Abstract
Purpose
The purpose of this study was to evaluate the effects of nonmodal phonation on estimates of subglottal pressure (Ps) derived from the magnitude of a neck-surface accelerometer (ACC) signal and to confirm previous findings regarding the impact of vowel contexts and pitch levels in a larger cohort of participants.
Method
Twenty-six vocally healthy participants (18 women, 8 men) were asked to produce a series of p-vowel syllables with descending loudness in 3 vowel contexts (/a/, /i/, and /u/), 3 pitch levels (comfortable, high, and low), and 4 elicited phonatory conditions (modal, breathy, strained, and rough). Estimates of Ps for each vowel segment were obtained by averaging the intraoral air pressure plateau before and after each segment. The root-mean-square magnitude of the neck-surface ACC signal was computed for each vowel segment. Three linear mixed-effects models were used to statistically assess the effects of vowel, pitch, and phonatory condition on the linear relationship (slope and intercept) between Ps and ACC signal magnitude.
Results
Results demonstrated statistically significant linear relationships between ACC signal magnitude and Ps within participants but with increased intercepts for the nonmodal phonatory conditions; slopes were affected to a lesser extent. Vowel and pitch contexts did not significantly affect the linear relationship between ACC signal magnitude and Ps.
Conclusion
The classic linear relationship between ACC signal magnitude and Ps is significantly affected when nonmodal phonation is produced by a speaker. Future work is warranted to further characterize nonmodal phonatory characteristics to improve the ACC-based prediction of Ps during naturalistic speech production.
When patients present to a voice clinic, they often complain of dysphonia, increased vocal effort, strain, and/or vocal fatigue. Recently, the American Speech-Language-Hearing Association set forth a recommended protocol for clinical voice assessment, including standards for endoscopic imaging, auditory-perceptual evaluation, and objective acoustic and aerodynamic measures (Patel et al., 2018). Objective measures of vocal function critically aid in documenting and supporting clinical decisions to enhance the quality of diagnostic and treatment approaches.
Clinical Utility of Subglottal Pressure Estimation
Average subglottal air pressure (Ps) during phonation is one of the recommended measures that assesses underlying glottal aerodynamics through the input air pressure that sets the vocal fold vibratory mucosa in motion. Ps, in combination with knowledge of aerodynamic and acoustic measurements, reflects the efficiency with which the larynx converts aerodynamic power to acoustic power and is theoretically related to changes in vocal effort and vocal health status (Baggott, Yuen, Hoffman, Zhou, & Jiang, 2007; Ramig & Dromey, 1996; Rosenthal, Lowell, & Colton, 2014; Titze, 1989). Ps is a central component of vocal efficiency and laryngeal resistance metrics (Björklund & Sundberg, 2016; Colton, Casper, & Leonard, 2006; Titze, 1992, 2013; Titze, Maxfield, & Palaparthi, 2016), which reflect the sum of active and passive forces and aerodynamics of the respiratory and laryngeal systems during voice production (Grillo & Verdolini, 2008). Vocal efficiency is defined as the ratio of sound power to the product of Ps and average airflow (Schutte, 1980), and laryngeal airway resistance is defined as the ratio of Ps to the corresponding average flow generated (Smitheran & Hixon, 1981).
Ps has also been used as an outcome measure to document postsurgical or posttherapy changes (Hartl, Hans, Vaissière, Riquet, & Brasnu, 2001; Holmberg, Doyle, Perkell, Hammarberg, & Hillman, 2003; Speyer, 2008; Zeitels, Hochman, & Hillman, 1998; Zeitels et al., 2009). In a series of studies, Zeitels et al. (Zeitels, Hillman, Bunting, & Vaughn, 1997; Zeitels, Hillman, Desloge, & Bunting, 1999; Zeitels, Hillman, Desloge, Mauri, & Doyle, 2002; Zeitels, Hillman, Franco, & Bunting, 2002; Zeitels et al., 1998, 2009) described pre- and postoperative aerodynamic measures compared to normative values established by Holmberg, Hillman, and Perkell (1988). In particular, the investigators reported Ps in relation to sound pressure level (SPL), given the strong relationship between the two measures. This SPL/Ps ratio was used as a short-hand version of vocal efficiency that reflected the input Ps required for voicing given the output vocal intensity (Holmberg et al., 1988; Holmberg, Hillman, Perkell, & Gress, 1994).
Discriminative aerodynamic profiles have been identified for patients with nonphonotraumatic vocal hyperfunction (i.e., diagnosis of muscle tension dysphonia with no evidence of phonotrauma) who exhibited elevated levels of Ps without concomitant changes in vocal intensity, representing a reduction in vocal efficiency (Hillman, Holmberg, Perkell, Walsh, & Vaughan, 1989). Gillespie, Gartner-Schmidt, Rubinstein, and Abbott (2013) expanded upon that work by employing estimates of Ps and glottal airflow to identify distinct subgroups of 90 women with nonphonotraumatic vocal hyperfunction based on aerodynamic profiles of combinations of high and low airflow and pressure that reflected differences in average laryngeal resistance. Gilman et al. (2017) corroborated these findings in a larger study of 192 patients with various voice disorders, noting that mean Ps was significantly higher across the patient group compared to that of a healthy control group. Espinoza, Zañartu, Van Stan, Mehta, and Hillman (2017) found that, across aerodynamic measures obtained, SPL-normalized estimates of Ps were the most salient measures in discriminating patients with phonotraumatic or nonphonotraumatic vocal hyperfunction from matched healthy controls. Grillo and Verdolini (2008) studied differences in aerodynamics of healthy speakers who imitated different voice qualities and found that vocal efficiency and laryngeal resistance distinguished among pressed, breathy, and normal voice qualities to varying degrees.
Traditional Methods of Ps Estimation
Despite the growing evidence of its clinical utility, measurements of Ps and thus Ps-based metrics of laryngeal resistance and vocal efficiency are largely underutilized due to cumbersome or invasive measurement procedures and/or the requirement of expensive, specialized equipment. Many of these methods for obtaining direct or indirect Ps also suffer from uncertainties about how well they reflect glottal aerodynamic function. However, the most common indirect, noninvasive method used for clinic voice assessment is the measurement of intraoral pressure (IOP) during controlled speech gestures. IOP signals are obtained during the repeated production of bilabial plosives followed by sustained vowels (i.e., /p/ or /b/ + vowel) at a constant pitch and loudness and at a set syllable rate. When the task is performed appropriately, the interruption of airflow due to lip closure temporarily equilibrates Ps throughout the subglottal and supraglottal tracts, manifesting as a plateau in the IOP signal (Rothenberg, 1973).
Although IOP-derived measures of Ps have shown the ability to discriminate vocal function mechanisms, the ecological validity of such static Ps estimates is inherently limited, as Ps is measured at one moment in time during isolated vowel contexts. Ps estimation during connected speech production is necessary to reflect naturalistic contexts of pitch, loudness, and speech rate. Despite its limitations, IOP measures are useful for obtaining a person's baseline Ps. Ambulatory estimates of Ps would complement these baseline measurements by capturing Ps during running speech and in natural environments where vocal status is likely to change. The ability to capture changes in Ps would provide important information about the underlying mechanisms of voice production, such as relationships among Ps, glottal airflow, and acoustic (Titze, 1992). Furthermore, capturing Ps changes and linking those changes to vocal dose, perceived vocal effort, or environmental factors could help identify specific, individualized targets for voice therapy and biofeedback to help patients develop more efficient voicing strategies.
Estimation of Vocal Function Measures From Neck-Surface Vibration
Over the past half century, small accelerometer (ACC) sensors have been used in voice and speech research to obtain estimates of acoustic and aerodynamic vocal function measures, as well as measures related to nasalization (Cheyne, Hanson, Genereux, Stevens, & Hillman, 2003; Lindstrom, Waye, Södersten, McAllister, & Ternström, 2011; Mehta, Zañartu, Feng, Cheyne, & Hillman, 2012; Popolo, Švec, & Titze, 2005; Stevens, Kalikow, & Willemain, 1975). Early motivations for using neck-surface ACC sensors included noise-robust measurements of fundamental frequency f 0 in high-noise environments (Porter, 1963; Sugimoto & Hiki, 1960). During phonation, the anterior neck-surface ACC signal consists of components related to tissue-to-tissue transmission of vocal fold collision forces through the thyroid cartilage and air-to-tissue transmission of aerodynamic energy through the trachea (Coleman, 1988; Gunter, Howe, Zeitels, Kobler, & Hillman, 2005). The periodicity of the neck-surface ACC signal closely approximates that of the corresponding acoustic voice signal (Mehta, Van Stan, & Hillman, 2016).
Neck-surface ACC sensors have also been used to obtain estimates of other vocal function measures, such as cepstral peak prominence (CPP; Castellana, Carullo, Corbellini, & Astolfi, 2018; Mehta et al., 2015), and perturbation measures, such as jitter, shimmer, and harmonics-to-noise ratio (Manfredi & Kob, 2009; Mehta et al., 2016). Furthermore, parameters of glottal airflow have been derived from the ACC signal using impedance-based inverse filtering to yield features such as peak-to-peak airflow and maximum flow declination rate (Zañartu, Ho, Mehta, Hillman, & Wodicka, 2013).
ACC-based estimation of vocal SPL has relied on the observation that the average magnitude of neck-surface vibration increases as individuals produce higher SPLs (Švec, Titze, & Popolo, 2005). However, there are limitations to the use of the neck-surface ACC signal to estimate voice SPL, as the short-time energy in the ACC signal predicts voice SPL with a high level of uncertainty (±6 dB on average) during running speech (Švec et al., 2005). Fryd, Van Stan, Hillman, and Mehta (2016) highlighted the wide variation of data points that resulted when relating ACC signal magnitude to SPL across multiple pitch and vowel contexts during repeated vowel productions. This degree of uncertainty is problematic, as SPL estimates obtained from ACC data are often used to derive higher level voice use parameters such as distance dose and energy dissipation dose (Titze & Hunter, 2015; Titze, Švec, & Popolo, 2003), which may be used to assess individuals in high-voice-use professions (Bottalico & Astolfi, 2012; Bottalico, Graetzer, Astolfi, & Hunter, 2017; Carroll et al., 2006; Lindstrom et al., 2011; Titze & Hunter, 2015), as well as patients with behaviorally based voice disorders (Mehta et al., 2015; Van Stan et al., 2015).
Ps Estimation From Neck-Surface Acceleration
The uncertainty of the relationship between ACC signal magnitude and voice SPL motivated investigations into the relationship between Ps estimates and ACC signal measures. Fryd et al. (2016) described this relationship in a study of 10 vocally healthy speakers and found a high degree of correlation between Ps and ACC magnitude estimates during modal voice production; less variation was observed than traditional ACC-based estimates of SPL, largely due to vowel dependencies. A high coefficient of determination (r 2) was found between Ps and ACC signal magnitude for each healthy speaker across vowel contexts and pitch levels. The current study seeks to confirm these results in a larger cohort of speakers.
McKenna, Llico, Mehta, Perkell, and Stepp (2017) explored the Ps–ACC relationship in healthy speakers who were asked to modulate levels of vocal effort at various intensities to simulate changes in vocal efficiency. A strong relationship was found between Ps and ACC signal magnitude estimates in 75% of the participants during productions with excessive vocal effort at an intensity that approximated a normal average speaking intensity. A statistically significant interaction of intensity was discovered (i.e., the Ps–ACC magnitude relationship changed depending on vocal intensity); however, this finding was not consistent across all participants. Those who exhibited moderate correlations had soft productions that did not follow the same pattern as the other intensities elicited; thus, it is possible that those with softer or louder voices produced different vocal qualities (i.e., using nonmodal phonation) that could have changed the relationship between Ps and ACC signal magnitude.
