Abstract
Purpose
Valid, reliable, and efficient patient-reported outcome measures are needed to quantify quality of life (QOL) outcomes after cochlear implantation to supplement information obtained from performance-based outcomes. We previously developed the Cochlear Implant Quality of Life (CIQOL) item bank to serve as the source of items for subsequent instruments. This study reports the development and psychometric properties for 2 of these new instruments, the CIQOL-35 Profile and the CIQOL-10 Global.
Method
Cochlear implant (CI) users referred from the CIQOL Development Consortium (n = 371), consisting of 20 CI centers across the United States, provided responses to the 81-item CIQOL item bank, which are grouped into 6 QOL domains (communication, emotional, entertainment, environment, listening effort, and social). Responses to the 81 CIQOL items were analyzed using item response theory to determine individual item difficulty, discrimination, and model fit to select the set of items for the profile instrument and global measure that would optimize their measurement characteristics.
Results
The 35-item CIQOL-35 Profile instrument assesses outcomes represented in the 6 domains of the CIQOL final item pool. The 10-item CIQOL-10 Global measure produces a single, overall QOL score. After ensuring the upper and lower ends of the item difficulty continuum were represented (item difficulty range: −2.48 to 2.47), the items with the highest discrimination ability for each domain were selected for the CIQOL-35 Profile instrument (discrimination range: 0.67–1.37). Items were selected for the CIQOL-10 Global measure in a similar manner.
Conclusion
The CIQOL-35 Profile and CIQOL-10 Global instruments provide psychometrically sound and efficient measures that can be used to assess QOL in adult CI users in both clinical and research settings.
Supplemental Material
Hearing loss has a dramatic impact that extends well beyond receptive communication ability. Multiple studies have shown that social isolation, emotional impact, and mental and listening effort that are a consequence of hearing loss decrease an individual's quality of life (QOL; Davis et al., 2016; Mick, Kawachi, & Lin, 2014; Zekveld, Kramer, & Festen, 2010). The effect of hearing loss on QOL is especially significant for adults with more severe hearing loss (Nirmalasari et al., 2017). Cochlear implantation is the standard treatment for those with bilateral moderate–profound hearing loss who no longer receive significant benefit from hearing aids. Although cochlear implantation is thought to provide benefits beyond communication, such as emotional state and social engagement, changes in speech recognition outcomes with cochlear implantation have been the primary research focus. Moreover, cochlear implant (CI) outcomes assessed using speech recognition (Adunka, Gantz, Dunn, Gurgel, & Buchman, 2018) correlate poorly with user self-reports of real-world communication ability and QOL (Capretta & Moberly, 2016; McRackan, Bauschard, Hatch, Franko-Tobin, Droghini, Nguyen, et al., 2018; McRackan, Bauschard, Hatch, Franko-Tobin, Droghini, Velozo, et al., 2018).
One reason for the lack of focus on outcomes beyond speech recognition is the time required to measure these outcomes. This highlights the need for valid, time-efficient, and precise instruments to measure QOL in adult patients with CIs. QOL instruments are widely used in other clinical populations to understand the effect of a medical intervention on a patient's life and, as they are largely based on patient report, are classified as patient-reported outcome measures (PROMs). Assessing QOL allows direct input from affected patients about how disease processes and how interventions impact their lives across several domains. A direct measure of patient report for patients with CI supplements the information obtained from performance-based outcome measures, such as speech recognition scores, and gives patients a means to report their outcomes using a validated tool.
One method by which researchers can develop valid, reliable, and efficient PROMs is through the use of item response theory (IRT), the modern standard for PROM development. The advantages of IRT over classical test theory are discussed in McRackan, Hand, Velozo, Dubno, & Cochlear Implant Quality of Life (CIQOL) Development Consortium, 2019 and are briefly reviewed here. First, instruments developed with IRT are considered to have psychometric properties that are sample and test independent (Prieto, Alonso, & Lamarca, 2003), whereas classical test theory is based on observed and true scores, which are sample dependent. IRT is focused on measuring an underlying latent trait (referred to as person ability or person measure), which is independent of test difficulty. Second, IRT is focused on item-level, rather than test-level, psychometrics. IRT analyses determine the characteristics of each item and the utility for inclusion of each item in subsequent instruments. Items can then be selected for each instrument based on highest discrimination across the ability range and best match between item difficulty and subject ability. This process generates optimized instruments with maximized capacity to differentiate individuals across a greater range of the latent trait—termed precision (Rose, Bjorner, Becker, Fries, & Ware, 2008). Third, relative to classical test theory, IRT has a more strict set of assumptions that must be met before analysis can be performed (Reeve et al., 2007), including (a) items can only contribute to one domain of QOL (unidimensionality), (b) responses to each item are unrelated to responses to other items (local independence), and (c) items fit the IRT measurement model (item fit). Confirmatory factor analysis (CFA) is used to confirm unidimensionality and local independence. Such strict preliminary analysis is not required before analysis using classical test theory.
CIQOL Item Bank Development
To address the critical need for PROMs for adult CI users, we first developed the 81-item CIQOL item bank consisting of six domain-specific CIQOL item banks (McRackan, Bauschard, Hatch, Franko-Tobin, Droghini, Nguyen, et al., 2018) following the Patient-Reported Outcomes Measurement Information System (PROMIS) and Consensus-Based Standards for the Selection of Health Status Measurement Instruments (COSMIN) guidelines (Mokkink et al., 2010; PROMIS, 2013). To briefly summarize these steps, a systematic literature search was first conducted to identify PROMs previously used in the adult CI user population (McRackan, Bauschard, Hatch, Franko-Tobin, Droghini, Nguyen, et al., 2018; McRackan, Bauschard, Hatch, Franko-Tobin, Droghini, Velozo, et al., 2018). Information found through the literature search guided the development of a focus group protocol for patients with CI. Central and minor themes identified from the focus group were used to generate initial items, consisting of 101 items grouped into seven hypothesized domain constructs: communication, emotional, entertainment, environment, independence, listening effort, and social (McRackan et al., 2017). To test the psychometric properties of the initial item pool, responses from 371 subjects were analyzed with CFA and IRT.
