score of 4 was given when there was no appreciable swallow initiation at any bolus location.
b. Bolus location at LVC
For scores of bolus location at the time of LVC, the scale was extended as follows: 0 = bolus head in the oral cavity or at the posterior angle of ramus, 1 = bolus head at the vallecular pit, 2 = bolus head at the posterior laryngeal surface of epiglottis, 3 = bolus head at the level of the pyriform sinuses, 4 = bolus head in the UES, and 5 = no appreciable swallow initiation.
10. Pixel-Based Tracing of Structural Movement of Area (ASPEKT Method Steps 6–8)
ImageJ allows for the measurement of structural position, movement, or area on radiographic images. In the ASPEKT method, pixel-based tracing is performed to capture spatial information regarding hyoid position and movement, pharyngeal area, and UESO. All of these measures are made in a coordinate system for which the C2–C4 cervical spine serves as the y-axis, with the origin located at the anterior inferior corner of C4, and for which the x-axis is derived perpendicular to the y-axis (see Figure A2).
Figure A2.
Image showing the coordinate system used for pixel-based measurements, with the origin located at the anterior–inferior corner of the C4 vertebra, the y-axis defined by the C2–C4 cervical spine, and the x-axis derived at 90° to the y-axis. The red dashed line between the anterior–inferior corners of the C2 and C4 vertebrae is used as an anatomical reference scalar.
In addition, all pixel-based measures in the ASPEKT are derived in anatomically scaled units (Molfenter & Steele, 2014; Perlman, Vandaele, & Otterbacher, 1995). Derivation in millimeters would be possible, if a radio-opaque scalar reference of known size was placed on the patient in the field of view. A coin has been used by previous researchers for this purpose (Logemann, 1986). However, studies have shown that height differences between men and women explain a significant amount of sex-based variation in hyoid movement measured in millimeters, suggesting that structural movements in swallowing may differ based on the length and size of the pharynx (Molfenter & Steele, 2014). For this reason, anatomical scalars are used in the ASPEKT method. The preferred scalar is a straight line running from the anterior–inferior corner of the C2 vertebra down to the anterior–inferior corner of the C4 vertebra. In cases where the cervical spine is altered through degeneration, abnormal curvature, or the presence of hardware, measurement of the anterior–posterior length of the vocal folds is recommended as an alternative. This alternative measure, sometimes called tracheal width, has been used in imaging studies of primate vocal tract evolution and speech production as a reference scalar (Badin, Bailly, & Revert, 2002; Boe et al., 2017; Captier et al., 2013; Serrurier & Badin, 2008) and has been shown to have a similar relationship to participant height as cervical spine scalars (Molfenter & Steele, 2014). Explorations across a large number of videofluoroscopies in our lab suggest that the vocal fold length measure corresponds closely to half of the C2–C4 cervical spine scalar.
a.Measures of Hyoid Position (ASPEKT Method Step 6)
Hyoid position is measured as distance from the anterior–inferior corner of C4. Measurements are taken in the anterior (X) and superior (Y) planes. The XY hypotenuse position is then derived using the Pythagorean theorem. This procedure can be performed on any frame of interest. For the current study, hyoid position was tracked frame by frame, beginning five frames prior to the HYB frame until approximately five frames after the beginning of hyoid descent from peak position. A MATLAB algorithm was then used to search through the series of hyoid position measures to confirm the frames of peak position in each plane (X, Y, and XY). In the case of a plateau in hyoid movement at its peak position, the first frame at peak position was used. In the example shown in Figure A3, the yellow triangle shows the three planes in which hyoid peak position is measured (as distance from the anterior–inferior corner of C4), relative to the green dashed line, which represents the C2–C4 reference scalar (i.e., one cervical unit). The position of the hyoid in this image is 1.23 cervical units (i.e., 123% of the scalar) anterior to the C4 origin along the x-axis, 0.88 cervical units superior to the C4 origin along the y-axis, and 1.55 cervical units away from the C4 origin along the XY hypotenuse. Additional instructions regarding the measurement of hyoid movement using ImageJ, together with a spreadsheet for calculating anatomically scaled measures, can be found at http://steeleswallowinglab.ca/srrl/best-practice/hyoid-movement/.
Figure A3.
Image showing measurement of peak hyoid position in X, Y, and XY planes of movement relative to the anterior–inferior corner of the C4 vertebra. The red dashed line between the anterior–inferior corners of the C2 and C4 vertebra is used as an anatomical reference scalar.
b.Measures of Hyoid Displacement and Kinematics (ASPEKT Method Step 6c)
For the current study, the hyoid parameter of interest was peak hyoid position, as described above. Measurement of hyoid displacement (i.e., change in position between two frames of interest) is also possible. In addition, the hyoid position histories that are captured through frame-by-frame tracing can be used to identify durations of hyoid movement between key frames and to calculate velocities and peak velocities (Nagy et al., 2013; Nagy, Molfenter, Peladeau-Pigeon, Stokely, & Steele, 2014).
c.Measures of UESO (ASPEKT Method Step 7a)
The ImageJ line tool is used to trace the diameter of UESO on the UESMax frame. In the example shown in Figure A4, the yellow line represents the UES diameter measure, and the green dashed line represents the C2–C4 reference scalar (i.e., one cervical unit). The UES diameter line measures 0.32 cervical units in length or 32% of the reference scalar.