The current study further investigates ACC-based estimation of Ps and the impact of nonmodal phonatory conditions on the typically linear relationship between Ps and ACC signal magnitude, which will help determine whether ACC-based estimation of Ps could be used to study patients with voice disorders who often exhibit nonmodal voice qualities. Employing a study design that used healthy speakers as their own controls, we compared the relationship of Ps and ACC signal magnitude during modal voice production to the relationship during nonmodal voice productions across a spectrum of vocal intensity levels. The purpose of this study was to statistically quantify the impact of nonmodal phonatory conditions on the relationship between Ps and ACC signal magnitude. We addressed the following research questions in a vocally healthy speaker group:
What is the impact of nonmodal phonatory conditions on magnitude-based ACC estimates of Ps?
What is the impact of pitch level on magnitude-based ACC estimates of Ps within each of the phonatory conditions?
A secondary aim was to replicate prior work (Fryd et al., 2016) with a larger sample size and more sophisticated statistical methods to determine the effects of vowel differences on the relationship between Ps and ACC signal magnitude.
Method
Participants
Twenty-six vocally healthy adult speakers (18 women, eight men) were recruited to participate in this study via convenience sampling. In the current study, the mean (standard deviation) participant age was 26 (7.6) years, ranging from 19 to 47 years in women; for men, the mean (standard deviation) participant age was 33 (9.9) years, ranging from 19 to 50 years. Inclusion criteria included an age range of 18–65 years, typical sounding voice, and vocal folds with straight edges exhibiting typical vibration, as assessed by a licensed speech-language pathologist via auditory perceptual evaluation and videostroboscopic examination. Exclusion criteria included a history of voice disorders, current complaints of voice problems, atypical vocal fold vibration, and/or atypical vocal fold edges.
Procedure
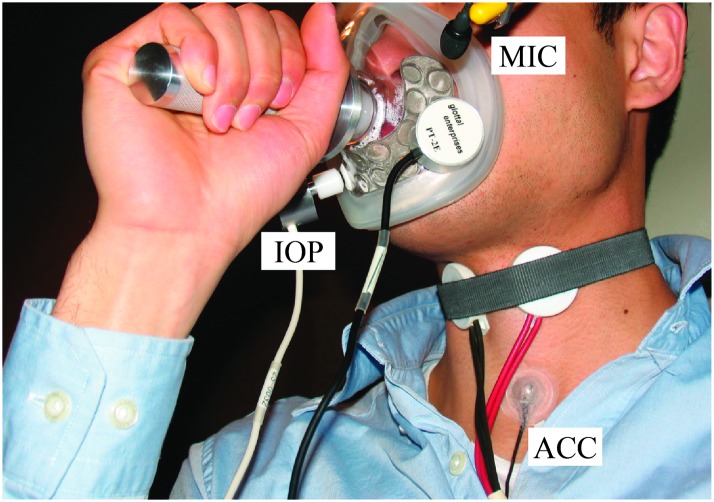
Since data were collected as part of a larger study involving ambulatory voice monitoring, the ACC signal was recorded at an 11025-Hz sampling rate and 16-bit quantization onto a smartphone whose audio drivers and filters were modified for high-quality sampling instead of default telephone-optimized settings (Mehta et al., 2012). Participants were recorded in an acoustically treated sound booth. An ACC sensor (BU-27135; Knowles Corp.) was positioned halfway between the thyroid prominence and the sternal notch to measure neck-surface vibration (see Figure 1). This location is ideal for sensor placement because signals of maximal magnitude can be expected there (Stevens et al., 1975), the sensor is relatively inconspicuous, and it is comfortable for long-term wear (Cheyne et al., 2003; Mehta et al., 2012). A circumferentially vented pneumotachograph mask was placed over the nose and mouth of each participant. Simultaneous acquisition of additional sensor signals was performed from the following devices:
Figure 1.
Illustration of data collection setup with pneumotachograph and neck-surface accelerometer (ACC) placement halfway between the thyroid prominence and the sternal notch. Also pictured are microphone (MIC) and intraoral pressure (IOP) sensors.
head-mounted, omnidirectional acoustic microphone placed 15 cm from the lips (ME 102; Sennheiser Electronic GmbH) and
IOP sensor (PT-75; Glottal Enterprises, Inc.).
These sensors underwent low-pass antialiasing filtering at 8 kHz prior to digital sampling at 20 kHz and 16-bit quantization. IOP, ACC, and microphone signals were calibrated to physical units of cm H2O, cm/s2, and Pa, respectively. The oral airflow signal was also recorded and calibrated but not used for the current investigation.
To determine the impact of nonmodal phonation on magnitude-based ACC estimates of Ps, participants were asked to produce four different voice conditions: modal, breathy, strained, and rough. The terms modal and nonmodal are defined using Gerratt and Kreiman's (2001) nonmodal taxonomy, where modal is the usual or baseline type of phonation and nonmodal is any phonation that differs from or contrasts with the typical voice. It is acknowledged that consistent and accurate terminology has proven challenging in this area. Since all the participants were speakers with healthy voices, modal phonation was used as the reference category when assessing the impact of nonmodal phonatory conditions, consistent with the methods of Grillo and Verdolini (2008). Nonmodal phonation refers to voicing that deviates from the most common type of voice qualities that are characterized by periodic vocal fold vibration (Gerratt & Kreiman, 2001). Examples of nonmodal phonation include categorical qualities such as vocal fry and diplophonia, as well as more continuously scaled qualities of breathiness, roughness, and strain.
For modal productions, participants were instructed to produce a string of /pa/ tokens in one breath, starting from a loud vocal intensity and gradually decreasing in loudness to a soft vocal intensity. This method allowed for the acquisition of a wide range of loudness levels and large number of data points in a short time (Björklund & Sundberg, 2016; Fryd et al., 2016), relative to the conventional method of eliciting one vocal intensity per syllable string. For breathy productions, participants were asked to produce the same task using a breathy or airy voice. For strained productions, participants were asked to perform the task using a voice as if they were lifting something heavy while speaking. For the rough productions, participants were asked to produce the task using a voice with a rough quality (“Cookie Monster” and “Batman” character voices were mentioned as models for those with familiarity). When necessary, the task was modeled by the investigators.
Trials were repeated at three pitch levels: comfortable (within speaking range), higher than comfortable, and lower than comfortable. Participants thus produced two to three trials per pitch level for each modal/nonmodal phonatory condition, yielding up to 36 trials (3 trials × 3 pitch levels × 4 phonatory conditions). It should be noted that, for most participants, it was difficult to change pitch when producing the rough condition tasks, so only one pitch level (comfortable) was included in our analysis for the rough condition. Trials were monitored by a voice-specialized speech-language pathologist to ensure that a consistent mode of production was maintained for each pitch condition. In addition, to confirm findings of a previous study of 10 participants (Fryd et al., 2016), multiple vowel contexts (/pa/, /pi/, and /pu/) were elicited in the modal voice condition only to simulate some of the articulatory variation that occurs during continuous speech. The entire recording session typically lasted approximately 20 min, and participants were encouraged to take breaks as needed to minimize any potential confounding effects of vocal fatigue.
In contrast to work by Lei, Kennedy, Fasanella, Li-Jessen, and Mongeau (2019), the intent of eliciting the nonmodal phonatory conditions was not to obtain pure examples of breathy, strained, and rough qualities but rather to elicit a variety of voice conditions that might influence the relationship between Ps and ACC signal magnitude. Even so, to validate that the elicited productions represented different modes of phonation and that they were categorized into the expected auditory-perceptual categories, a second voice-specialized speech-language pathologist independently rated randomized trials of each elicited phonatory and pitch condition. Percent agreement for each phonatory condition was calculated as 85%. Intrarater agreement was assessed on 20% of the productions using Cohen's kappa, resulting in substantial agreement (κ = .78).
Signal Analysis
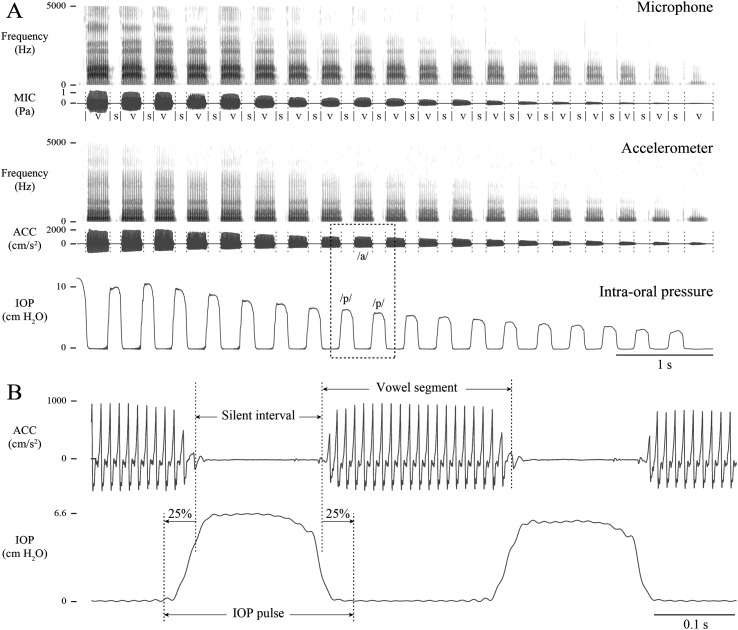
Figure 2 displays an example of microphone, ACC, and IOP signals for one trial in a modal phonatory condition for one male participant, M2. The voiceless /p/ plosives of the p-vowel gestures created a sequence of descending pulses in the IOP signal. Vowel segments can be seen in the microphone and ACC signals between IOP pulses.
Figure 2.
An example of the repeated /pa/ gesture with descending loudness for one male participant, M2. (A) Time-aligned signals from the acoustic microphone (MIC), neck-surface accelerometer (ACC), and intraoral pressure (IOP) sensor are displayed on a common timescale, along with the Praat TextGrid tier (S = silence, V = vowel). The boxed region is expanded in (B) to illustrate the boundary detection of each vowel segment and IOP pulse.
First, boundaries of the vowel segments were determined in the microphone signal using Praat Version 6.0.30, which identified sounding/silent intervals (Boersma & Weenink, 2013). The built-in algorithm was configured to detect a −25-dB change in signal intensity from the maximum intensity within 32-ms sliding windows (minimum silent interval = 25 ms, minimum sounding interval = 50 ms). Figure 2A displays the resulting TextGrid of labeled vowel segment and silent interval boundaries. Boundaries for the first and last plosive of each breath group were verified visually to create a trial label for each permutation of pitch, vowel, and phonatory conditions.
Second, boundaries of each intervocalic IOP pulse were detected automatically using a custom algorithm (see Figure 2B). The IOP signal was low-pass filtered with a fifth-order Butterworth filter (80-Hz 3-dB cutoff frequency) to remove harmonic information that might confound the boundary determination. Next, the silent interval boundaries were extended by 25% to the left and right, resulting in IOP pulse boundaries that compensated for the slight overlap between the preceding vowel segment and the rise of the subsequent IOP signal.