For the CIQOL item bank, three items were removed based on local dependence, and six items misfit the IRT model. One domain was removed (independence; 11 items) due to poor psychometric properties and misfit to the IRT model. Thus, the final CIQOL item banks consist of 81 items in six domains: communication (28 items), emotional (15 items), entertainment (eight items), environment (six items), listening effort (eight items), and social (16 items). These represent the six domain-specific CIQOL item banks (McRackan, Hand, Velozo, Dubno, & CIQOL Development Consortium, 2019).
None of the existing hearing-related and CI-specific PROMs has followed the rigorous PROMIS and COSMIN guidelines to establish face and construct validity. In fact, the most commonly used PROMs in the adult CI user population were developed and validated primarily for individuals with mild-to-moderate hearing loss and hearing aid users; these include the Hearing Handicap Inventory for Adults/Elderly (Newman, Weinstein, Jacobson, & Hug, 1990; Ventry & Weinstein, 1982); the Speech, Spatial and Qualities of Hearing Scale (Gatehouse & Noble, 2004); and the Abbreviated Profile of Hearing Aid Benefit (Cox & Alexander, 1995). By not including CI users or those with more severe hearing loss in the development process, researchers and clinicians cannot be confident that these PROMs accurately and reliably reflect the themes and constructs they purport to measure for adult CI users. Our CI focus group data provide preliminary support for this as themes and domains were identified that have not been included in previous hearing and CI-specific PROMs (McRackan, Hand, Velozo, Dubno, & CIQOL Development Consortium, 2018; McRackan et al., 2019). Such stakeholder engagement is now considered a standard procedure for PROM development (Mokkink et al., 2010; PROMIS, 2013).
The Nijmegen Cochlear Implant Questionnaire (NCIQ) is by far the most commonly used CI-specific PROM (Hinderink, Krabbe, & Van Den Broek, 2000). It consists of 60 items separated into six domains (basic sound perception, advanced sound perception, speech production, self-esteem, activity, and social interactions). The included items and domains were developed by expert opinion, rather than from information provided by CI users, and therefore do not include certain domains that CI users value as important (Hughes, Hutchings, Rapport, McMahon, & Boisvert, 2018; McRackan et al., 2017). In addition, the NCIQ was validated and tested on 91 Dutch-speaking participants (including 46 controls) from a single clinical site in the Netherlands. As detailed earlier, more rigorous psychometric testing for PROM development has become standard since the NCIQ was developed, which can improve the measurement properties of such instruments. Given this knowledge gap, there is a need to develop and validate a CI-specific PROM using rigorous psychometric techniques and recruitment of CI users from large, multi-institutional samples.
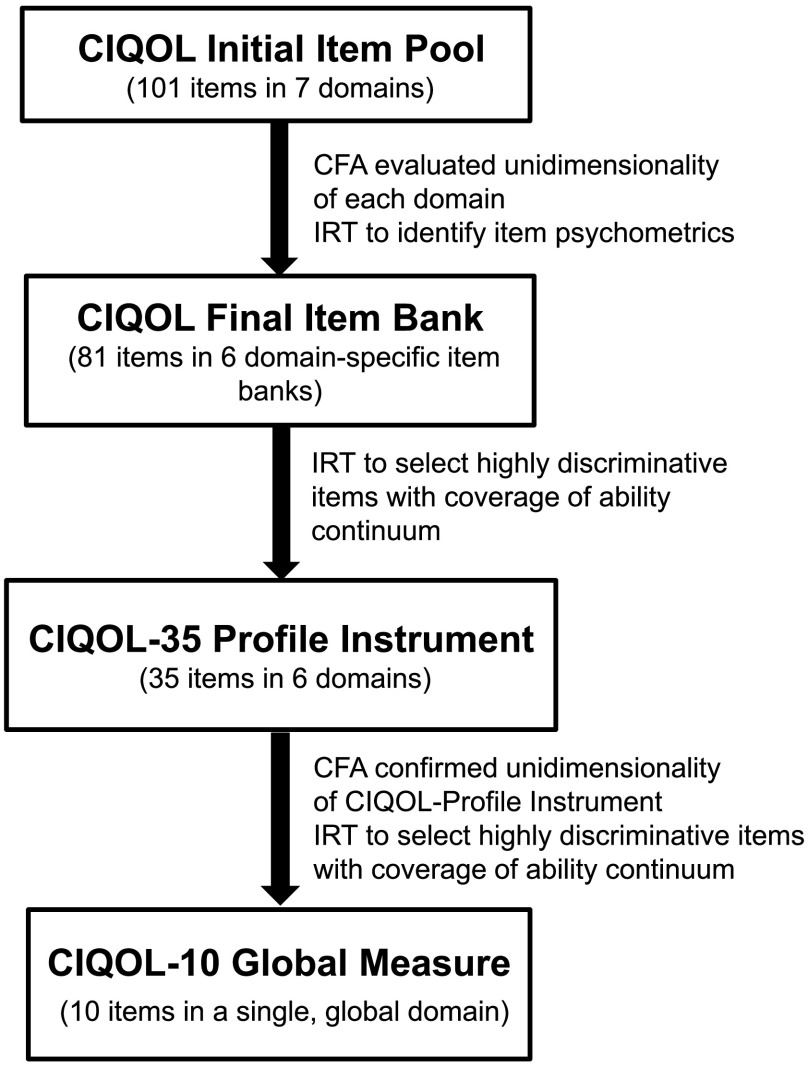
The current study aims to report how results from psychometric analyses guided the development of the new CIQOL instruments. This work represents the first time the rigorous PROMIS and COSMIN guidelines have been followed from item to instrument development in hearing research. The study consisted of three components (see Figure 1). First, we used the results of the CFA and IRT analyses of the CIQOL item bank to develop a profile instrument consisting of 35 items that provides outcomes for each of the six QOL domains (CIQOL-35 Profile). Second, we performed new CFA and IRT analyses on the CIQOL-35 Profile instrument to confirm that it is psychometrically sound. Third, based on these results, we developed a 10-item CIQOL instrument that provides an efficient global assessment of QOL of CI users (CIQOL-10 Global).