Figure A4.
Image showing measurement of the maximum upper esophageal sphincter distension. The red dashed line between the anterior–inferior corners of the C2 and C4 vertebrae is used as an anatomical reference scalar.
d.Measures of Pharyngeal Area (ASPEKT Method Steps 7b and 7c)
For the current study, it was of interest to measure the area of the pharynx at rest and on the frame of MPC. Area measures made using the ImageJ tool are expressed relative to the squared C2–C4 reference area (Stokely, Peladeau-Pigeon, Leigh, Molfenter, & Steele, 2015). The boundaries of the pharynx are defined to include all space above the UES, below the top of C2, posterior to the arytenoids, base of the tongue, and pharyngeal surface of the epiglottis and anterior to the posterior pharyngeal wall. This is illustrated in Figure A5, in which the red dashed square shows the squared C2–C4 reference scalar and the yellow outline shows the boundaries of the area of the pharynx. In this example, the pharyngeal area measure is 103% of the size of the squared reference area. It should be noted that the boundaries of the pharynx in the ASPEKT measurement approach differ from the boundaries used by Leonard, Rees, Belafsky, and Allen (2011) by excluding the nasopharynx and the laryngeal vestibule.
Figure A5.
Image showing measurement of the pharyngeal area at rest. The red dashed lines illustrate the squared C2 and C4 reference area that is used as an anatomical reference scalar.
Our convention is to use the anatomically normalized pharyngeal area measure on the MPC frame to represent the degree of pharyngeal constriction seen during a swallow. Calculation of a pharyngeal constriction ratio, by dividing the pharyngeal area at constriction by the pharyngeal area at rest, is also possible (Leonard et al., 2011). It should be noted that the frame selected for measurement of the pharyngeal area at rest in the ASPEKT method is the swallow rest frame at the end of the swallow, whereas the frame used by Leonard et al. is a static frame at the beginning of the videofluoroscopy procedure, in which a 1-ml thin liquid bolus is held in the mouth. As shown in Figure A6, the traceable unobliterated area of the pharynx, shown by the yellow outline, has an area that measures 4% of the red dashed square reference area and would yield a pharyngeal constriction ratio measure of 3.8% compared to the pharyngeal area measured in Figure A5.
Figure A6.
Image showing measurement of the unobligated pharyngeal area on the frame of maximum pharyngeal constriction. The red dashed lines illustrate the squared C2 and C4 reference area that is used as an anatomical reference scalar.
11. Measures of Residue Severity (ASPEKT Method Step 8)
Postswallow residue can be measured in a variety of ways. Ordinal ratings, using 3- or 4-point scales, are commonly used in the literature (Eisenhuber et al., 2002; Robbins et al., 2007) but show limited sensitivity to change in treatment outcome studies (Logemann et al., 2009; Robbins et al., 2007; Steele et al., 2013). Other authors have proposed measurements of residue for which the percentage of the bolus remaining in the pharynx is judged perceptually (Rademaker, Pauloski, Logemann, & Shanahan, 1994) or measured relative to a frame showing the complete bolus in the pharynx (Leonard, 2017). For the current study, and as part of the ASPEKT method, residue severity was measured on the “swallow rest” frame in a manner similar to the pixel-based measures of area described above. Residue can be measured in various locations. For the current study, residue was measured separately in the valleculae, in the pyriform sinuses, and elsewhere within the pharynx. Pixel-based tracing allows for the calculation of lateral-view residue area relative to the lateral-view area of the spatial housing, expressed as percent full. By convention, the upper boundaries for tracing the spatial housing of the valleculae and the pyriform sinuses are defined as the apex of the epiglottic leaf and the apex of the arytenoid process, respectively, with these lines drawn perpendicular to the spine. Alternatively, residue area can be expressed relative to the squared C2–C4 reference scalar. Finally, these different approaches can be combined to calculate the Normalized Residue Ratio Scale (NRRS) measure (Pearson, Molfenter, Smith, & Steele, 2012). Additional instructions regarding the NRRS and residue measurement using ImageJ, together with a spreadsheet for calculating anatomically scaled measures, can be found at http://steeleswallowinglab.ca/srrl/best-practice/nrrs-residue/.
The NRRS measure does not provide a conceptually interpretable unit and does not easily allow for the summation of total pharyngeal residue, given that the area of the spatial housing for residue that falls outside the valleculae and pyriform sinuses is not easily defined. Therefore, in order to appreciate the severity of total pharyngeal residue, we recommend expressing the sum of all residue areas relative to the squared C2–C4 reference scalar.
Residue measurement is illustrated in Figures A7, A8, and A9. In Figure A7, the frame of swallow rest is shown, with residue seen in both the valleculae and pyriform sinuses. In Figure A8, the ImageJ tracings for residue measurement in the valleculae and pyriform sinuses are shown. The residue in each space is outlined in yellow, and the spatial housing areas are shown in white. The squared C2–C4 reference scalar is shown by the red dashed lines. In this example, the ratio of the vallecular residue to the vallecular housing area provides a measure of 64% full, which translates to 19% of the squared C2–C4 reference scalar and an NRRSv score of 0.68. The ratio of the pyriform sinus residue to its housing area yields a measure of 74% full, translating to 25% of the squared C2–C4 reference scalar and an NRRSp score of 1.04. In addition to residue in these spaces, Figure A7 shows some residue in the pharynx, between the valleculae and pyriform sinuses. This “extra” residue is traced in Figure A9 and occupies 5% of the C2–C42 reference scalar. The total pharyngeal residue area can be expressed as the sum of the three measured areas (vallecular, pyriform, and extra residue), translating to 49% of the C2–C42 reference scalar.