Third, estimates of Ps for each vowel segment were determined by computing the mean of the IOP pulse peak amplitudes preceding and following each vowel. Alignment of the smartphone-recorded ACC signal was achieved using a custom algorithm in MATLAB that shifted the ACC signal (resampled to the acoustic sampling rate of 20 kHz), such that the absolute value of the cross-correlation between the two signals was maximized. ACC signal magnitude was computed as the root-mean-square level from the mid–50 ms of each vowel segment.
Statistical Analysis
Several statistical metrics were computed to analyze the relationship between Ps and ACC signal magnitude within and across vowel, pitch, and modal/nonmodal phonatory conditions. First, using Excel, the coefficient of determination (r 2) analyzed the strength of the relationship between Ps and ACC signal magnitude within and across the different vowel contexts and pitch levels within each of the modal and nonmodal phonatory conditions. Linear regression models for these relationships were computed and plotted to allow for the comparison of slopes and intercepts within and across participants. The root-mean-square error between Ps and ACC signal magnitude was computed after pooling all vowel, pitch, and phonatory conditions to obtain a measure of uncertainty if ACC signal magnitude were to be used as a surrogate of Ps in practice.
Next, using SPSS (Version 25), linear mixed-effects (LME) models were fit by restricted maximum likelihood. The data set was cleaned by removing Ps values of less than 1 cm H2O, as those values likely reflected whispered or pulsed p-vowel productions. Random effects terms were chosen based on variance explained. Because normative values for some of the glottal aerodynamic measures differ for men and women (Holmberg, Hillman, & Perkell, 1989), the data for men and women were analyzed separately. Within each participant group, three separate LME models were derived to compare the slopes and intercepts of the Ps–ACC magnitude relationship: LME Model 1 assessed the impact of nonmodal phonatory conditions on the Ps–ACC magnitude relationship, LME Model 2 assessed the impact of pitch level on the Ps–ACC magnitude relationship within each phonatory condition, and LME Model 3 assessed the impact of vowel context on the Ps–ACC magnitude relationship within the modal phonatory phonation to confirm previous results (Fryd et al., 2016). Random effects were estimated for participant ACC signal magnitude.
For LME Model 1, nonmodal phonatory conditions were dummy coded using the modal phonatory condition as the reference with only comfortable /a/ vowel productions analyzed. For LME Model 2, nonmodal phonatory conditions were also dummy coded using the modal phonatory condition as the reference, and high- and low-pitch levels were dummy coded using comfortable pitch as the reference (/a/ vowel productions only). For LME Model 3, vowels were dummy coded using the /a/ vowel as the reference category for modal, comfortable pitch productions. The distributions of ACC signal magnitude and Ps both exhibited a positive skew. Because regression models are typically robust to skew with a large sample size, we initially tested models without any transformations. However, regression diagnostics indicated heteroscedasticity in the residuals; therefore, we applied a base 10 logarithmic transformation to both ACC signal magnitude and Ps. Plots of predicted Ps versus residuals indicated that the assumption of homoscedasticity was no longer violated. The Appendix reports the results of the three LME models using the original, nontransformed data to allow for interpretation of the intercepts in terms of cm H2O.
The LME model outputs included the estimates of the fixed-effects coefficients, the standard error associated with the estimate, the degrees of freedom, p value, and effect size correlation. Due to the large amount of data collected, a Bonferroni-adjusted alpha of .0125 was used for all statistical tests, which was derived by dividing the standard alpha value of .05 by the number of independent variables. The effect size correlation r was interpreted using Pearson's suggested values of .1, .3, and .5 as cutoffs for small, medium, and large effects, respectively (Cohen, 1988).
Results
Table 1 displays descriptive statistics for the female and male participant groups. These results verified that a wide range of SPL and Ps values was elicited by the descending loudness p-vowel protocol within each phonatory condition. Driven by anatomical and physiological differences among individuals, the variety of productions illustrates the need for specific, well-controlled calibration procedures to obtain participant-specific relationships between Ps and ACC signal magnitude. The f 0 produced by each participant was relatively stable, with a standard deviation of approximately two semitones within each pitch level.
Table 1.
Group-based statistics demonstrating the spectrum of conditions elicited by the descending loudness p-vowel protocol.
| Pitch level | Modal |
Breathy |
Strained |
Rough |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| f 0 | SPL | Ps | f 0 | SPL | Ps | f 0 | SPL | Ps | f 0 | SPL | Ps | |
| Females | ||||||||||||
| Comf | 263.9 (26.1) | 62.0–94.5 | 2.8–15.2 | 248.6 (15.5) | 62.9–85.3 | 4.8–13.3 | 258.7 (29.6) | 67.2–95.1 | 5.3–18.5 | 229.2 (52.3) | 71.3–91.6 | 7.8–21.5 |
| High | 386.2 (27.9) | 67.5–96.6 | 3.8–17.2 | 326.2 (9.7) | 67.2–87.1 | 5.4–13.9 | 375.2 (11.1) | 71.3–96.4 | 5.5–19.3 | — | — | — |
| Low | 220.1 (21.9) | 62.4–91.1 | 2.4–13.0 | 212.4 (11.8) | 64.7–82.2 | 4.6–10.6 | 218.1 (23.5) | 66.4–91.9 | 4.8–15.4 | — | — | — |
| Males | ||||||||||||
| Comf | 146.6 (17.7) | 61.2–94.9 | 2.2–14.1 | 145.6 (5.4) | 62.9–84.7 | 3.7–13.2 | 170.5 (16.6) | 71.6–94.1 | 6.4–19.9 | 134.3 (13.5) | 75.1–93.1 | 8.5–22.2 |
| High | 228.8 (15.4) | 67.8–97.4 | 3.7–17.1 | 203.9 (6.8) | 64.2–88.9 | 4.5–14.1 | 236.6 (15.9) | 71.9–98.0 | 6.1–23.0 | — | — | — |
| Low | 123.5 (11.7) | 64.4–91.7 | 2.5–12.7 | 120.4 (7.4) | 63.0–83.9 | 3.9–11.8 | 138.8 (15.8) | 70.8–90.7 | 6.2–17.6 | — | — | — |
Note. Reported measures are mean (standard deviation) of the average fundamental frequency (f 0; Hz), mean range of the sound pressure level (SPL; dB SPL @15 cm), and mean range of the subglottal pressure estimates (Ps; cm H2O) exhibited within each phonatory condition (modal, breathy, strained, rough) and pitch level (comfortable [Comf], high, low). Note that only one pitch level was elicited for the rough phonatory condition. Em dashes indicate that the task was not elicited.
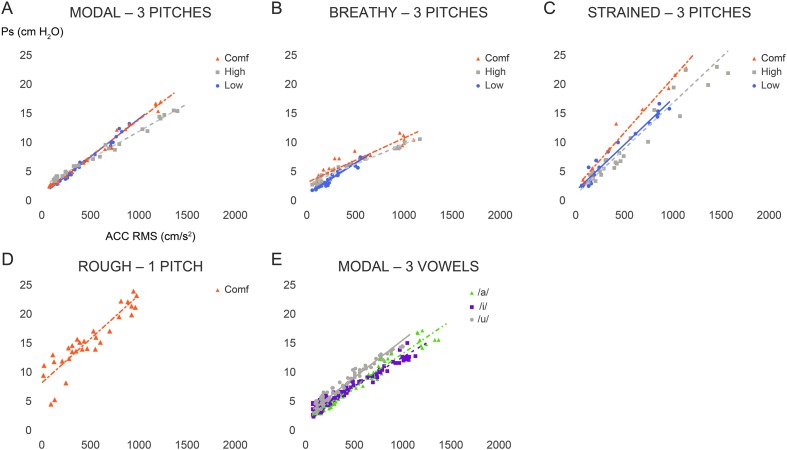
Figure 3 displays illustrative scatter plots of the relationship between Ps and ACC signal magnitude for female participant F6 for each pitch level within the four phonatory conditions. As noted in the methods, high- and low-pitch levels in the rough condition were not elicited due to the difficult nature of the task. For the statistical analyses, a base 10 logarithmic transformation was performed on both the Ps and ACC signal magnitude values and are therefore presented in the results and tables as such.
Figure 3.
Illustration of the relationship between subglottal air pressure (Ps) estimates and neck-surface accelerometer (ACC) root-mean-square (RMS) magnitude for the female participant F6. Data points for the (A) modal, (B) breathy, (C) strained, and (D) rough phonatory conditions are color coded for pitch level (vowel /a/ only). (E) The impact of vowel (/a/, /i/, and /u/ color coded) is shown, pooling across pitch levels within each vowel context. The linear regression equation and coefficient of determination (r 2) are reported for each color-coded set of data points. Comf = comfortable.
Impact of Nonmodal Phonation on the Relationship Between Ps and ACC Magnitude
Table 2 reports estimates of the fixed-effects variables of LME Model 1, which compared the slope and intercept of the “modal” condition to the respective slopes and intercepts of the Ps–ACC magnitude relationship for the nonmodal phonatory conditions (“breathy,” “strained,” and “rough”). For females, the intercepts of the nonmodal conditions were all statistically different from the intercept for modal (p < .001), with small to medium effect sizes (r = .30 for breathy, r = .14 for strained, and r = .41 for rough) compared to the modal condition. The slopes (ACC × Nonmodal interaction) for nonmodal conditions were also all statistically different (p < .001) than the slope for modal (ACC × Modal interaction), with small to medium effect sizes (r = .27 for breathy, r = .11 for strained, and r = .32 for rough) compared to the modal condition.
Table 2.
Linear mixed-effects Model 1 results quantifying the impact of nonmodal phonation on the linear relationship between subglottal pressure (Ps) and accelerometer (ACC) signal magnitude.
| Condition | Estimate ± SE | Estimate ± SE wrt modal | df | p | r |
|---|---|---|---|---|---|
| Females | |||||
| Intercept | |||||
| Modal | −.69 ± .13 | — | — | — | — |
| Breathy | — | .61 ± .04 | 1988.28 | < .001* | .30 |
| Strained | — | .30 ± .05 | 1988.48 | < .001* | .14 |
| Rough | — | .89 ± .04 | 2000.13 | < .001* | .41 |
| Slope | |||||
| ACC × Modal | .60 ± .05 | — | — | — | — |
| ACC × Breathy | — | −.21 ± .02 | 1985.85 | < .001* | .27 |
| ACC × Strained | — | −.09 ± .02 | 1987.29 | < .001* | .11 |
| ACC × Rough | — | −.26 ± .02 | 1999.23 | < .001* | .32 |
| Males | |||||
| Intercept | |||||
| Modal | −.41 ± .15 | — | — | — | — |
| Breathy | — | .30 ± .05 | 1039.32 | < .001* | .19 |
| Strained | — | .37 ± .05 | 1078.92 | < .001* | .23 |
| Rough | — | .55 ± .05 | 1079.39 | < .001* | .33 |
| Slope | |||||
| ACC × Modal | .49 ± .04 | — | — | — | — |
| ACC × Breathy | — | −.09 ± .02 | 1049.16 | < .001* | .14 |
| ACC × Strained | — | −.04 ± .40 | 1079.31 | .03 | .07 |
| ACC × Rough | — | −.07 ± .02 | 1080.44 | .002 | .10 |
Note. For the modal phonatory reference condition, the estimates of intercept and slope are shown. For the nonmodal phonatory conditions, the estimates of intercepts and slopes are shown with reference to (wrt) those for the modal phonatory condition. The standard error (SE), degrees of freedom (df), p value, and effect size (r) are reported for each estimate. A base-10 logarithmic transformation was computed for Ps and ACC values. The values in the breathy, rough, and strained estimate columns are in reference to the associated modal estimate values. Em dashes indicate data not applicable.
p < .01.