Figure 1.
Outline of the CIQOL-35 Profile instrument and the CIQOL-10 Global measure development process. CIQOL = Cochlear Implant Quality of Life; CFA = confirmatory factor analysis; IRT = item response theory.
Method
Subject Recruitment
This study was approved by the institutional review board of the Medical University of South Carolina. The CIQOL Development Consortium was established to enroll a large and diverse sample of CI users with respect to age, sex, CI listening modalities, and communication abilities. The consortium consists of 20 CI centers across the United States that distributed recruitment flyers to CI recipients (electronically and on paper). Interested patients then e-mailed our research team to be enrolled. Subjects must have been 18–89 years of age, a CI user for at least 1 year, and not have received a CI for single-sided deafness.
Data Collection
Data collection methods are described in detail in McRackan, Bauschard, Hatch, Franko-Tobin, Droghini, Nguyen, et al. (2018) and are briefly reviewed here. The secure, web-based data collection platform REDCap (Research Electronic Data Capture) was used for all data collection. Assuming a 60% response rate, questionnaires were sent to the first 500 subjects who contacted the research team. The questionnaire consisted of three sections: (a) subject demographics, (b) hearing and CI history (including speech recognition scores), and (c) the 101-item CIQOL initial item pool. Duration of CI use was calculated based on the date subjects had their first (or only) CI activated. Subjects requested their most recent best aided speech recognition scores from their audiologist and, if available, entered the results into the questionnaire. At least one of these speech recognition scores was available for 236 of the 371 subjects (63.6%). Subjects were not excluded from the current analysis if they were unable to obtain their scores. Finally, subjects provided responses to the 101 CIQOL initial item pool, although only responses to the 81 items in the six CIQOL item banks were used in the current analysis. Item response options used one of the option sets recommended by PROMIS: never, rarely, sometimes, often, or always.
Data Analysis
Following PROMIS guidelines (PROMIS, 2013), the item-level subject responses to the 81 items in the six CIQOL item banks were analyzed with IRT to generate the CIQOL-35 Profile instrument and the CIQOL-10 Global measure (see Figure 1). As described earlier, IRT focuses on the measurement of an underlying latent trait (e.g., communication, emotion) and provides psychometric properties at the person and item level. The person parameter produced by IRT is called person ability, which refers to an individual's score on the underlying latent trait. Item parameters produced by IRT include difficulty, discrimination, and fit statistics. Table 1 provides a description of commonly used person- and item-level and interpretation guidelines. Additional IRT primers that provide more detail on these parameters and IRT in general have been published (Boone, 2016; De Champlain, 2010; Hays, Morales, & Reise, 2000).
Table 1.
Item response theory terminology and interpretation guidelines.
| Term | Description | Interpretation |
|---|---|---|
| Person ability | A person's score on the latent trait, given on the same interval-level scale as item difficulty parameters (Linacre, 2016) | High-ability parameters indicate high QOL, while low ability parameters indicate poor QOL. |
| Item difficulty | The point on the unidimensional continuum of the latent trait at which the highest and lowest categories of the rating scale have equal probabilities of being observed (Linacre, 2016) | High-difficulty parameters indicate an item is less likely to be endorsed at the upper end of the rating scale (i.e., few individuals respond “always” to the item). |
| Item discrimination | The extent to which an individual's response to a single item corresponds with their responses on all items in the domain (Kelley et al., 2002) | High-discrimination parameters indicate a strong correlation between a response to a single item and responses on all items in a domain (e.g., an individual who scores low on an item with high discrimination likely scored low on all items, indicating poor QOL in that domain). |
| Infit | Identifies unexpected patterns of responses to the item by persons whose ability level is well matched with the item difficulty | High-infit/outfit parameters indicate that items show misfit with the IRT model. Items with mean square values of > 1.20 and standardized z values of > 2.0 demonstrate significant misfit to the model (Linacre, 2002). |
| Outfit | Identifies unexpected item response patterns by persons whose ability level is far from the item difficulty (i.e., the item is either very easy or very difficult) |
Note. QOL = quality of life; IRT = item response theory.
CIQOL-35 Profile Instrument
After demonstrating that the six CIQOL item banks represented unidimensional constructs (McRackan, Hand, Velozo, Dubno, & CIQOL Development Consortium, 2019), the six domains were analyzed individually with one-parameter logistic IRT analyses with rating scale models and joint maximum likelihood estimation using WINSTEPS, Version 3.90.0 (Linacre, 2016). Item fit was examined first, as the removal of items that misfit the IRT model from a measure (completed in the development of the six CIQOL item banks) can result in the misfit of other items. Items that demonstrated significant misfit to the IRT model (defined as infit or outfit mean square values of > 1.20 and standardized z values of > 2.0; Wright & Linacre, 1994) were not considered for inclusion in the CIQOL-35 Profile instrument. Next, items in each domain across a range of item difficulties with the highest discrimination parameters were selected. It was decided a priori that five items would be selected from each domain, except for the communication domain that had the largest number of items, from which 10 items would be selected.