Figure A7.
Image of a swallow rest frame with residue located in both the valleculae and pyriform sinuses.
Figure A8.
Tracings of residue area relative to spatial housing in the valleculae and pyriform sinuses. Residue area can also be expressed relative to the squared C2–C4 reference scalar illustrated by the red dashed lines.
Figure A9.
Tracing of additional residue in the area between the valleculae and pyriform sinuses. Tracings of additional residue can be added to tracings of vallecular and pyriform sinus residue for a total pharyngeal area measure, with all subcomponents expressed relative to the squared C2–C4 reference scalar illustrated by the red dashed lines.
12. Interrater Agreement
Many studies in the dysphagia literature have reported poor interrater agreement for videofluoroscopy rating. In order for ASPEKT method ratings to be useful for research or in the clinical identification of swallowing pathophysiology, it is critical that good interrater and intrarater agreement can be established. Checking agreement is a key part of the ASPEKT method and occurs at two key time points in the process. First, agreement for ratings of the number of swallows, penetration–aspiration, and event timing is inspected at the end of the event identification phase (Step 4). This ensures that the frames that are carried forward for ordinal or pixel-based ratings are confirmed and that these subsequent measurements are made on identical frames by different raters. After a set of initial ratings has been made independently by two raters, an Excel macro program is run to inspect them for agreement and identify cases that require discrepancy resolution. Strict criteria are used to handle discrepancies between raters. Any difference (of any magnitude) in ratings of the number of swallows per bolus, PAS scores, LVC (complete/incomplete), or bolus location at swallow onset is sent to a consensus meeting for resolution. For timing measures, any difference of more than five frames between raters regarding the frame at which key events occurred is sent for resolution. Consensus meetings are attended by a minimum of three trained raters and involve review, repeat measurement, and debate regarding the discrepant ratings until consensus is achieved. In cases where ratings are in close enough agreement to not require resolution, a priori rules guide selection of the frame of record. For event timing, where discrepancies are five frames or less, the earlier frame is used as the frame of record for the timing of penetration–aspiration events, BPM, HYB, LVC, and UESO, whereas the later frame is used as the frame of record for MPC, UESMax, UESC, LVCoff, and swallow rest. Once discrepancies are resolved, the sequence of events can be derived and timing intervals between events are calculated.
In the current study, thresholds for differences in pixel-based measurement that required resolution were set on a parameter-by-parameter basis, using the 95% confidence interval upper boundary of rater differences from a previous data set. The thresholds were as follows: normalized UES diameter differences > 0.08, normalized measures of pharyngeal area at rest > 0.2, normalized measures of MPC > 0.16, and NRRS measures > 0.1 for the valleculae and > 0.22 for the pyriform sinuses. Each of these measures is further explained below. Where rater differences did not require resolution, the smaller of the two rating values was taken as the rating of record. In the absence of a historical data set for a rating team, upon which to base the decision about the need for discrepancy resolution of pixel-based measures, we recommend reviewing cases for which the ratio of the larger value to the smaller value exceeds a value of 1.6.
For the current study, interrater agreement for the initial ratings (i.e., before discrepancy resolution) was strong for all types of measures:
a. For the number of swallows per bolus, absolute agreement was achieved in 97.9% of cases, with a Fleiss κ of .938 (95% CI [.893, .983]).
b. For PAS scores, absolute agreement was found in 94% of cases, with 4.5% of cases differing by 1 point on the 8-point Penetration–Aspiration Scale and 1% of cases differing by more than 1 point. The Fleiss κ score was .338, suggesting fair agreement prior to the resolution of discrepancies by consensus (95% CI [.296, .379]).
c. For the identification of frame numbers corresponding to key events in the swallowing sequence, a mean absolute difference of 1.6 frames was found (95% CI [1.5, 1.7]), with an intraclass coefficient of 1 (95% CI [1, 1]).
d. For the binary categorical rating of LVC being complete or incomplete, absolute agreement was seen for 96.6% of the boluses in the data set, with a Fleiss κ of .926 (95% CI [.882, .97]).
e. For the ordinal rating measures of bolus location, absolute agreement was seen in 72% of cases, with 20% of cases differing by one level and 8% of cases differing by more than one level and with a moderate Fleiss κ of .563 (95% CI [.544, .582]).
f. For pixel-based measures of distance or length, a mean difference of 4.3 pixels (95% CI [4.08, 4.57]) and an intraclass coefficient of .972 (95% CI [.97, .973]).
g. For pixel-based measures of area, a mean difference of 181.5 pixels2 (95% CI [174, 189]) and an intraclass coefficient of .965 (95% CI [.964, .967]).