For males, the intercepts of the nonmodal conditions were all statistically different than the intercept for the modal condition (p < .001), with small to medium effect sizes (r = .19 for breathy, r = .23 for strained, and r = .33 for rough) compared to the modal condition. The slopes for breathy (ACC × Breathy interaction) and rough (ACC × Rough interaction) were statistically different from the modal condition (p < .001 and p = .002, respectively), with small effect sizes (r = .14 and r = .10, respectively). The slope for strained (ACC × Strained) was not statistically different from the slope for modal (ACC × Modal).
Impact of Pitch on the Relationship Between Ps and ACC Magnitude
Table 3 reports estimates of the fixed-effects variables of LME Model 2, which assessed the effects of pitch level within each phonatory condition. Pitch conditions within the rough condition were not elicited due to the difficult nature of the task.
Table 3.
Linear mixed-effects Model 2 results quantifying the impact of pitch level on the linear relationship between subglottal pressure (Ps) and accelerometer (ACC) signal magnitude within each phonatory condition.
| Condition | Estimate ± SE | Estimate ± SE wrt Comf | df | p | r |
|---|---|---|---|---|---|
| Females | |||||
| Modal | |||||
| Intercept | |||||
| Comf | −.65 ± .11 | — | — | — | — |
| High | — | .16 ± .05 | 4918.03 | < .001* | .05 |
| Low | — | .07 ± .05 | 4923.60 | .17 | .02 |
| Slope | |||||
| ACC × Comf | .59 ± .04 | — | — | — | — |
| ACC × High | — | −.06 ± .02 | 4917.81 | .002* | .04 |
| ACC × Low | — | −.04 ± .02 | 4922.65 | .03 | .03 |
| Breathy | |||||
| Intercept | |||||
| Comf | −.06 ± .05 | — | — | — | — |
| High | — | −.07 ± .07 | 4920.07 | .34 | .01 |
| Low | — | −.09 ± .07 | 4923.60 | .21 | .02 |
| Slope | |||||
| ACC × Comf | .38 ± .02 | — | — | — | — |
| ACC × High | — | .03 ± .03 | 4919.89 | .29 | .02 |
| ACC × Low | — | .02 ± .03 | 4922.93 | .53 | .01 |
| Strained | |||||
| Intercept | |||||
| Comf | −.39 ± .05 | — | — | — | — |
| High | — | −.28 ± .08 | 4917.11 | < .001* | .05 |
| Low | — | .17 ± .07 | 4918.92 | .02 | .03 |
| Slope | |||||
| ACC × Comf | .51 ± .02 | — | — | — | — |
| ACC × High | — | .11 ± .03 | 4916.99 | .001* | .06 |
| ACC × Low | — | −.04 ± .03 | 4918.54 | .15 | .02 |
| Males | |||||
| Modal | |||||
| Intercept | |||||
| Comf | −.40 ± .13 | — | — | — | — |
| High | — | −.01 ± .04 | 2770.61 | .78 | .01 |
| Low | — | .30 ± .04 | 2775.84 | < .001* | .14 |
| Slope | |||||
| ACC × Comf | .50 ± .04 | — | — | — | — |
| ACC × High | — | .03 ± .02 | 2770.58 | .07 | .05 |
| ACC × Low | — | −.11 ± .02 | 2775.53 | < .001* | .11 |
| Breathy | |||||
| Intercept | |||||
| Comf | −.06 ± .04 | — | — | — | — |
| High | — | .04 ± .06 | 2770.27 | .54 | .01 |
| Low | — | −.24 ± .05 | 2769.41 | < .001* | .08 |
| Slope | |||||
| ACC × Comf | .38 ± .02 | — | — | — | — |
| ACC × High | — | −.02 ± .03 | 2769.64 | .33 | .02 |
| ACC × Low | — | −.01 ± .03 | 2768.06 | < .001* | .10 |
| Strained | |||||
| Intercept | |||||
| Comf | .0004 ± .04 | — | — | — | |
| High | — | −.12 ± .07 | 2767.49 | .06 | .04 |
| Low | — | −.34 ± .06 | 2774.28 | < .001* | .11 |
| Slope | |||||
| ACC × Comf | .44 ± .02 | — | — | — | |
| ACC × High | — | .02 ± .03 | 2767.66 | .37 | .02 |
| ACC × Low | — | .12 ± .03 | 2774.03 | < .001* | .08 |
Note. For the comfortable pitch (Comf) reference condition within each phonatory condition, the estimates of intercept and slope are shown. For the high- and low-pitch conditions, the estimates of intercept and slope are shown with reference to (wrt) those for the Comf pitch category within each phonatory condition. The standard error (SE), degrees of freedom (df), p value, and effect size (r) are reported for each estimate. A base 10 logarithmic transformation was computed for Ps and ACC values. The values in the high- and low-pitch columns are in reference to the associated comfortable pitch estimates within each phonatory condition. Em dashes indicate data not applicable.
p < .0125.
Pitch Within the Modal Condition
For females within the modal condition, the intercept for high-pitch condition was statistically different from the intercept for the comfortable pitch condition (p = .002), but with a negligible effect size (r = .05), and the intercept for the low-pitch condition was not statistically different from the intercept for the comfortable pitch condition. Similarly, the slope (ACC × Pitch interaction) for the high-pitch condition was statistically different from the slope for the comfortable pitch condition (p < .001), but with a negligible effect size (r = .04), and the slope for the low-pitch condition was not statistically different from the slope for the comfortable pitch condition.
For males, within the modal condition, the intercept for the high pitch was not statistically different from the intercept for the comfortable pitch, but the intercept for the low pitch was statistically different from modal (p < .001), with a small effect size (r = .14). The slope (ACC × Pitch interaction) for the high-pitch condition was not statistically different from the slope of the comfortable pitch condition, but the slope for the low-pitch condition was statistically different from the slope of the comfortable pitch condition (p < .001), with a small effect size (r = .11).
Pitch Within the Breathy Condition
For females, within the breathy condition, no statistical differences were found in either the intercepts or the slopes (ACC × Pitch interaction) of the high-pitch condition or the low-pitch condition compared to those of the comfortable pitch condition. For males, within the elicited breathy condition, the intercept for the high-pitch condition was not statistically different from the comfortable pitch condition, whereas the intercept for the low-pitch condition was statistically different from the intercept of the comfortable pitch condition (p < .001), but with a negligible effect size (r = .08). Similarly, the slope (ACC × Pitch interaction) for the high-pitch condition was not statistically different from the slope for the comfortable pitch condition, but the slope for the low-pitch condition was statistically different from the comfortable pitch condition (p < .001), with a small effect size (r = .10).
Pitch Within the Strained Condition
For females, within the elicited strained condition, the intercept for the high-pitch condition was statistically different from the intercept for the comfortable pitch condition (p < .001), but with a negligible effect size (r = .05). The intercept for the low-pitch condition was not statistically different from the intercept for the comfortable pitch condition. Similarly, the slope (ACC × Pitch interaction) for the high-pitch condition was statistically different from the slope for the comfortable pitch condition (p < .001), but with a negligible effect size (r = .06), and the slope for the low-pitch condition was not statistically different from the slope for the comfortable pitch condition.
For males, within the elicited strained condition, no statistical difference was found between the intercepts for comfortable and high-pitch levels; however, a statistical difference was found in the intercepts of the low-pitch and comfortable pitch conditions (p < .001), with a small effect size (r = .11). The slope (ACC × Pitch interaction) for the high-pitch condition was not statistically different from the slope for ACC for the comfortable pitch condition; however, the slope for the low-pitch condition was statistically different from the slope for the comfortable pitch condition (p < .001), but with a negligible effect size (r = .08).
Impact of Vowel on the Relationship Between Ps and ACC Magnitude
Table 4 shows results of LME Model 3 that compared intercepts and slopes (ACC × vowel) of /i/ and /u/ vowels to the intercept and slope of /a/ vowels produced in modal voice at a comfortable pitch across participants. For females, the intercept for the /i/ vowel condition was statistically different from the intercept for the /a/ vowel condition (p = .001), but with a negligible effect size (r = .08). The intercept for the /u/ vowel condition was also statistically different from the intercept for the /a/ vowel condition (p = .01), also with a negligible effect size (r = .06). Similarly, the slopes for the /i/ vowel and /u/ vowel conditions were statistically different from the slope for ACC for the /a/ vowel condition (p = .001), with negligible effect sizes (r = .08).
Table 4.
Linear mixed-effects Model 3 results quantifying the impact of vowel on the linear relationship between subglottal pressure (Ps) and accelerometer (ACC) signal magnitude.
| Condition | Estimate ± SE | Estimate ± SE wrt /a/ | df | p | r |
|---|---|---|---|---|---|
| Females | |||||
| Intercept | |||||
| /a/ | −.70 ± .11 | — | — | — | — |
| /i/ | — | .08 ± .03 | 1667.43 | .001* | .08 |
| /u/ | — | .04 ± .03 | 1657.69 | .012* | .06 |
| Slope | |||||
| ACC × /a/ | .61 ± .04 | — | — | — | — |
| ACC × /i/ | — | −.04 ± .01 | 1667.17 | .001* | .08 |
| ACC × /u/ | — | −.02 ± .01 | 1667.45 | .001* | .08 |
| Males | |||||
| Intercept | |||||
| /a/ | −.80 ± .17 | — | — | — | — |
| /i/ | — | .41 ± .02 | 1112.80 | < .001* | .47 |
| /u/ | — | .35 ± .02 | 946.40 | < .001* | .42 |
| Slope | |||||
| ACC × /a/ | .66 ± .04 | — | — | — | — |
| ACC × /i/ | — | −.15 ± .01 | 1113.20 | < .001* | .42 |
| ACC × /u/ | — | −.13 ± .01 | 1112.37 | < .001* | .37 |
Note. For the vowel /a/ reference condition, the estimates of interceptand slope are shown. For the vowels /i/ and /u/, the estimates of intercepts and slopes are shown with reference to (wrt) those for vowel /a/. The standard error (SE), degrees of freedom (df), p value, and effect size (r) are reported for each estimate. A base 10 logarithmic transformation was computed for Ps and ACC values. The values in the /i/ and /u/ estimate columns are in reference to the associated /a/ estimate values. Em dashes indicate data not applicable.
p < .0125.
For males, the intercepts for both the /i/ vowel condition and /u/ vowel condition were statistically different from the intercept for the /a/ vowel condition (p < .001), with medium effect sizes (r = .47 and .42, respectively). The slopes for ACC for /i/ vowel and /u/ vowel conditions were also statistically different from the slope for ACC for the /a/ vowel condition (p < .001), also with medium effect sizes (r = .42 and .37, respectively).
Individual Participant Data
Table 5 is a comprehensive summary of the strength of the linear relationship between Ps and ACC magnitude within each elicited phonatory conditions. The coefficient of determination (r 2) and the relative change in slope and intercept from those in each participant's modal phonatory condition are reported. These statistics were computed after pooling data points across vowel contexts and pitch levels since the LME models showed that the only significant effects were due primarily to the type of nonmodal phonatory condition produced (and not to vowel and pitch). Across all participants in the study, the mean (standard deviation) r 2 was highest for the modal condition at .72 (.14), with decreased but still statistically significant values for the nonmodal conditions: .58 (.25) for breathy, .61 (.21) for strained, and .55 (.28) for rough. When applied in practice, the uncertainty of estimating Ps with ACC signal magnitude must be computed without knowledge of the type of phonatory condition produced. In that scenario, the mean (standard deviation) root-mean-square error across subjects was 2.9 (1.3) cm H2O, with one subject exhibiting a minimum error of 1.4 cm H2O and another subject exhibiting maximum error of 7.4 cm H2O.