CIQOL-10 Global Measure
The CIQOL-10 Global measure provides one score derived from a total of 10 items representing all six CIQOL domains. The items in the CIQOL-10 Global measure were selected from the 35 items in the CIQOL-35 Profile instrument. To ensure that it was psychometrically sound to develop the CIQOL-10 Global measure in this manner, the unidimensionality of the CIQOL-35 Profile instrument was examined first with CFA, as this is a prerequisite for one-parameter IRT models. An ordered-category, single-factor CFA with diagonal weighted least squares estimation was performed using the package “lavaan” in the statistical software R (Rosseel et al., 2017). Multiple types of fit indicators were examined, including those that are reflective of absolute fit, those with parsimony corrections, and comparative fit indicators. Acceptable CFA model fit was defined a priori by standardized root mean square residual (SRMR) < 0.08, root mean square error of approximation (RMSEA) < 0.08, comparative fit index (CFI) > .95, and Tucker–Lewis index (TLI) > 0.95 (Brown, 2015). In addition, to examine the association between items and the latent construct, standardized item factor loadings were examined. Standardized factor loadings of ≥ 0.32 indicated a significant relationship between the item and the latent construct (Tabachnick & Fidell, 2013). Item residual correlations were examined for local dependence (responses to one item are not associated to responses to other items), with correlations > .2 indicating dependence (Pilkonis et al., 2011).
After establishing unidimensionality and removing locally dependent items, a one-parameter logistic IRT analysis was performed using a rating scale model and joint maximum likelihood estimation. Item fit was examined, and items with significant model misfit (mean square values of > 1.20 and standardized z values of > 2.0; Wright & Linacre, 1994) were excluded from consideration for inclusion in the CIQOL-10 Global measure. From the remaining items, 10 items were selected that covered the range of item difficulties and had the highest discrimination parameters. Based on a priori criteria, at least one item from each of the six domains was included in the CIQOL-10 Global measure.
Power Analysis
Sample size needs were determined based on the most sample size–dependent portion of the analysis—the CFA. Sample sizes of 300 are considered conservative for CFA under a variety of sample conditions based on Monte Carlo simulations (MacCallum, Widaman, Zhang, & Hong, 1999). Rasch measurement, a one-parameter model, is the most robust of the IRT models with regard to sample size; a sample size of 150 will result in item calibrations stable within 0.5 logits, with a 99% confidence interval (Linacre, 1994).
Results
Subjects
Of the 500 subjects e-mailed, 371 (74.2%) completed the questionnaires. Subjects represented the full range of age, duration of CI use, speech recognition abilities, and listening modalities of the adult CI user population and used all three CI manufacturers' devices (see Tables 2 and 3; see also McRackan, Bauschard, Hatch, Franko-Tobin, Droghini, Nguyen, et al., 2018, Tables 1, 2, and 3).
Table 2.
Demographic and cochlear implant (CI) characteristics of the study sample.
| Variable | n (%) |
|---|---|
| Sex | |
| Male | 149 (40.2) |
| Female | 222 (59.8) |
| Marital status | |
| Married/domestic partnership | 251 (67.7) |
| Not married/no domestic partnership | 120 (32.3) |
| Combined annual household income | |
| $0–$20,000 | 26 (7.0) |
| $20,001–$50,000 | 63 (16.9) |
| $50,001–$80,000 | 87 (23.4) |
| $80,001–$110,000 | 66 (17.7) |
| > $110,000 | 93 (25.0) |
| Unknown/not reported | 36 (9.7) |
| Highest level of education | |
| Did not complete high school | 3 (0.8) |
| High school graduate or equivalent | 27 (7.3) |
| Some college/trade/technical/vocational training | 109 (29.4) |
| Bachelor's degree | 112 (30.2) |
| Master's degree or higher | 120 (32.3) |
| Employment status | |
| Employed | 160 (43.1) |
| Not employed | 45 (12.1) |
| Retired | 166 (44.7) |
| Residential setting | |
| Urban | 81 (21.8) |
| Suburban | 214 (57.7) |
| Rural | 76 (20.5) |
| U.S. region | |
| West | 89 (24.0) |
| Midwest | 90 (24.3) |
| Northeast | 50 (13.5) |
| South/Southwest | 142 (38.3) |
| CI company | |
| Advanced Bionics | 43 (11.5) |
| Cochlear | 216 (58.2) |
| MED-EL | 112 (30.1) |
| Listening modality | |
| Unilateral CI with no contralateral HA | 87 (23.4) |
| Unilateral CI with contralateral HA | 96 (25.8) |
| Bilateral CI | 188 (50.6) |
| Hybrid/EAS | |
| Yes | 12 (3.2) |
| No | 358 (96.4) |
Note. HA = hearing aid; EAS = Electric Acoustic Stimulation.
Table 3.
Age, hearing, and cochlear implant (CI) characteristics of the study sample.
| Variable | M ± SD | Range | Response rate n (%) |
|---|---|---|---|
| Age (years) | 59.5 ± 14.9 | 19–88 | 371 (100) |
| Duration of hearing loss prior to CI (years) | 27.1 ± 18.4 | 0–80 | 371 (100) |
| Duration of CI use (years) | 7.6 ± 6.5 | 1–33 | 371 (100) |
| AzBio quiet score (%) | 81.2 ± 23.0 | 0–100 | 185 (49.9) |
| AzBio +10 dB SNR score (%) | 64.3 ± 27.5 | 0–100 | 121 (32.6) |
| CNC word score (%) | 69.6 ± 24.4 | 0–100 | 173 (46.6) |
| HINT score (%) | 76.1 ± 30.2 | 0–100 | 78 (21.0) |
Note. SNR = signal-to-noise ratio; CNC = consonant–nucleus–consonant; HINT = Hearing in Noise Test.