Appendix References
- Badin P., Bailly G., & Revert L. (2002). Three-dimensional linear articulatory modeling of tongue, lips and face, based on MRI and video images. Journal of Phonetics, 30, 533–553. [Google Scholar]
- Boe L. J., Berthommier F., Legou T., Captier G., Kemp C., Sawallis T. R., … Fagot J. (2017). Evidence of a vocalic proto-system in the baboon (papio papio) suggests pre-hominin speech precursors. PLOS ONE, 12(1), e0169321 https://doi.org/10.1371/journal.pone.0169321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Captier G., Boe L. J., Badin P., Guihard-Costa A. M., Canovas F., & Larroche J. C. (2013). Geometrical growth models of the fetal forebrain, cerebellum, brainstem and change of the cranial base angles during fetal period. Morphologie, 97(317), 38–47. https://doi.org/10.1016/j.morpho.2012.12.002 [DOI] [PubMed] [Google Scholar]
- Eisenhuber E., Schima W., Schober E., Pokieser P., Stadler A., Scharitzer M., … Oschatz E. (2002). Videofluoroscopic assessment of patients with dysphagia: Pharyngeal retention is a predictive factor for aspiration. American Journal of Roentgenology, 178(2), 393–398. [DOI] [PubMed] [Google Scholar]
- Kahrilas P. J., Logemann J. A., Lin S., & Ergun G. A. (1992). Pharyngeal clearance during swallowing: A combined manometric and videofluoroscopic study. Gastroenterology, 103(1), 128–136. [DOI] [PubMed] [Google Scholar]
- Leonard R., Kendall K., & McKenzie S. (2004). UES opening and cricopharyngeal bar in nondysphagic elderly and nonelderly adults. Dysphagia, 19(3), 182–191. [DOI] [PubMed] [Google Scholar]
- Leonard R., Rees C. J., Belafsky P., & Allen J. (2011). Fluoroscopic surrogate for pharyngeal strength: The pharyngeal constriction ratio (PCR). Dysphagia, 26(1), 13–17. https://doi.org/10.1007/s00455-009-9258-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard R. J. (2017). Two methods for quantifying pharyngeal residue on fluoroscopic swallow studies: Reliability assessment. Annals of Otolaryngology and Rhinology, 4(3), 1168. [Google Scholar]
- Logemann J. A. (1986). Manual for the videofluorographic study of swallowing. Austin, TX: Pro-Ed. [Google Scholar]
- Logemann J. A., Rademaker A., Pauloski B. R., Kelly A., Stangl-Mcbreen C., Antinoja J., … Shaker R. (2009). A randomized study comparing the Shaker exercise with traditional therapy: A preliminary study. Dysphagia, 24(4), 403–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Harris B., Brodsky M. B., Michel Y., Castell D. O., Schleicher M., Sandidge J., … Blair J. (2008). MBS measurement tool for swallow impairment—MBSImp: Establishing a standard. Dysphagia, 23(4), 392–405. https://doi.org/10.1007/s00455-008-9185-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molfenter S. M., & Steele C. M. (2014). Use of an anatomical scalar to control for sex-based size differences in measures of hyoid excursion during swallowing. Journal of Speech, Language, and Hearing Research, 57(3), 768–778. https://doi.org/10.1044/2014_JSLHR-S-13-0152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy A., Leigh C., Hori S. F., Molfenter S. M., Shariff T., & Steele C. M. (2013). Timing differences between cued and noncued swallows in healthy young adults. Dysphagia, 28, 428–434. https://doi.org/10.1007/s00455-013-9456-y [DOI] [PubMed] [Google Scholar]
- Nagy A., Molfenter S. M., Peladeau-Pigeon M., Stokely S., & Steele C. M. (2014). The effect of bolus volume on hyoid kinematics in healthy swallowing. Biomed Research International, 2014, Article ID 738971. https://doi.org/10.1155/2014/738971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson W. G. Jr., Molfenter S. M., Smith Z. M., & Steele C. M. (2012). Image-based measurement of post-swallow residue: The Normalized Residue Ratio Scale. Dysphagia, 28, 167–177. https://doi.org/10.1007/s00455-012-9426-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman A. L., Vandaele D. J., & Otterbacher M. S. (1995). Quantitative assessment of hyoid bone displacement from video images during swallowing. Journal of Speech and Hearing Research, 38(3), 579–585. [DOI] [PubMed] [Google Scholar]
- Rademaker A. W., Pauloski B. R., Logemann J. A., & Shanahan T. K. (1994). Oropharyngeal swallow efficiency as a representative measure of swallowing function. Journal of Speech and Hearing Research, 37(2), 314–325. [DOI] [PubMed] [Google Scholar]
- Robbins J., Hamilton J. W., Lof G. L., & Kempster G. B. (1992). Oropharyngeal swallowing in normal adults of different ages. Gastroenterology, 103(3), 823–829. [DOI] [PubMed] [Google Scholar]
- Robbins J., Kays S. A., Gangnon R. E., Hind J. A., Hewitt A. L., Gentry L. R., & Taylor A. J. (2007). The effects of lingual exercise in stroke patients with dysphagia. Archives of Physical Medicine and Rehabilitation, 88(2), 150–158. [DOI] [PubMed] [Google Scholar]
- Rosenbek J. C., Robbins J. A., Roecker E. B., Coyle J. L., & Wood J. L. (1996). A penetration–aspiration scale. Dysphagia, 11(2), 93–98. [DOI] [PubMed] [Google Scholar]
- Serrurier A., & Badin P. (2008). A three-dimensional articulatory model of the velum and nasopharyngeal wall based on MRI and CT data. The Journal of the Acoustical Society of America, 123(4), 2335–2355. https://doi.org/10.1121/1.2875111 [DOI] [PubMed] [Google Scholar]
- Steele C. M., Bailey G. L., Polacco R. E., Hori S. F., Molfenter S. M., Oshalla M., & Yeates E. M. (2013). Outcomes of tongue-pressure strength and accuracy training for dysphagia following acquired brain injury. International Journal of Speech-Language Pathology, 15(5), 492–502. https://doi.org/10.3109/17549507.2012.752864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokely S. L., Peladeau-Pigeon M., Leigh C., Molfenter S. M., & Steele C. M. (2015). The relationship between pharyngeal constriction and post-swallow residue. Dysphagia, 30(3), 349–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
Funding Statement
Funding for this study was provided through an RO1 grant from the National Institute on Deafness and Other Communication Disorders (Grant DC011020) to the first author.