Table 5.
Slope, intercept, and coefficient of determination (r 2) for the linear regression equation predicting subglottal pressure given accelerometer signal magnitude for each subject per phonatory condition, pooling across pitch level and vowel contexts.
| Subject ID | Modal |
Breathy |
Strained |
Rough |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Slope | Intercept | r 2 | ΔSlope | ΔIntercept | r 2 | ΔSlope | ΔIntercept | r 2 | ΔSlope | ΔIntercept | r 2 | |
| F1 | 16.21 | 1.77 | .85 | 4.82 | 2.55 | .58 | 3.08 | 0.88 | .78 | 27.80 | 1.16 | .15 |
| F2 | 6.99 | 2.83 | .84 | 1.27 | −0.53 | .71 | — | — | — | −1.89 | 3.76 | .07 |
| F3 | 8.41 | 3.83 | .62 | 4.90 | −0.76 | .59 | 2.27 | −1.09 | .44 | −2.40 | 1.74 | .00 |
| F4 | 12.88 | 2.66 | .76 | −9.34 | 3.60 | .18 | 0.43 | 3.06 | .60 | 5.50 | 0.97 | .81 |
| F5 | 5.73 | 2.53 | .77 | −4.10 | 2.56 | .04 | −0.14 | 0.66 | .85 | 2.92 | 7.46 | .20 |
| F6 | 1.08 | 2.66 | .91 | −2.61 | −0.36 | .90 | 6.05 | −1.23 | .95 | 7.30 | 4.97 | .83 |
| F7 | 11.69 | 3.75 | .80 | −1.49 | −0.63 | .64 | −5.34 | 2.83 | .24 | −8.25 | 1.10 | .26 |
| F8 | 13.70 | 3.96 | .61 | 8.63 | −0.77 | .64 | −8.57 | 3.23 | .36 | 7.86 | 4.31 | .82 |
| F9 | 4.37 | 3.63 | .48 | −2.61 | 3.28 | .10 | −0.62 | 0.76 | .54 | 0.90 | −2.01 | .31 |
| F10 | 17.26 | 3.64 | .83 | −4.30 | 4.25 | .54 | −5.78 | 4.08 | .67 | −6.12 | 13.49 | .73 |
| F11 | 6.89 | 2.73 | .71 | — | — | — | 6.77 | 1.06 | .90 | 1.35 | 4.92 | .67 |
| F12 | 17.05 | 3.20 | .72 | −7.50 | 2.72 | .30 | −7.05 | 3.39 | .28 | −6.74 | 7.43 | .36 |
| F13 | 37.95 | 4.46 | .65 | −22.02 | 1.22 | .24 | 13.69 | −1.61 | .74 | 26.70 | −5.09 | .22 |
| F14 | 5.08 | 4.29 | .82 | −0.91 | −1.88 | .81 | −0.66 | 1.11 | .81 | −0.90 | 5.81 | .68 |
| F15 | 5.04 | 4.06 | .85 | −0.75 | −0.20 | .79 | −0.65 | 3.33 | .72 | −0.14 | 3.46 | .76 |
| F16 | 9.69 | 2.12 | .85 | −0.84 | 1.03 | .84 | −1.44 | 7.52 | .63 | 2.01 | 5.29 | .89 |
| F17 | 11.51 | 3.71 | .71 | 0.71 | 19.04 | .85 | 11.08 | 6.42 | .67 | −3.79 | 8.31 | .69 |
| F18 | 16.87 | 3.15 | .71 | −9.62 | 1.87 | .38 | −3.79 | 0.44 | .60 | −8.15 | 4.13 | .68 |
| M1 | 7.08 | 2.42 | .83 | 0.35 | 2.16 | .74 | 4.87 | 4.57 | .65 | 4.98 | 7.32 | .96 |
| M2 | 21.88 | 3.54 | .49 | −4.82 | 0.89 | .73 | −1.51 | 4.89 | .51 | 1.98 | 8.29 | .54 |
| M3 | 3.31 | 4.63 | .39 | 2.87 | −0.65 | .81 | 1.28 | 0.72 | .63 | — | — | — |
| M4 | 5.06 | 2.87 | .70 | −0.66 | 0.67 | .71 | 3.41 | 7.38 | .36 | −0.66 | 4.79 | .62 |
| M5 | 25.75 | 4.57 | .68 | −7.16 | −0.74 | .58 | −0.65 | −0.25 | .88 | 35.12 | −4.37 | .77 |
| M6 | 5.74 | 2.12 | .85 | 0.71 | 3.44 | .71 | 0.65 | 6.82 | .17 | 5.18 | 9.14 | .43 |
| M7 | 77.93 | 5.76 | .48 | 146.81 | −1.12 | .70 | 17.25 | −1.13 | .69 | 93.42 | 0.83 | .75 |
| M8 | 15.42 | 3.02 | .90 | −5.35 | 2.52 | .39 | 11.00 | 1.26 | .59 | 11.09 | 8.94 | .49 |
|
M (SD) |
14.60 (15.09) |
3.38 (.93) |
.72 (.14) |
3.48 (3.46) |
1.77 (4.00) |
.58 (.25) |
7.94 (34.27) |
2.36 (2.77) |
.61 (.21) |
8.16 (2.90) |
4.61 (4.42) |
.55 (.28) |
Note. The change in the slope (ΔSlope) and intercept (ΔIntercept) are computed for each nonmodal phonatory condition relative to the respective slope and intercept in the modal phonatory condition. Mean (M) and standard deviation (SD) of each parameter across all subjects are reported in the last rows. An em dash indicates that the participant did not complete tasks for the indicated condition. All Slope and ΔSlope values were multiplied by 1,000 for readability.
Discussion
The goals of this line of research are to (a) measure Ps during connected speech to better assess the underlying phonatory mechanisms that individuals are using as they speak naturally; (b) further improve Ps estimation accuracy to target specific, individualized therapy strategies (e.g., biofeedback during voice therapy); and (c) enhance ambulatory monitoring and biofeedback during real-life contexts. The use of neck-placed ACCs has allowed ambulatory monitoring investigations to capture an array of vocal function measures. If Ps can be estimated via an ACC sensor, then Ps can be added to the suite of measures available from ambulatory monitoring. These aerodynamic measures can then be used not only to document changes before and after interventions but also track the fluctuations in vocal status that occur throughout one's day. Furthermore, Ps can be used in conjunction with voice therapy to provide patient-specific biofeedback during natural voicing tasks. Toward the goal of monitoring Ps in an ambulatory setting, it was first necessary to understand the impact that voice quality variations have on the relationship of Ps and ACC in participants with typical voices (i.e., no pathology).
Healthy participants were selected for this study so that each person's modal voice served as his or her own control to the elicited nonmodal conditions. We chose to use conventional voice quality terms used in clinical voice assessment—“breathy,” “strained,” and “rough”— to elicit exemplars of nonmodal phonatory behavior that was hypothesized to change the relationship between Ps and ACC signal magnitude. While not every person produced each nonmodal condition the same way, the intent was not to obtain pure examples of breathy, strained, or rough voices but rather to elicit atypical voice characteristics commonly associated with many voice disorders.
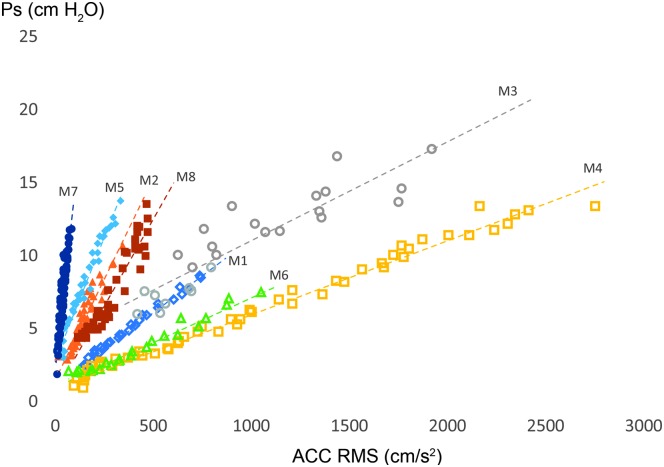
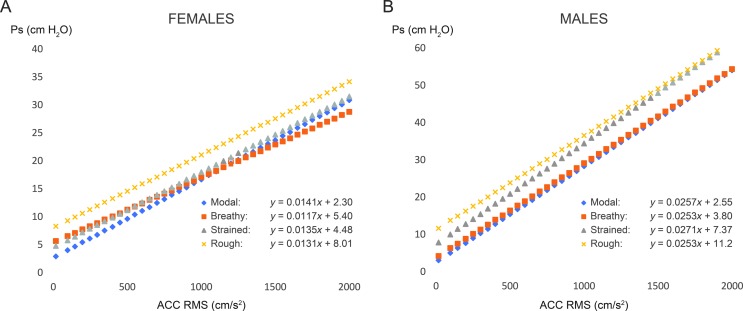
The first research question yielded the main findings of the study that phonatory condition had a significant impact on the linear Ps–ACC magnitude relationship within each participant. For females, both the intercepts and slopes for nonmodal conditions were statistically different from those of the modal condition. For males, the intercepts for all nonmodal conditions were statistically different from the intercept for modal, and the slopes for breathy and rough were statistically different from the slope for modal. The slope for strained was not statistically different from the slope for modal, which suggests that, for some males, their strained productions were not much different than their modal productions. The variation in intercept, without concomitant variation in slope, is thought to reflect changes in Ps required to initiate and maintain phonation across loudness levels, which are theoretically related to changes in vocal effort and vocal efficiency across vocal intensity levels (Chang & Karnell, 2004; Ramig & Dromey, 1996; Rosenthal et al., 2014). Therefore, it is reasonable that the Ps required for a person to initiate and maintain phonation would increase when his or her voice sounds more breathy, strained, or rough, compared to his or her modal phonatory characteristics, as shown in the results. A better understanding of the effect of phonatory condition on the relationship between Ps and the ACC signal takes us one step closer to the goal of measuring Ps in patients with voice disorders during natural running speech, including ambulatory monitoring contexts. As illustrated in Figure 4, there is wide variation in the ACC–Ps relationship within male participants, so individualized calibration will be necessary when estimating Ps from the ACC, regardless of phonatory condition, pitch, or vowel.
Figure 4.
Regression lines derived from raw data demonstrating the variation within male subjects for the modal condition (comfortable pitch, vowel /a/). Ps = subglottal pressure; ACC = accelerometer; RMS = root-mean-square.
The second research question further dissected the effects of nonmodal phonatory conditions by looking at the effects of pitch within each phonatory condition, thus gaining a better understanding about how variations in typical running speech (variations in vowels, pitch, and loudness) may affect our ability to predict Ps using ACC signal magnitude. Results of the analysis of pitch differences within modal phonation revealed negligible effects in the Ps–ACC magnitude relationship among pitch levels in women and small effects in men.
Fryd et al. (2016) used descriptive statistics to document r 2 changes between Ps and ACC signal magnitude pooling pitch and vowel contexts. The current study evaluated these differences using more formal statistical analyses to test for the impact of vowel and pitch on the relationship between Ps and ACC magnitude. Results of the current study confirm that pitch differences may have negligible to small effects on the Ps–ACC magnitude relationship, which suggests that pitch differences may not need to be taken into account when estimating Ps using the ACC signal in women, but further investigation of the effects of pitch differences in men is warranted, particularly within individuals.