Overall, more women were enrolled than men, most were married and did not have children aged < 18 years living in the household. The majority had some education beyond a high school diploma and were employed or retired. Subjects were evenly split among the household income categories, except in the lowest bracket. All regions of the United States were represented, with the South/Southwest having the highest percentage of subjects (38.3%). Individuals from the local institution represented only 2.9% of those who completed the questionnaire.
CIQOL-35 Profile Instrument
Based on the IRT analysis of the domain-specific CIQOL item banks, a total of 16 items (six from the communication domain, three from emotional, one from entertainment, one from environment, one from listening effort, and four from social) were excluded from consideration in the CIQOL-35 Profile instrument due to model misfit. Next, items were selected across a range of item difficulties with the highest discrimination parameters. Care was also taken to ensure that the upper and lower ends of the item difficulty continuum were represented to minimize ceiling and floor effects. For example, after excluding misfitting items, both the easiest item (“conversation in quiet without asking for repeat”) and the most difficult item (“follow conversation in a group of five”) were selected from the communication domain. When deciding between two items of similar difficulty (e.g., “withdraw in social situations” and “avoid social situations” in the social domain), the item with the highest discrimination parameter (“avoid social situations”) was selected. This process resulted in the selection of 10 items from the communication domain and five items each from the emotional, entertainment, environment, listening effort, and social domains for the short forms for each domain (and together they are the CIQOL-35 Profile instrument).
The item parameters and fit statistics for the items retained in the CIQOL-35 Profile instrument are presented in Table 4 (item parameters and fit statistics for full CIQOL item banks are available in Supplemental Material S1). The communication construct showed the largest item difficulty range (−2.01 to 2.44), and the entertainment construct showed the smallest (−0.71 to 0.71). All items had discrimination above 0.84, and all items fit the measurement model (mean square < 1.2 and standardized z < 2.0).
Table 4.
Item parameter estimations and fit statistics for items in the CIQOL-35 Profile instrument.
| Item stem | Item parameters |
Fit statistics |
||||
|---|---|---|---|---|---|---|
| Difficulty (SE) | Discrimination | Infit MnSq | Infit Zstd | Outfit MnSq | Outfit Zstd | |
| Communication | ||||||
| Follow conversation in a group of five | 2.44 (0.08) | 1.00 | 0.99 | 0.0 | 1.02 | 0.3 |
| Understand strangers without lipreading in a noisy place | 1.52 (0.08) | 1.09 | 0.94 | −0.8 | 0.91 | −1.2 |
| Conversation in a noisy place without asking for repeat | 1.13 (0.08) | 1.37 | 0.67 | −5.2 | 0.66 | −5.1 |
| Understand conversation in a crowded room | 0.90 (0.08) | 1.31 | 0.73 | −4.1 | 0.71 | −4.3 |
| Ask a lot of questions about what is being said | 0.69 (0.08) | 1.12 | 0.85 | −2.2 | 0.97 | −0.4 |
| Hear and understand without looking | −0.07 (0.08) | 1.36 | 0.67 | −5.0 | 0.66 | −5.1 |
| Conversation without asking for repeat | −0.26 (0.08) | 1.36 | 0.67 | −5.0 | 0.67 | −4.7 |
| Conversation with 3+ people | −0.62 (0.08) | 1.21 | 0.82 | −2.6 | 0.79 | −2.8 |
| Other people's voices sound clear and natural | −1.14 (0.09) | 0.84 | 1.20 | 2.5 | 1.13 | 1.4 |
| Conversation in quiet without asking for repeat | −2.01 (0.09) | 0.89 | 1.10 | 1.3 | 1.14 | 1.3 |
| Emotional | ||||||
| Frustrated when cannot follow conversation | 1.78 (0.08) | 0.94 | 1.03 | 0.4 | 1.04 | 0.6 |
| Keep quiet in conversation to avoid saying wrong things | 1.10 (0.08) | 0.92 | 1.06 | 0.8 | 1.06 | 0.9 |
| Hearing loss makes me irritable | −0.10 (0.08) | 1.16 | 0.87 | −1.8 | 0.80 | −2.4 |
| Hearing loss makes me feel inadequate | −0.36 (0.08) | 1.29 | 0.80 | −2.8 | 0.72 | −3.4 |
| Feel comfortable being myself | −2.48 (0.11) | 0.92 | 1.12 | 1.3 | 0.92 | −0.3 |
| Entertainment | ||||||
| Music sounds clear and natural | 0.71 (0.07) | 1.11 | 1.00 | 0.0 | 0.95 | −0.6 |
| Recognize melodies in music | 0.09 (0.07) | 1.32 | 0.73 | −4.1 | 0.72 | −4.0 |
| Able to enjoy music | −0.25 (0.07) | 1.05 | 1.09 | 1.2 | 0.97 | −0.4 |
| Able to enjoy listening to radio and TV | −0.61 (0.08) | 1.18 | 0.85 | −2.0 | 0.84 | −2.0 |
| Due to hearing loss, listen to TV less often than I like | −0.71(0.08) | 0.89 | 1.16 | 2.0 | 1.06 | 0.7 |
| Environment | ||||||
| Hear someone approach from behind | 1.29 (0.07) | 1.25 | 0.78 | −3.3 | 0.78 | −3.3 |
| Hear cars approaching in traffic | −0.18 (0.08) | 1.13 | 0.90 | −1.3 | 0.86 | −1.9 |
| Distinguish sounds in nature | −0.34 (0.08) | 1.40 | 0.62 | −5.8 | 0.62 | −5.8 |
| Everyday sounds sound natural | −0.95 (0.09) | 1.01 | 1.07 | 0.9 | 0.98 | −0.2 |
| Everyday sounds are clear | −1.03 (0.09) | 1.31 | 0.74 | −3.7 | 0.68 | −4.3 |