References
- American Dietetic Association. (2002). National dysphagia diet: Standardization for optimal care. Chicago, IL: Author. [Google Scholar]
- Baijens L. W. J., Speyer R., Passos V. L., Pilz W., Roodenburg N., & Clavé P. (2011). Swallowing in Parkinson patients versus healthy controls: Reliability of measurements in videofluoroscopy. Gastroenterology Research and Practice, 2011, Article ID 380682. https://doi.org/10.1155/2011/380682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbon C. E. A., & Steele C. M. (2018). Characterizing the flow of thickened barium and non-barium liquid recipes using the IDDSI flow test. Dysphagia, 34, 73–79. https://doi.org/10.1007/s00455-018-9915-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett J. W., Van Lieshout P. H. H. M., Pelletier C. A., & Steele C. M. (2009). Sip-sizing behaviors in natural drinking conditions compared to instructed experimental conditions. Dysphagia, 24(2), 152–158. [DOI] [PubMed] [Google Scholar]
- Bisch E. M., Logemann J. A., Rademaker A. W., Kahrilas P. J., & Lazarus C. L. (1994). Pharyngeal effects of bolus volume, viscosity, and temperature in patients with dysphagia resulting from neurologic impairment and in normal subjects. Journal of Speech and Hearing Research, 37(5), 1041–1059. [DOI] [PubMed] [Google Scholar]
- Bonilha H. S., Blair J., Carnes B., Huda W., Humphries K., McGrattan K., … Martin-Harris B. (2013). Preliminary investigation of the effect of pulse rate on judgments of swallowing impairment and treatment recommendations. Dysphagia, 28(4), 528–538. https://doi.org/10.1007/s00455-013-9463-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnaby G. D., & Harenberg L. (2013). What is “usual care” in dysphagia rehabilitation: A survey of USA dysphagia practice patterns. Dysphagia, 28(4), 567–574. https://doi.org/10.1007/s00455-013-9467-8 [DOI] [PubMed] [Google Scholar]
- Chi-Fishman G., & Sonies B. C. (2002). Effects of systematic bolus viscosity and volume changes on hyoid movement kinematics. Dysphagia, 17(4), 278–287. [DOI] [PubMed] [Google Scholar]
- Cichero J. A. Y., Lam P., Steele C. M., Hanson B., Chen J., Dantas R. O., … Stanschus S. (2017). Development of international terminology and definitions for texture-modified foods and thickened fluids used in dysphagia management: The IDDSI framework. Dysphagia, 32(2), 293–314. https://doi.org/10.1007/s00455-016-9758-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cichero J. A. Y., Steele C. M., Duivestein J., Clavé P., Chen J., Kayashita J., … Murray J. (2013). The need for international terminology and definitions for texture-modified foods and thickened liquids used in dysphagia management: Foundations of a global initiative. Current Physical Medicine and Rehabilitation Reports, 1, 280–291. https://doi.org/10.1007/s40141-013-0024-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clavé P., Arreola V., Romea M., Medina L., Palomera E., & Serra-Prat M. (2008). Accuracy of the volume–viscosity swallow test for clinical screening of oropharyngeal dysphagia and aspiration. Clinical Nutrition, 27(6), 806–815. [DOI] [PubMed] [Google Scholar]
- Clavé P., de Kraa M., Arreola V., Girvent M., Farré R., Palomera E., & Serra-Prat M. (2006). The effect of bolus viscosity on swallowing function in neurogenic dysphagia. Alimentary Pharmacology & Therapeutics, 24(9), 1385–1394. [DOI] [PubMed] [Google Scholar]
- Coster S. T., & Schwarz W. H. (1987). Rheology and the swallow-safe bolus. Dysphagia, 1(3), 113–118. [Google Scholar]
- Curran J., & Groher M. E. (1990). Development and dissemination of an aspiration risk reduction diet. Dysphagia, 5(1), 6–12. [DOI] [PubMed] [Google Scholar]
- Cutler A. N., Morris E. R., & Taylor L. J. (1983). Oral perception of viscosity in fluid foods and model systems. Journal of Texture Studies, 14, 377–395. [Google Scholar]
- Daggett A., Logemann J., Rademaker A., & Pauloski R. (2006). Laryngeal penetration during deglutition in normal subjects of various ages. Dysphagia, 21(4), 270–274. https://doi.org/10.1007/s00455-006-9051-6 [DOI] [PubMed] [Google Scholar]
- Daniels S. K., Schroeder M. F., DeGeorge P. C., Corey D. M., & Rosenbek J. C. (2007). Effects of verbal cue on bolus flow during swallowing. American Journal of Speech-Language Pathology, 16(2), 140–147. [DOI] [PubMed] [Google Scholar]
- Dantas R. O., Dodds W. J., Massey B. T., & Kern M. K. (1989). The effect of high- vs low-density barium preparations on the quantitative features of swallowing. American Journal of Roentgenology, 153(6), 1191–1195. [DOI] [PubMed] [Google Scholar]
- Garcia J. M., Chambers E., & Molander M. (2005). Thickened liquids: Practice patterns of speech-language pathologists. American Journal of Speech-Language Pathology, 14(1), 4–13. [DOI] [PubMed] [Google Scholar]