In contrast to Fryd et al. (2016), our results indicated that vowel differences may have a small effect on the relationship between Ps and ACC magnitude in women and a moderate effect in men. The differences in vowels observed may be due, in part, to nonlinear source filter and supra-/subglottal coupling (Titze, 2008) and also, in part, to individual differences that reflect the need for subject-specific calibration. It is also possible that some men changed phonatory conditions (e.g., became breathier or more tense) when producing different vowels or that the pneumotachograph mask altered the way in which they produced the vowels. Table 5 describes the comprehensive summary of the strength of the linear relationship between Ps and ACC magnitude within each elicited phonatory conditions, and M2, M3, and M7, in particular, exhibited lower coefficients in the modal condition of determination compared with the other participants. Further examination of the underlying data revealed an effect of vowel category in the same three male participants, which was driving the effect size of Model 3 in male participants that was not observed in prior work (Fryd et al., 2016). A larger sample of male participants may diminish the effects of vowel on the relationship and would require further investigation, as a small sample of men was a limitation of the current study.
Clinical Implications
Ambulatory monitoring has largely relied on estimates of f 0 and SPL from the ACC signal with the primary objective of quantifying typical daily vocal behavior and the accumulated impact of prolonged voice use (Bottalico & Astolfi, 2012; Buekers, Bierens, Kingma, & Marres, 1995; Hillman, Heaton, Masaki, Zeitels, & Cheyne, 2006; Lindstrom et al., 2011; Mehta et al., 2015; Nacci et al., 2013; Švec, Popolo, & Titze, 2003; Titze & Hunter, 2015; Titze et al., 2003; Van Stan et al., 2015). User-friendly platforms have been developed to allow for unobtrusive, noninvasive ambulatory monitoring that allows for data acquisition using a small ACC placed on the neck skin below the glottis (Carullo, Vallan, & Astolfi, 2013; Cheyne et al., 2003; KayPENTAX, 2009; Lindstrom, 2011; Mehta et al., 2012; Szabo, Hammarberg, Håkansson, & Södersten, 2001). A variety of vocal function measures have been derived from the ACC during connected speech, but Ps has yet been derived in an ambulatory environment.
This study confirms that the relationship between Ps and ACC magnitude estimates remains strong across multiple pitch contexts, which is a promising evidence that Ps could be measured with an ACC in connected speech contexts and during natural voicing tasks of daily life, providing further insight to how Ps changes over the course of a day, week, or during specific voicing scenarios. Ambulatory Ps measurement would lend itself to in-field estimates of laryngeal resistance and vocal efficiency (requiring simultaneous measurement of acoustic SPL and flow), which would be valuable information to clinicians, as the measures would provide a more comprehensive view of a patient's voice that could be used to better understand, diagnose, treat, or prevent voice disorders.
Future Directions
Results of this study confirmed that the relationship between ACC magnitude and Ps is participant specific, as seen in Fryd et al. (2016). Individual differences such as neck skin morphology, laryngeal anatomy, and glottal configuration are assumed to account for some of this between-participants variability. Individual-specific calibrations are expected to be necessary to track changes over time. Data can be used to further develop this measurement method, with a goal of monitoring patients with voice disorders during running speech in natural speaking environments.
Future work could employ other vocal function measures to detect the presence and degree of nonmodal phonatory characteristics, so that ACC-based estimates of Ps can be made in both healthy and patient populations. Multiple regression techniques may prove useful to improve ACC-based predictions of Ps when modal and nonmodal phonation is exhibited by an individual. Distinguishing among voice modes and vocal pathologies is hypothesized to be crucial to obtaining accurate ACC-based estimates of Ps that employ more than just simple magnitude metrics. Other ACC-based measures are hypothesized to aid in characterizing nonmodal characteristics, such as CPP, that have been shown to correlate highly between acoustic and ACC signal domains (Mehta et al., 2016).
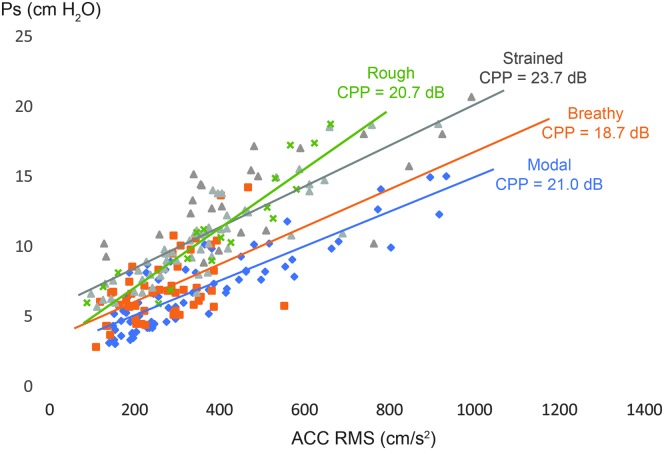
Figure 5 plots the Ps–ACC magnitude regression lines per phonatory condition for female participant F4 for an initial view into how Ps prediction could be improved using measures that can detect nonmodal phonatory characteristics. As expected, significant relationships between ACC magnitude and Ps were found for modal, breathy, strained, and rough voice qualities, whose linear regression equations exhibited similar slopes but different intercepts (rough phonation exhibited more variability in slope). The mean CPP within the modal, breathy, strained, and rough trials were 21.0, 18.7, 23.7, and 20.7 dB, respectively. It is possible that incorporating CPP into the linear regression equation would help to minimize the error in predicting Ps. Other potential metrics sensitive to voice mode include open quotient (Yokonishi et al., 2016), which could be obtained from ACC-based glottal airflow estimates derived using, for example, impedance-based inverse filtering (Cortés et al., 2018; Zañartu et al., 2013). Incorporation of additional ACC-based measures may be applied to help delineate different voice modes and characterize pathological glottal conditions that are associated with varying degrees of glottal closure, vocal fold stiffness/tension, and adduction forces.
Figure 5.
Data from the female participant F4 illustrating the potential of cepstral peak prominence (CPP) to characterize nonmodal phonation and, thus, be incorporated in models to improve the accelerometer (ACC)-based prediction of subglottal pressure (Ps). RMS = root-mean-square.
Conclusion
This study assessed the impact of nonmodal phonation on the linear relationship between Ps and ACC magnitude. Overall, the effects of vowel and pitch contexts on the relationship of Ps and ACC magnitude were small/negligible; however, the relationship between Ps and ACC magnitude changed significantly in the presence of nonmodal phonation. Future work is warranted to further characterize nonmodal phonation so that models of Ps can compensate for nonmodal characteristics. A noninvasive, inexpensive, and accurate method for estimating Ps during natural speech has the potential to significantly enhance the study and clinical assessment of voice, particularly for application to ambulatory monitoring and biofeedback as individuals go about their usual activities in home, work, and social settings.
Acknowledgments
This work is supported by National Institute on Deafness and Other Communication Disorders Grants R21 DC015877 awarded to Daryush Mehta and P50 DC015446 awarded to Bob Hillman. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors thank Bob Hillman for his mentorship and contributions to the project and Olivia Murton, AJ Ortiz, Sarah DeRosa, Jarrad Van Stan, Víctor Espinoza, and Matías Zañartu for their contributions and support.
Appendix
Linear Mixed-Effects Models Without Log-Transformation of Signal Measures
Tables A1, A2, and A3 mirror Tables 2, 3, and 4, respectively, using the analysis of nontransformed subglottal pressure and accelerometer signal magnitude data. These tables provide estimates of the intercept and slope for the linear mixed-effects models to allow for interpretation of the intercepts in terms of cm H2O.
Table A1.
Linear mixed-effects Model 1 results quantifying the impact of nonmodal phonation on the linear relationship between subglottal pressure and accelerometer (ACC) signal magnitude.
| Condition | Estimate ± SE | Estimate ± SE wrt modal | df | p | r |
|---|---|---|---|---|---|
| Females | |||||
| Intercept (cm H2O) | |||||
| Modal | 2.60 ± 0.69 | — | — | — | — |
| Breathy | — | 2.80 ± 0.24 | 1981.61 | < .001* | .25 |
| Strained | — | 1.88 ± 0.26 | 1981.22 | < .001* | .16 |
| Rough | — | 5.42 ± 0.28 | 1988.90 | < .001* | .40 |
| Slope ([cm H2O]/[cm/s2]) | |||||
| ACC × Modal | 14.11 ± 2.47 | — | — | — | — |
| ACC × Breathy | — | −2.43 ± 0.42 | 1975.14 | < .001* | .13 |
| ACC × Strained | — | −0.61 ± 0.40 | 1976.76 | .13 | .03 |
| ACC × Rough | — | −1.05 ± 0.37 | 1984.95 | .01* | .06 |
| Males | |||||
| Intercept (cm H2O) | |||||
| Modal | 2.55 ± 0.51 | — | — | — | — |
| Breathy | — | 1.25 ± 0.29 | 1080.77 | < .001* | .13 |
| Strained | — | 4.82 ± 0.31 | 1084.24 | < .001* | .43 |
| Rough | — | 8.65 ± 0.30 | 1076.40 | < .001* | .66 |
| Slope ([cm H2O]/[cm/s2]) | |||||
| ACC × Modal | 25.73 ± 11.09 | — | — | — | — |
| ACC × Breathy | — | −0.42 ± 1.38 | 1078.53 | .44 | .02 |
| ACC × Strained | — | 1.38 ± 0.40 | 1080.93 | .03 | .07 |
| ACC × Rough | — | −0.40 ± 0.00 | 1077.05 | .49 | .02 |
Note. For the modal phonatory reference condition, the estimates of intercept and slope are shown. For the nonmodal phonatory conditions, the estimates of intercepts and slopes are shown with reference to (wrt) those for the modal phonatory condition. The standard error (SE), degrees of freedom (df), p value, and effect size (r) are reported for each estimate. All slope values were multiplied by 1,000 for readability. The values in the breathy, rough, and strained estimate columns are in reference to the associated modal estimates. Em dashes indicate data not applicable.
p < .01.
Table A2.