| Listening effort | ||||||
| Have to concentrate during conversation with strangers in noisy place | 2.47 (0.08) | 1.20 | 0.81 | −2.6 | 0.76 | −2.1 |
| Have to concentrate during conversation | 1.04 (0.08) | 1.12 | 0.92 | −1.2 | 0.90 | −1.2 |
| Easily have conversation in a noisy place | 0.16 (0.08) | 1.00 | 1.03 | 0.5 | 1.01 | 0.2 |
| Ignore competing sounds and focus on who is speaking | −1.19 (0.08) | 0.98 | 1.00 | 0.1 | 0.97 | −0.3 |
| Takes minimal effort to follow conversation | −1.62 (0.08) | 1.16 | 0.81 | −2.7 | 0.90 | −1.2 |
| Social | ||||||
| Feel left out in a group | 1.19 (0.08) | 1.17 | 0.87 | −1.8 | 0.86 | −2.0 |
| Avoid social situations | 0.49 (0.08) | 1.30 | 0.75 | −3.7 | 0.75 | −3.5 |
| Hearing loss keeps me from socializing | −0.33 (0.08) | 1.36 | 0.72 | −4.0 | 0.65 | −4.2 |
| Have confidence to socialize | −0.59 (0.09) | 1.25 | 0.78 | −3.0 | 0.72 | −3.0 |
| If interested, will join family/friends for social event | −1.72 (0.10) | 0.96 | 1.11 | 1.2 | 0.97 | −0.1 |
Note. Data for the full item bank are available in Supplemental Material S1. SE = standard error; MnSq = mean square; Zstd = standardized residual.
CIQOL-10 Global Measure
A single-factor CFA was performed on the CIQOL-35 Profile instrument to ensure that it was unidimensional and psychometrically sound to use as a source of items for the CIQOL-10 Global measure (see Figure 1). Results of the single-factor CFA on the CIQOL-35 Profile instrument revealed adequate-to-good model fit (SRMR = 0.08, RMSEA = 0.12, CFI = .98, TLI = 0.98) relative to a priori criteria (SRMR < 0.08, RMSEA < 0.08, CFI > .95, TLI > 0.95). Three sets of items demonstrated local dependence. Care was taken to ensure that no two dependent items were retained in the CIQOL-10 Global measure.
Next, we performed an additional one-parameter logistic IRT analysis on the CIQOL-35 Profile instrument to guide selection of items for the CIQOL-10 Global measure. In this analysis, all items in the CIQOL-35 Profile were analyzed together in a single, unidimensional model, excluding only those items that were identified as locally dependent in the CFA. After exclusion of two items due to misfit (“able to enjoy music” and “music sounds clear and natural”), 10 items were selected across a range of item difficulties with high discrimination parameters. The 10 items included at least one item from each of the six QOL domains. The easiest item (“feel comfortable being myself”) and the most difficult item (“have to concentrate during conversation with strangers in noisy place”) were selected to ensure breadth of coverage across the latent trait continuum and to minimize ceiling and floor effects. The CIQOL-10 Global measure includes three items from the communication domain, two items each from the emotional and listening effort domains, and one item each from the entertainment, environment, and social domains.
Table 5 displays the item parameters and fit statistics for the items selected for the CIQOL-10 Global measure (Supplemental Material S2 displays item parameters and fit statistics from an additional IRT analysis of the CIQOL-35 Profile items). The item difficulties ranged from −2.63 to 3.18, and all items had discrimination parameters above 0.67. Although the item “Due to hearing loss, listen to TV less often than I like” showed relatively high infit and outfit statistics, it was retained due to its clinical relevance and to ensure representation of the entertainment domain.
Table 5.
Item parameter estimations and fit statistics for items in the CIQOL-10 Global measure.
| Item stem | Domain | Item parameters |
Fit statistics |
||||
|---|---|---|---|---|---|---|---|
| Difficulty (SE) | Discrimination | Infit MnSq | Infit Zstd | Outfit MnSq | Outfit Zstd | ||
| Have to concentrate during conversation with strangers in noisy place | Lis | 3.18 (0.08) | 0.92 | 1.07 | 0.9 | 1.09 | 0.9 |
| Understand strangers without lipreading in a noisy place | Com | 1.26 (0.07) | 1.18 | 0.85 | −2.1 | 0.84 | −2.3 |
| Hear someone approach from behind | Env | 0.87 (0.07) | 0.95 | 1.00 | 0.0 | 1.03 | 0.4 |
| Keep quiet in conversation to avoid saying wrong things | Emo | 0.43 (0.07) | 1.00 | 0.96 | −0.5 | 1.12 | 1.5 |
| Hear and understand without looking | Com | 0.05 (0.07) | 1.35 | 0.70 | −4.6 | 0.68 | −4.7 |
| Takes minimal effort to follow conversation | Lis | −0.31 (0.07) | 1.29 | 0.72 | −4.3 | 0.77 | −3.0 |
| Due to hearing loss, listen to TV less often than I like | Ent | −0.58 (0.07) | 0.67 | 1.41 | 5.0 | 1.28 | 3.1 |
| Hearing loss keeps me from socializing | Soc | −0.90 (0.08) | 1.09 | 1.00 | 0.0 | 0.92 | −0.9 |
| Conversation in quiet without asking for repeat | Com | −1.44 (0.08) | 1.03 | 0.91 | −1.1 | 1.07 | 0.7 |
| Feel comfortable being myself | Emo | −2.63 (0.10) | 0.88 | 1.21 | 2.2 | 1.05 | 0.3 |
Note. Data for the full CIQOL-35 Profile instrument are available in Supplemental Material S2. SE = standard error; MnSq = mean square; Zstd = standardized residual; Lis = listening effort; Com = communication; Env = environment; Emo = emotional; Ent = entertainment; Soc = social.