- Guedes R., Azola A., Macrae P., Sunday K., Mejia V., Vose A., & Humbert I. A. (2017). Examination of swallowing maneuver training and transfer of practiced behaviors to laryngeal vestibule kinematics in functional swallowing of healthy adults. Physiology & Behavior, 174, 155–161. https://doi.org/10.1016/j.physbeh.2017.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson B. (2016). A review of diet standardization and bolus rheology in the management of dysphagia. Current Opinion Otolaryngology & Head and Neck Surgery, 24(3), 183–190. https://doi.org/10.1097/MOO.0000000000000251 [DOI] [PubMed] [Google Scholar]
- Herzberg E. G., Lazarus C. L., Steele C. M., & Molfenter S. M. (2018). Swallow event sequencing: Comparing healthy older and younger adults. Dysphagia, 33, 759–767. https://doi.org/10.1007/s00455-018-9898-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hind J., Divyak E., Zielinski J., Taylor A., Hartman M., Gangnon R., & Robbins J. (2012). Comparison of standardized bariums with varying rheological parameters on swallowing kinematics in males. Journal of Rehabilitation Research and Development, 49(9), 1399–1404. [DOI] [PubMed] [Google Scholar]
- Humbert I. A., Sunday K. L., Karagiorgos E., Vose A. K., Gould F., Greene L., … Rivet A. (2018). Swallowing kinematic differences across frozen, mixed, and ultrathin liquid boluses in healthy adults: Age, sex, and normal variability. Journal of Speech, Language, and Hearing Research, 61(7), 1544–1559. https://doi.org/10.1044/2018_JSLHR-S-17-0417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jardine M., Miles A., & Allen J. (2018). Dysphagia onset in older adults during unrelated hospital admission: Quantitative videofluoroscopic measures. Geriatrics, 3(4), 66 https://doi.org/10.3390/geriatrics3040066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendall K. A., & Leonard R. J. (2001). Pharyngeal constriction in elderly dysphagic patients compared with young and elderly nondysphagic controls. Dysphagia, 16(4), 272–278. [DOI] [PubMed] [Google Scholar]
- Kendall K. A., Leonard R. J., & McKenzie S. W. (2003). Sequence variability during hypopharyngeal bolus transit. Dysphagia, 18(2), 85–91. [DOI] [PubMed] [Google Scholar]
- Kendall K. A., McKenzie S., Leonard R. J., Gonçalves M. I., & Walker A. (2000). Timing of events in normal swallowing: A videofluoroscopic study. Dysphagia, 15(2), 74–83. [DOI] [PubMed] [Google Scholar]
- Kotrlik J. W., & Williams H. A. (2003). The incorporation of effect size in informaton technology, learning, and performance research. Information Technology, Learning, and Performance Journal, 21(1), 1–7. [Google Scholar]
- Lazarus C. L., Logemann J. A., Rademaker A. W., Kahrilas P. J., Pajak T., Lazar R., & Halper A. (1993). Effects of bolus volume, viscosity, and repeated swallows in nonstroke subjects and stroke patients. Archives of Physical Medicine and Rehabilitation, 74(10), 1066–1070. [DOI] [PubMed] [Google Scholar]
- Leonard R., Kendall K. A., & McKenzie S. (2004). Structural displacements affecting pharyngeal constriction in nondysphagic elderly and nonelderly adults. Dysphagia, 19(2), 133–141. [DOI] [PubMed] [Google Scholar]
- Leonard R., Kendall K. A., McKenzie S., Gonçalves M. I., & Walker A. (2000). Structural displacements in normal swallowing: A videofluoroscopic study. Dysphagia, 15(3), 146–152. [DOI] [PubMed] [Google Scholar]
- Leonard R., & McKenzie S. (2006). Hyoid-bolus transit latencies in normal swallow. Dysphagia, 21(3), 183–190. [DOI] [PubMed] [Google Scholar]
- Linden P., Tippett D., Johnston J., Siebens A., & French J. (1989). Bolus position at swallow onset in normal adults: Preliminary observations. Dysphagia, 4(3), 146–150. [DOI] [PubMed] [Google Scholar]
- Lof G. L., & Robbins J. (1990). Test–retest variability in normal swallowing. Dysphagia, 4, 236–242. [DOI] [PubMed] [Google Scholar]
- Logemann J. A., Gensler G., Robbins J., Lindblad A. S., Brandt D., Hind J. A., … Miller Gardner P. J. (2008). A randomized study of three interventions for aspiration of thin liquids in patients with dementia or Parkinson's disease. Journal of Speech, Language, and Hearing Research, 51(1), 173–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logemann J. A., Pauloski B. R., Rademaker A. W., Colangelo L. A., Kahrilas P. J., & Smith C. H. (2000). Temporal and biomechanical characteristics of oropharyngeal swallow in younger and older men. Journal of Speech, Language, and Hearing Research, 43, 1264–1274. [DOI] [PubMed] [Google Scholar]
- Logemann J. A., Pauloski B. R., Rademaker A. W., & Kahrilas P. J. (2002). Oropharyngeal swallow in younger and older women: Videofluoroscopic analysis. Journal of Speech, Language, and Hearing Research, 45, 434–445. [DOI] [PubMed] [Google Scholar]