Linear mixed-effects Model 2 results quantifying the impact of pitch level on the linear relationship between subglottal pressure and accelerometer (ACC) signal magnitude within each phonatory condition.
| Condition | Estimate ± SE | Estimate ± SE wrt Comf | df | p | r |
|---|---|---|---|---|---|
| Females | |||||
| Modal | |||||
| Intercept (cm H2O) | |||||
| Comf | 2.87 ± 0.72 | — | — | — | — |
| High | — | 0.99 ± 0.26 | 4916.54 | < .001* | .05 |
| Low | — | −0.57 ± 0.25 | 4917.27 | .02 | .03 |
| Slope ([cm H2O]/[cm/s2]) | |||||
| ACC × Comf | 13.44 ± 2.15 | — | — | — | — |
| ACC × High | — | −2.13 ± 0.40 | 4916.40 | < .001* | .08 |
| ACC × Low | — | 0.48 ± 0.56 | 4916.42 | .39 | .01 |
| Breathy | |||||
| Intercept (cm H2O) | |||||
| Comf | 5.64 ± 0.25 | — | — | — | — |
| High | — | 0.17 ± 0.38 | 4915.25 | .66 | .08 |
| Low | — | 0.29 ± 0.37 | 4915.80 | .42 | .01 |
| Slope ([cm H2O]/[cm/s2]) | |||||
| ACC × Comf | 11.20 ± 0.43 | — | — | — | — |
| ACC × High | — | 0.39 ± 0.59 | 4915.29 | .66 | .01 |
| ACC × Low | — | −2.35 ± 0.79 | 4915.66 | .42 | .04 |
| Strained | |||||
| Intercept (cm H2O) | |||||
| Comf | 4.60 ± 0.26 | — | — | — | — |
| High | — | −0.55 ± 0.38 | 4916.52 | .15 | .02 |
| Low | — | 0.26 ± 0.37 | 4916.23 | .49 | .01 |
| Slope ([cm H2O]/[cm/s2]) | |||||
| ACC × Comf | 13.06 ± 0.41 | — | — | — | — |
| ACC × High | — | 1.98 ± 0.54 | 4915.39 | < .001* | .05 |
| ACC × Low | — | 1.93 ± 0.75 | 4915.28 | .012* | .04 |
| Males | |||||
| Modal | |||||
| Intercept (cm H2O) | |||||
| Comf | 2.84 ± 0.44 | — | — | — | — |
| High | — | −0.52 ± 0.28 | 2773.70 | .07 | .03 |
| Low | — | 1.78 ± 0.25 | 2776.71 | < .001* | .13 |
| Slope ([cm H2O]/[cm/s2]) | |||||
| ACC × Comf | 28.58 ± 15.71 | — | — | — | — |
| ACC × High | — | 1.58 ± 0.47 | 2771.49 | .02 | .03 |
| ACC × Low | — | −2.29 ± 0.54 | 2770.57 | .04 | .05 |
| Breathy | |||||
| Intercept (cm H2O) | |||||
| Comf | 4.26 ± 0.28 | — | — | — | — |
| High | — | 1.01 ± 0.42 | 2771.49 | .02 | .05 |
| Low | — | −0.83 ± 0.41 | 2770.57 | .04 | .04 |
| Slope ([cm H2O]/[cm/s2]) | |||||
| ACC × Comf | 27.76 ± 0.54 | — | — | — | — |
| ACC × High | — | −2.00 ± 0.74 | 2770.88 | .01* | .05 |
| ACC × Low | — | 4.22 ± 1.09 | 2771.17 | < .001* | .07 |
| Strained | |||||
| Intercept (cm H2O) | |||||
| Comf | 7.69 ± 0.28 | — | — | — | — |
| High | — | 0.73 ± 0.42 | 2770.32 | < .001* | .03 |
| Low | — | −1.99 ± 0.40 | 2774.34 | .09 | .09 |
| Slope ([cm H2O]/[cm/s2]) | |||||
| ACC × Comf | 30.34 ± 0.61 | — | — | — | — |
| ACC × High | — | −1.40 ± 0.82 | 2771.72 | .09 | .03 |
| ACC × Low | — | −0.41 ± 0.93 | 2774.52 | .66 | .01 |
Note. For the comfortable pitch (Comf) reference condition within each phonatory condition, the estimates of intercept and slope are shown. For the high- and low-pitch conditions, the estimates of intercept and slope are shown with reference to (wrt) those for the comfortable pitch category within each phonatory condition. The standard error (SE), degrees of freedom (df), p value, and effect size (r) are reported for each estimate. Em dashes indicate data not applicable. All slope values were multiplied by 1,000 for readability. The values in the high- and low-pitch columns are in reference to the associated comfortable pitch estimates within each phonatory condition.
p < .0125.
Table A3.
Linear mixed-effects Model 3 results quantifying the impact of vowel on the linear relationship between subglottal pressure and accelerometer (ACC) signal magnitude.
| Condition | Estimate ± SE | Estimate ± SE wrt /a/ | df | p | r |
|---|---|---|---|---|---|
| Females | |||||
| Intercept (cm H2O) | |||||
| /a/ | 3.01 ± 0.20 | — | — | — | — |
| /i/ | — | 0.31 ± 0.09 | 4936.55 | < .001* | .05 |
| /u/ | — | 0.48 ± 0.09 | 4948.54 | < .001* | .08 |
| Slope ([cm H2O]/[cm/s2]) | |||||
| ACC × /a/ | 13.05 ± 2.23 | — | — | — | — |
| ACC × /i/ | — | 0.17 ± 0.15 | 4960.00 | .27 | .02 |
| ACC × /u/ | — | −0.07 ± 0.15 | 4959.39 | .62 | .01 |
| Males | |||||
| Intercept (cm H2O) | |||||
| /a/ | 3.30 ± 0.58 | — | — | — | — |
| /i/ | — | 0.81 ± 0.09 | 3331.88 | < .001* | .15 |
| /u/ | — | 0.83 ± 0.09 | 3330.21 | < .001* | .17 |
| Slope ([cm H2O]/[cm/s2]) | |||||
| ACC × /a/ | 23.18 ± 9.83 | — | — | — | — |
| ACC × /i/ | — | −0.98 ± 0.18 | 3332.38 | < .001* | .09 |
| ACC × /u/ | — | −1.87 ± 0.17 | 3332.40 | < .001* | .19 |
Note. For the vowel /a/ reference condition, the estimates of intercept and slope are shown. For the vowels /i/ and /u/, the estimates of intercepts and slopes are shown with reference to (wrt) those for vowel /a/. The standard error (SE), degrees of freedom (df), p value, and effect size (r) are reported for each estimate. Em dashes indicate data not applicable. All slope values were multiplied by 1,000 for readability. The values in the /i/ and /u/ estimate columns are in reference (±) to the modal estimate values to the left. The values in the /i/ and /u/ estimate columns are in reference to the associated /a/ estimate values.
p < .0125.
Figure A1.
Regression lines derived from linear mixed-effects Model 1 for estimating subglottal pressure (Ps) using accelerometer (ACC) root-mean-square (RMS) magnitude for the modal, breathy, strained, and rough phonatory conditions within the (A) female and (B) male groups.
Funding Statement
This work is supported by National Institute on Deafness and Other Communication Disorders Grants R21 DC015877 awarded to Daryush Mehta and P50 DC015446 awarded to Bob Hillman. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- Baggott C. D., Yuen A. K., Hoffman M. R., Zhou L., & Jiang J. J. (2007). Estimating subglottal pressure via airflow redirection. The Laryngoscope, 117(8), 1491–1495. [DOI] [PubMed] [Google Scholar]
- Björklund S., & Sundberg J. (2016). Relationship between subglottal pressure and sound pressure level in untrained voices. Journal of Voice, 30(1), 15–20. [DOI] [PubMed] [Google Scholar]
- Boersma P., & Weenink D. (2013). Praat: Doing phonetics by computer (Version 5.3.39) [Computer program]. Retrieved from https://www.praat.org [Google Scholar]
- Bottalico P., & Astolfi A. (2012). Investigations into vocal doses and parameters pertaining to primary school teachers in classrooms. The Journal of the Acoustical Society of America, 131(4), 2817–2827. [DOI] [PubMed] [Google Scholar]
- Bottalico P., Graetzer S., Astolfi A., & Hunter E. J. (2017). Silence and voicing accumulations in Italian primary school teachers with and without voice disorders. Journal of Voice, 31(2), 260.e11–260.e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buekers R., Bierens E., Kingma H., & Marres E. (1995). Vocal load as measured by the voice accumulator. Folia Phoniatrica et Logopaedica, 47(5), 252–261. [DOI] [PubMed] [Google Scholar]
- Carroll T., Nix J., Hunter E., Emerich K., Titze I., & Abaza M. (2006). Objective measurement of vocal fatigue in classical singers: A vocal dosimetry pilot study. Otolaryngology—Head & Neck Surgery, 135(4), 595–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carullo A., Vallan A., & Astolfi A. (2013). A low-cost platform for voice monitoring. Proceedings of the IEEE International Instrumentation and Measurement Technology Conference, Minneapolis, MN, 67–72. [Google Scholar]
- Castellana A., Carullo A., Corbellini S., & Astolfi A. (2018). Discriminating pathological voice from healthy voice using cepstral peak prominence smoothed distribution in sustained vowel. IEEE Transactions on Instrumentation and Measurement, 67(3), 646–654. [Google Scholar]
- Chang A., & Karnell M. P. (2004). Perceived phonatory effort and phonation threshold pressure across a prolonged voice loading task: A study of vocal fatigue. Journal of Voice, 18(4), 454–466. [DOI] [PubMed] [Google Scholar]
- Cheyne H. A., Hanson H. M., Genereux R. P., Stevens K. N., & Hillman R. E. (2003). Development and testing of a portable vocal accumulator. Journal of Speech, Language, and Hearing Research, 46(6), 1457–1467. [DOI] [PubMed] [Google Scholar]
- Cohen J. (1988). Statistical power analysis for the behavioral sciences. Hillsdale, NJ: Erlbaum. [Google Scholar]
- Coleman R. F. (1988). Comparison of microphone and neck-mounted accelerometer monitoring of the performing voice. Journal of Voice, 2(3), 200–205. [Google Scholar]
- Colton R. H., Casper J. K., & Leonard R. J. (2006). Understanding voice problems: A physiological perspective for diagnosis and treatment. Baltimore, MD: Lippincott Williams & Wilkins. [Google Scholar]
- Cortés J. P., Espinoza V. M., Ghassemi M., Mehta D. D., Van Stan J. H., Hillman R. E., … Zañartu M. (2018). Ambulatory assessment of phonotraumatic vocal hyperfunction using glottal airflow measures estimated from neck-surface acceleration. PLOS ONE, 13(12), e0209017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinoza V. M., Zañartu M., Van Stan J. H., Mehta D. D., & Hillman R. E. (2017). Glottal aerodynamic measures in women with phonotraumatic and nonphonotraumatic vocal hyperfunction. Journal of Speech, Language, and Hearing Research, 60(8), 2159–2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryd A. S., Van Stan J. H., Hillman R. E., & Mehta D. D. (2016). Estimating subglottal pressure from neck-surface acceleration during normal voice production. Journal of Speech, Language, and Hearing Research, 59(6), 1335–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerratt B. R., & Kreiman J. (2001). Toward a taxonomy of nonmodal phonation. Journal of Phonetics, 29(4), 365–381. [Google Scholar]
- Gillespie A. I., Gartner-Schmidt J., Rubinstein E. N., & Abbott K. V. (2013). Aerodynamic profiles of women with muscle tension dysphonia/aphonia. Journal of Speech, Language, and Hearing Research, 56(2), 481–488. [DOI] [PubMed] [Google Scholar]
- Gilman M., Petty B., Maira C., Pethan M., Wang L., Hapner E. R., & Johns M. M. III (2017). Aerodynamic patterns in patients with voice disorders: A retrospective study. Journal of Voice, 31(5), 545–549. [DOI] [PubMed] [Google Scholar]
- Grillo E. U., & Verdolini K. (2008). Evidence for distinguishing pressed, normal, resonant, and breathy voice qualities by laryngeal resistance and vocal efficiency in vocally trained subjects. Journal of Voice, 22(5), 546–552. [DOI] [PubMed] [Google Scholar]
- Gunter H. E., Howe R. D., Zeitels S. M., Kobler J. B., & Hillman R. E. (2005). Measurement of vocal fold collision forces during phonation: Methods and preliminary data. Journal of Speech, Language, and Hearing Research, 48(3), 567–576. [DOI] [PubMed] [Google Scholar]
- Hartl D. M., Hans S., Vaissière J., Riquet M., & Brasnu D. F. (2001). Objective voice quality analysis before and after onset of unilateral vocal fold paralysis. Journal of Voice, 15(3), 351–361. [DOI] [PubMed] [Google Scholar]