Discussion
With an increased emphasis on assessment of QOL, development and reporting standards for instruments have become more stringent. Following the PROMIS guidelines, the current study presents the process through which items were selected from the six domain-specific CIQOL item banks to create the CIQOL-35 Profile instrument and CIQOL-10 Global measure. Although other hearing- and CI-specific PROMs are available to measure QOL in individuals with hearing loss who use CIs (Gatehouse & Noble, 2004; Hinderink et al., 2000; Newman et al., 1990), the CIQOL-35 Profile instrument and the CIQOL-10 Global measure have both practical and measurement advantages. By using adult CI patient focus groups to create the CIQOL initial item pool and rigorous psychometric analysis to select items for the six CIQOL item banks, qualitative and quantitative research was used to engage the appropriate stakeholders to provide face, content, and construct validity.
The use of IRT to develop the CIQOL item banks allowed for psychometric analysis at the item level. Primarily, this includes the level of person ability at which the item provides the most information (item difficulty), degree to which the item differentiates individuals according to ability (item discrimination), and how well each item fits the measurement model (item fit). These data were then used to select items that measure the full ability continuum on the unidimensional latent construct and minimize ceiling and floor effects. As a result, the CIQOL-35 Profile instrument and the CIQOL-10 Global measure demonstrate optimized precision in differentiating individuals across the range of the latent trait (Rose et al., 2008). The CIQOL item banks, the CIQOL-35 Profile instrument, and the CIQOL-10 Global measure are among the few PROMs developed using these rigorous methods.
Table 6 provides a summary description of the CIQOL item banks, the CIQOL-35 Profile instrument, and the CIQOL-10 Global measure. The CIQOL item banks provide the greatest precision in each of the six domains, but the length (81 items) is likely prohibitive for use in many research or clinical settings. As such, the CIQOL item banks were not designed to be administered as a whole; rather, they serve as the source of items to create other CIQOL measures. A significant advantage of this approach is that the items within each of the six domains are unidimensional and psychometrically sound, so that each can be used individually. For example, if the goal is to assess the impact of CI use on emotional well-being, five items in the emotional domain could be administered, but if the most precise assessment of emotional well-being is needed, all 15 items in the item bank of this construct should be used. Similar to the CIQOL item banks, the CIQOL-35 Profile instrument produces domain-level scores, includes a selected number of items from each of the six domains, and provides a more efficient means to measure domain-specific CIQOL.
Table 6.
Descriptions of the CIQOL-35 Profile instrument, CIQOL-10 Global measure, and domain-specific item banks from the Cochlear Implant Quality of Life (CIQOL) final item pool.
| CIQOL Instrument | No. items | Provides domain-specific data | Provides global QOL data | Suggestions for use |
|---|---|---|---|---|
| CIQOL-35 Profile Instrument | 35 |

|

|
• Efficient evaluation of CI-related QOL in six domains • Appropriate for use in clinical or research settings |
| CIQOL-10 Global measure | 10 |

|
• Busy clinical settings or research studies with many instruments to limit clinician and patient burden and survey fatique | |
| CIQOL final item pool | 81 |

|
• Not intended to be used as a whole due to length • Source of six domain-specific item banks (see below) |
|
| Communication item bank | 28 |

|
• Provides greatest information regarding receptive and expressive communication ability | |
| Emotional item bank | 15 |

|
• Provides greatest information regarding emotional well-being | |
| Entertainment item bank | 8 |

|
• Provides greatest information regarding enjoyment and clarity of TV, radio, music, etc. | |
| Environment item bank | 6 |

|
• Provides greatest information regarding ability to distinguish and localize environmental sounds | |
| Listening effort item bank | 8 |

|
• Provides greatest information regarding degree of effort and resulting fatigue associated with listening | |
| Social item bank | 16 |

|
• Provides greatest information regarding ability to interact in groups and attend and enjoy social functions |
Note. QOL = quality of life; CI = cochlear implant.
The CIQOL-10 Global measure is an efficient method of obtaining a global assessment of a CI user's QOL without domain-specific data. Measures of this type are important for routine use in the busy clinical setting where clinicians may not have sufficient time for patients to complete measures with large numbers of items. The CIQOL-10 Global measure is also appropriate for inclusion in research protocols that use multiple PROMs to minimize survey fatigue (Porter, Whitcomb, & Weitzer, 2004). Another important benefit is that items for the CIQOL-10 Global measure were drawn from the CIQOL-35 Profile instrument so that the score from the CIQOL-10 Global measure can be derived from item-level responses to the CIQOL-35 Profile instrument. In this manner, the CIQOL-35 Profile instrument provides both domain-specific data and a global assessment of CIQOL.