- Martin-Harris B., Brodsky M. B., Michel Y., Castell D. O., Schleicher M., Sandidge J., … Blair J. (2008). MBS measurement tool for swallow impairment—MBSImp: Establishing a standard. Dysphagia, 23(4), 392–405. https://doi.org/10.1007/s00455-008-9185-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Harris B., Brodsky M. B., Michel Y., Lee F.-S., & Walters B. (2007). Delayed initiation of the pharyngeal swallow: Normal variability in adult swallows. Journal of Speech, Language, and Hearing Research, 50(3), 585–594. [DOI] [PubMed] [Google Scholar]
- Martin-Harris B., & Jones B. (2008). The videofluorographic swallowing study. Physical Medicine and Rehabilitation Clinics of North America, 19(4), 769–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer J., Rogus-Pulia N., Peppler W., & Thibeault S. (2018, March). Effects of recording rate, screen calibration, and room ambient lighting on videofluoroscopic swallow study outcomes. Paper presented at the Dysphagia Research Society Meeting, Baltimore, MD. [Google Scholar]
- Molfenter S. M., Leigh C., & Steele C. M. (2014). Event sequence variability in healthy swallowing: Building on previous findings. Dysphagia, 29(2), 234–242. https://doi.org/10.1007/s00455-013-9501-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molfenter S. M., Lenell C., & Lazarus C. L. (2018). Volumetric changes to the pharynx in healthy aging: Consequence for pharyngeal swallow mechanics and function. Dysphagia, 34(1), 129–137. https://doi.org/10.1007/s00455-018-9924-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molfenter S. M., & Steele C. M. (2012a). Variation in temporal measures of swallowing: Sex and volume effects. Dysphagia, 28(2), 226–233. https://doi.org/10.1007/s00455-012-9437-6 [DOI] [PubMed] [Google Scholar]
- Molfenter S. M., & Steele C. M. (2012b). Temporal variability in the deglutition literature. Dysphagia, 27(2), 162–177. https://doi.org/10.1007/s00455-012-9397-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molfenter S. M., & Steele C. M. (2014). Use of an anatomical scalar to control for sex-based size differences in measures of hyoid excursion during swallowing. Journal of Speech, Language, and Hearing Research, 57(3), 768–778. https://doi.org/10.1044/2014_JSLHR-S-13-0152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy A., Leigh C., Hori S. F., Molfenter S. M., Shariff T., & Steele C. M. (2013). Timing differences between cued and noncued swallows in healthy young adults. Dysphagia, 28(3), 428–434. https://doi.org/10.1007/s00455-013-9456-y [DOI] [PubMed] [Google Scholar]
- Nagy A., Molfenter S. M., Péladeau-Pigeon M., Stokely S., & Steele C. M. (2014). The effect of bolus volume on hyoid kinematics in healthy swallowing. BioMed Research International, 2014, 738971 https://doi.org/10.1155/2014/738971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy A., Molfenter S. M., Péladeau-Pigeon M., Stokely S., & Steele C. M. (2015). The effect of bolus consistency on hyoid velocity in healthy swallowing. Dysphagia, 30(4), 445–451. https://doi.org/10.1007/s00455-015-9621-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman R., Vilardell N., Clavé P., & Speyer R. (2016). Effect of bolus viscosity on the safety and efficacy of swallowing and the kinematics of the swallow response in patients with oropharyngeal dysphagia: White paper by the European Society for Swallowing Disorders (ESSD). Dysphagia, 31(2), 232–249. https://doi.org/10.1007/s00455-016-9696-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicosia M. A., Hind J. A., Roecker E. B., Carnes M., Doyle J., Dengel G. A., & Robbins J. (2000). Age effects on the temporal evolution of isometric and swallowing pressure. Journals of Gerontology: Series A: Biological Sciences and Medical Sciences, 55(11), M634–M640. [DOI] [PubMed] [Google Scholar]
- Ong J. J., Steele C. M., & Duizer L. M. (2018a). Challenges to assumptions regarding oral shear rate during oral processing and swallowing based on sensory testing with thickened liquids. Food Hydrocolloids, 84, 173–180. https://doi.org/10.1016/j.foodhyd.2018.05.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong J. J., Steele C. M., & Duizer L. M. (2018b). Sensory characteristics of liquids thickened with commercial thickeners to levels specified in the International Dysphagia Diet Standardization Initiative (IDDSI) framework. Food Hydrocolloids, 79, 208–217. https://doi.org/10.1016/j.foodhyd.2017.12.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson W. G. Jr., Molfenter S. M., Smith Z. M., & Steele C. M. (2012). Image-based measurement of post-swallow residue: The Normalized Residue Ratio Scale. Dysphagia, 28(2), 167–177. https://doi.org/10.1007/s00455-012-9426-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Péladeau-Pigeon M., & Steele C. M. (2013). Technical aspects of a videofluoroscopic swallowing study. Canadian Journal of Speech-Language Pathology and Audiology, 37(3), 216–226. [Google Scholar]