- Hillman R. E., Heaton J. T., Masaki A., Zeitels S. M., & Cheyne H. A. (2006). Ambulatory monitoring of disordered voices. Annals of Otology, Rhinology & Laryngology, 115(11), 795–801. [DOI] [PubMed] [Google Scholar]
- Hillman R. E., Holmberg E. B., Perkell J. S., Walsh M., & Vaughan C. (1989). Objective assessment of vocal hyperfunction: An experimental framework and initial results. Journal of Speech, Language, and Hearing Research, 32(2), 373–392. [DOI] [PubMed] [Google Scholar]
- Holmberg E. B., Doyle P., Perkell J. S., Hammarberg B., & Hillman R. E. (2003). Aerodynamic and acoustic voice measurements of patients with vocal nodules: Variation in baseline and changes across voice therapy. Journal of Voice, 17(3), 269–282. [DOI] [PubMed] [Google Scholar]
- Holmberg E. B., Hillman R. E., & Perkell J. S. (1988). Glottal airflow and transglottal air pressure measurements for male and female speakers in soft, normal, and loud voice. The Journal of the Acoustical Society of America, 84(2), 511–529. [DOI] [PubMed] [Google Scholar]
- Holmberg E. B., Hillman R. E., & Perkell J. S. (1989). Glottal airflow and transglottal air pressure measurements for male and female speakers in low, normal, and high pitch. Journal of Voice, 3(4), 294–305. [DOI] [PubMed] [Google Scholar]
- Holmberg E. B., Hillman R. E., Perkell J. S., & Gress C. (1994). Relationships between intra-speaker variation in aerodynamic measures of voice production and variation in SPL across repeated recordings. Journal of Speech and Hearing Research, 37(3), 484–495. [DOI] [PubMed] [Google Scholar]
- KayPENTAX. (2009). Ambulatory phonation monitor: Applications for speech and voice. Lincoln Park, NJ: Author. [Google Scholar]
- Lei Z., Kennedy E., Fasanella L., Li-Jessen N. Y.-K., & Mongeau L. (2019). Discrimination between modal, breathy and pressed voice for single vowels using neck-surface vibration signals. Applied Sciences, 9(7), 1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindstrom F. (2011). VoxLog manual: For efficient voice measurement. Umea, Sweden: Sonovox. [Google Scholar]
- Lindstrom F., Waye K. P., Södersten M., McAllister A., & Ternström S. (2011). Observations of the relationship between noise exposure and preschool teacher voice usage in day-care center environments. Journal of Voice, 25(2), 166–172. [DOI] [PubMed] [Google Scholar]
- Manfredi C., & Kob M. (2009). New trends in voice pathology detection and classification. Biomedical Signal Processing and Control, 3(4), 171–172. [Google Scholar]
- McKenna V. S., Llico A. F., Mehta D. D., Perkell J. S., & Stepp C. E. (2017). Magnitude of neck-surface vibration as an estimate of subglottal pressure during modulations of vocal effort and intensity in healthy speakers. Journal of Speech, Language, and Hearing Research, 60(12), 3404–3416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta D. D., Van Stan J. H., & Hillman R. E. (2016). Relationships between vocal function measures derived from an acoustic microphone and a subglottal neck-surface accelerometer. IEEE/ACM Transactions on Audio, Speech and Language Processing, 24(4), 659–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta D. D., Van Stan J. H., Zañartu M., Ghassemi M., Guttag J. V., Espinoza V. M., … Hillman R. E. (2015). Using ambulatory voice monitoring to investigate common voice disorders: Research update. Frontiers in Bioengineering and Biotechnology, 3(155), 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta D. D., Zañartu M., Feng S. W., Cheyne H. A., & Hillman R. E. (2012). Mobile voice health monitoring using a wearable accelerometer sensor and a smartphone platform. IEEE Transactions on Biomedical Engineering, 59(11), 3090–3096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nacci A., Fattori B., Mancini V., Panicucci E., Ursino F., Cartaino F. M., & Berrettini S. (2013). The use and role of the ambulatory phonation monitor (APM) in voice assessment. Acta Otorhinolaryngologica Italica, 33(1), 49–55. [PMC free article] [PubMed] [Google Scholar]
- Patel R. R., Awan S. N., Barkmeier-Kraemer J., Courey M., Deliyski D., Eadie T., … Hillman R. E. (2018). Recommended protocols for instrumental assessment of voice: American Speech-Language-Hearing Association expert panel to develop a protocol for instrumental assessment of vocal function. American Journal of Speech-Language Pathology, 27(3), 887–905. [DOI] [PubMed] [Google Scholar]
- Popolo P. S., Švec J. G., & Titze I. R. (2005). Adaptation of a pocket PC for use as a wearable voice dosimeter. Journal of Speech, Language, and Hearing Research, 48(4), 780–791. [DOI] [PubMed] [Google Scholar]
- Porter H. C. (1963). Extraction of pitch from the trachea. Retrieved from https://apps.dtic.mil/dtic/tr/fulltext/u2/401920.pdfa
- Ramig L. O., & Dromey C. (1996). Aerodynamic mechanisms underlying treatment-related changes in vocal intensity in patients with Parkinson disease. Journal of Speech and Hearing Research, 39(4), 798–807. [DOI] [PubMed] [Google Scholar]
- Rosenthal A. L., Lowell S. Y., & Colton R. H. (2014). Aerodynamic and acoustic features of vocal effort. Journal of Voice, 28(2), 144–153. [DOI] [PubMed] [Google Scholar]
- Rothenberg M. (1973). A new inverse-filtering technique for deriving the glottal air flow waveform during voicing. The Journal of the Acoustical Society of America, 53(6), 1632–1645. [DOI] [PubMed] [Google Scholar]
- Schutte H. K. (1980). The efficiency of voice production. Gröningen, the Netherlands: State University Hospital. [Google Scholar]
- Smitheran J. R., & Hixon T. J. (1981). A clinical method for estimating laryngeal airway resistance during vowel production. Journal of Speech and Hearing Disorders, 46(2), 138–146. [DOI] [PubMed] [Google Scholar]
- Speyer R. (2008). Effects of voice therapy: A systematic review. Journal of Voice, 22(5), 565–580. [DOI] [PubMed] [Google Scholar]
- Stevens K. N., Kalikow D. N., & Willemain T. R. (1975). A miniature accelerometer for detecting glottal waveforms and nasalization. Journal of Speech and Hearing Research, 18(3), 594–599. [DOI] [PubMed] [Google Scholar]
- Sugimoto T., & Hiki S. (1960). Extraction of the pitch of a voice from the vibration of the outer skin of the trachea. Journal of the Acoustical Society of Japan, 1(4), 291–293. [Google Scholar]
- Švec J. G., Popolo P. S., & Titze I. R. (2003). Measurement of vocal doses in speech: Experimental procedure and signal processing. Logopedics Phoniatrics Vocology, 28(4), 181–192. [DOI] [PubMed] [Google Scholar]
- Švec J. G., Titze I. R., & Popolo P. S. (2005). Estimation of sound pressure levels of voiced speech from skin vibration of the neck. The Journal of the Acoustical Society of America, 117(3), 1386–1394. [DOI] [PubMed] [Google Scholar]
- Szabo A., Hammarberg B., Håkansson A., & Södersten M. (2001). A voice accumulator device: Evaluation based on studio and field recordings. Logopedics Phoniatrics Vocology, 26(3), 102–117. [DOI] [PubMed] [Google Scholar]
- Titze I. R. (1989). On the relation between subglottal pressure and fundamental frequency in phonation. The Journal of the Acoustical Society of America, 85(2), 901–906. [DOI] [PubMed] [Google Scholar]
- Titze I. R. (1992). Vocal efficiency. Journal of Voice, 6(2), 135–138. [Google Scholar]
- Titze I. R. (2008). Nonlinear source–filter coupling in phonation: Theory. The Journal of the Acoustical Society of America, 123(5), 2733–2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titze I. R. (2013). Quantifying vocal efficiency and economy—How can computation augment clinical assessment? Proceedings of Meetings on Acoustics, 19(1), 060244. [Google Scholar]
- Titze I. R., & Hunter E. J. (2015). Comparison of vocal vibration-dose measures for potential-damage risk criteria. Journal of Speech, Language, and Hearing Research, 58(5), 1425–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titze I. R., Maxfield L., & Palaparthi A. (2016). An oral pressure conversion ratio as a predictor of vocal efficiency. Journal of Voice, 30(4), 398–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titze I. R., Švec J. G., & Popolo P. S. (2003). Vocal dose measures: Quantifying accumulated vibration exposure in vocal fold tissues. Journal of Speech, Language, and Hearing Research, 46(4), 919–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Stan J. H., Mehta D. D., Zeitels S. M., Burns J. A., Barbu A. M., & Hillman R. E. (2015). Average ambulatory measures of sound pressure level, fundamental frequency, and vocal dose do not differ between adult females with phonotraumatic lesions and matched control subjects. Annals of Otology, Rhinology & Laryngology, 124(11), 864–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokonishi H., Imagawa H., Sakakibara K., Yamauchi A., Nito T., Yamasoba T., & Tayama N. (2016). Relationship of various open quotients with acoustic property, phonation types, fundamental frequency, and intensity. Journal of Voice, 30(2), 145–157. [DOI] [PubMed] [Google Scholar]
- Zañartu M., Ho J. C., Mehta D. D., Hillman R. E., & Wodicka G. R. (2013). Subglottal impedance-based inverse filtering of voiced sounds using neck surface acceleration. IEEE Transactions on Audio, Speech, and Language Processing, 21(9), 1929–1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeitels S. M., Hillman R. E., Bunting G. W., & Vaughn T. (1997). Reinke's edema: Phonatory mechanisms and management strategies. Annals of Otology, Rhinology & Laryngology, 106(7 Pt 1), 533–543. [DOI] [PubMed] [Google Scholar]
- Zeitels S. M., Hillman R. E., Desloge R. B., & Bunting G. A. (1999). Cricothyroid subluxation: A new innovation for enhancing the voice with laryngoplastic phonosurgery. Annals of Otology, Rhinology & Laryngology, 108(12), 1126–1131. [DOI] [PubMed] [Google Scholar]
- Zeitels S. M., Hillman R. E., Desloge R., Mauri M., & Doyle P. (2002). Phonomicrosurgery in singers and performing artists: Treatment outcomes, management theories, and future directions. Annals of Otology, Rhinology & Laryngology, 111, 21–40. [DOI] [PubMed] [Google Scholar]
- Zeitels S. M., Hillman R. E., Franco R. A., & Bunting G. W. (2002). Voice and treatment outcome from phonosurgical management of early glottic cancer. Annals of Otology, Rhinology & Laryngology, 111(12), 3–20. [DOI] [PubMed] [Google Scholar]
- Zeitels S. M., Hochman I., & Hillman R. E. (1998). Adduction arytenopexy: A new procedure for paralytic dysphonia with implications for implant medialization. Annals of Otology, Rhinology & Laryngology, 107(9), 2–24. [PubMed] [Google Scholar]
- Zeitels S. M., Lopez-Guerra G., Burns J. A., Lutch M., Friedman A. M., & Hillman R. E. (2009). Microlaryngoscopic and office-based injection of bevacizumab (Avastin) to enhance 532-nm pulsed KTP laser treatment of glottal papillomatosis. Annals of Otology, Rhinology & Laryngology, 118(9), 1–24. [DOI] [PubMed] [Google Scholar]