- Perlman A. L., Van Daele D. J., & Otterbacher M. S. (1995). Quantitative assessment of hyoid bone displacement from video images during swallowing. Journal of Speech and Hearing Research, 38(3), 579–585. [DOI] [PubMed] [Google Scholar]
- Power M. L., Hamdy S., Singh S., Tyrrell P. J., Turnbull I., & Thompson D. G. (2007). Deglutitive laryngeal closure in stroke patients. Journal of Neurology, Neurosurgery, & Psychiatry, 78(2), 141–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins J., Hamilton J. W., Lof G. L., & Kempster G. B. (1992). Oropharyngeal swallowing in normal adults of different ages. Gastroenterology, 103(3), 823–829. [DOI] [PubMed] [Google Scholar]
- Robbins J., Nicosia M. A., Hind J. A., Gill G. D., Blanco R., & Logemann J. A. (2002). Defining physical properties of fluids for dysphagia evaluation and treatment. Perspectives on Swallowing and Swallowing Disorders (Dysphagia), 11, 16–19. [Google Scholar]
- Rosenbek J. C., Robbins J. A., Roecker E. B., Coyle J. L., & Wood J. L. (1996). A penetration–aspiration scale. Dysphagia, 11(2), 93–98. [DOI] [PubMed] [Google Scholar]
- Shama F., & Sherman P. (1973). Identification of stimuli controlling the sensory evaluation of viscosity II. Oral methods. Journal of Texture Studies, 4(1), 111–118. [Google Scholar]
- Steele C. M., Alsanei W. A., Ayanikalath S., Barbon C. E., Chen J., Cichero J. A., … Wang H. (2015). The influence of food texture and liquid consistency modification on swallowing physiology and function: A systematic review. Dysphagia, 30(1), 2–26. https://doi.org/10.1007/s00455-014-9578-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele C. M., Bailey G. L., & Molfenter S. M. (2010). Tongue pressure modulation during swallowing: Water versus nectar-thick liquids. Journal of Speech, Language, and Hearing Research, 53(2), 273–283. https://doi.org/10.1044/1092-4388(2009/09-0076) [DOI] [PubMed] [Google Scholar]
- Steele C. M., Molfenter S. M., Péladeau-Pigeon M., Polacco R. C., & Yee C. (2014). Variations in tongue–palate swallowing pressures when swallowing xanthan gum–thickened liquids. Dysphagia, 29(6), 678–684. https://doi.org/10.1007/s00455-014-9561-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele C. M., Molfenter S. M., Péladeau-Pigeon M., & Stokely S. (2013). Challenges in preparing contrast media for videofluoroscopy. Dysphagia, 28(3), 464–467. https://doi.org/10.1007/s00455-013-9476-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele C. M., Peladeau-Pigeon M., Barbon C. E. A., Guida B. T., Tapson M. S., Valenzano T. J., … Duizer L. M. (2019). Modulation of tongue pressure according to liquid flow properties in healthy swallowing. Journal of Speech, Language, and Hearing Research, 62(1), 22–33. https://pubs.asha.org/doi/10.1044/2018_JSLHR-S-18-0229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele C. M., Péladeau-Pigeon M., Tam K. L., Zohouri-Haghian N., & Mukhurjee R (2015, March). Poster #97: Variations in sip volume as a function of pre-sip cup volume. Poster presented at the Dysphagia Research Society 23rd Annual Meeting, Chicago, IL. [Google Scholar]
- Steele C. M., & Van Lieshout P. H. (2004). Influence of bolus consistency on lingual behaviors in sequential swallowing. Dysphagia, 19(3), 192–206. https://doi.org/10.1007/s00455-004-0006-5 [DOI] [PubMed] [Google Scholar]
- Stephen J. R., Taves D. H., Smith R. C., & Martin R. E. (2005). Bolus location at the initiation of the pharyngeal stage of swallowing in healthy older adults. Dysphagia, 20(4), 266–272. https://doi.org/10.1007/s00455-005-0023-z [DOI] [PubMed] [Google Scholar]
- Stokely S. L., Molfenter S. M., & Steele C. M. (2014). Effects of barium concentration on oropharyngeal swallow timing measures. Dysphagia, 29(1), 78–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickers Z., Damodhar H., Grummer C., Mendenhall H., Banaszynski K., Hartel R., … Robbins J. (2015). Relationships among reological, sensory yexture, and swallowing pressure measurements of hydrocolloid-thickened fluids. Dysphagia, 30(6), 702–713. https://doi.org/10.1007/s00455-015-9647-9 [DOI] [PubMed] [Google Scholar]
- Waito A. A., Steele C. M., Péladeau-Pigeon M., Genge A., & Argov Z. (2018). A preliminary videofluoroscopic investigation of swallowing physiology and function in individuals with oculopharyngeal muscular dystrophy (OPMD). Dysphagia, 33(6), 789–802. https://doi.org/10.1007/s00455-018-9904-9 [DOI] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a