Additional validation studies are necessary prior to the research and clinical application of the CIQOL instruments. These studies will assess the theoretical advantages of using stakeholder engagement and rigorous psychometric analyses in PROM development by directly comparing the measurement properties of the CIQOL-35 Profile and CIQOL-10 Global instruments to legacy PROMs. After validation, a wide range of applications for the CIQOL instruments will be available in clinical and research settings. Data from previous studies confirm absent to low correlations of CIQOL outcomes with speech recognition outcomes (McRackan, Hand, Velozo, & Dubno, 2019), which is consistent with published literature (Capretta & Moberly, 2016; McRackan, Bauschard, Hatch, Franko-Tobin, Droghini, Nguyen, et al., 2018; McRackan, Bauschard, Hatch, Franko-Tobin, Droghini, Velozo, et al., 2018). Thefore, how people listen, communicate, and interact with their environment is far more complex than commonly used speech recogntion tasks, even those that include background noise. In addition, by focusing solely on speech recognition outcomes, we limit our ability to learn about and understand the communication, social, emotional, and other experiences of CI users. Thus, the CIQOL instruments will provide a more comprehensive understanding of how cochlear implantation impacts its users and can be used to evaluate not only postimplantation progress but also how new CI device technologies, CI listening modalities, novel processing strategies, and other postimplantation interventions impact CI outcomes. In contrast to speech recognition tests, these instruments can provide a more comprehensive, domain-specific understanding of the impact that specific interventions have on CI users.
Limitations
There are a few methodological limitations for this study that should be considered. First, the sample used in this analysis consisted of adult CI users in the United States. Future studies may be warranted to examine the extent to which the CIQOL-35 Profile and the CIQOL-10 Global are appropriate tools to measure CIQOL in other cultures and where American English is not the primary language. Second, although the sample used in this study was representative of adult CI users in the United States with regard to age, employment status, living environment, duration of hearing loss, duration of CI use, device types, and listening modality, the extent to which the education level and household income of our sample generalize to the broader adult CI user population is unknown. Third, the analyses in this study used to create the CIQOL-35 Profile instrument and CIQOL-10 Global measure and the analyses used to generate the CIQOL item banks were conducted on the same samples (McRackan, Hand, Velozo, Dubno, & CIQOL Development Consortium, 2019).
Summary and Conclusions
The current study reports the development of the CIQOL-35 Profile instrument and CIQOL-10 Global measure from six domain-specific CIQOL item banks. Psychometric analyses and item-level parameters were used to select items from the item banks to create tools with optimal measurement characteristics. The descriptions of the development and analyses of these tools can assist researchers and clinicians in selecting the CIQOL instruments that best meet their needs and the needs of their patients. However, additional studies are needed to validate the CIQOL-35 Profile and CIQOL-10 Global instruments against legacy instruments prior to their use.
Supplementary Material
Acknowledgments
Additional information, including access to the CIQOL-35 Profile and CIQOL-10 Global instruments, can be found at https://education.musc.edu/CIQOL. This study was supported (in part) by the National Institutes of Health/National Institute on Deafness and Other Communication Disorders Grant K23 DC016911 (PI: T. R. M.); a K12 award through the South Carolina Clinical & Translational Research Institute, with an academic home at the Medical University of South Carolina; National Center for Advancing Translational Sciences Grant UL1TR001450 (PI: T. R. M.); a grant from the American Cochlear Implant Alliance (PI: T. R. M.); and a grant from the Doris Duke Charitable Foundation (PI: T. R. M.).
The Cochlear Implant Quality of Life Development Consortium consists of the following institutions (and individuals): Columbia University (Justin S. Golub), House Ear Clinic (Eric P. Wilkinson and Dawna Mills), Johns Hopkins University (John P. Carey), Kaiser Permanente–Los Angeles (Nopawan Vorasubin), Kaiser Permanente–San Diego (Vickie Brunk), Mayo Clinic Rochester (Matthew L. Carlson, Colin L. Driscoll and Douglas P. Sladen), Medical University of South Carolina (Elizabeth L. Camposeo, Meredith A. Holcomb, Paul R. Lambert, Ted A. Meyer and Cameron Thomas), Ohio State University (Aaron C. Moberly), Stanford University (Nikolas H. Blevins and Jannine B. Larky), University of Maryland (Ronna P. Herzano), University of Miami (Michael E. Hoffer and Sandra M. Prentiss), University of Cincinnati (Ravi N. Samy), University of Colorado (Samuel P. Gubbels), University of Pennsylvania (Jason Brant), University of Texas Southwestern (Jacob B. Hunter, Brandon Isaacson and J. Walter Kutz), University of Utah (Richard K. Gurgel), Virginia Mason Medical Center (Daniel M. Zeitler), Washington University in St. Louis (Craig A. Buchman and Jill B. Firszt) and Vanderbilt University (Rene H. Gifford, David S. Haynes and Robert F. Labadie). T. R. M. had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Funding Statement
Additional information, including access to the CIQOL-35 Profile and CIQOL-10 Global instruments, can be found at https://education.musc.edu/CIQOL. This study was supported (in part) by the National Institutes of Health/National Institute on Deafness and Other Communication Disorders Grant K23 DC016911 (PI: T. R. M.); a K12 award through the South Carolina Clinical & Translational Research Institute, with an academic home at the Medical University of South Carolina; National Center for Advancing Translational Sciences Grant UL1TR001450 (PI: T. R. M.); a grant from the American Cochlear Implant Alliance (PI: T. R. M.); and a grant from the Doris Duke Charitable Foundation (PI: T. R. M.).
References
- Adunka O. F., Gantz B. J., Dunn C., Gurgel R. K., & Buchman C. A. (2018). Minimum reporting standards for adult cochlear implantation. Otolaryngology—Head & Neck Surgery, 159(2), 215–219. https://doi.org/10.1177/0194599818764329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boone W. J. (2016). Rasch analysis for instrument development: Why, when, and how? CBE Life Science Education, 15(4). pii: rm4. https://doi.org/10.1187/cbe.16-04-0148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown T. A. (2015). Confirmatory factor analysis for applied research (2nd ed.). New York, NY: Guilford. [Google Scholar]
- Capretta N. R., & Moberly A. C. (2016). Does quality of life depend on speech recognition performance for adult cochlear implant users? Laryngoscope, 126(3), 699–706. https://doi.org/10.1002/lary.25525 [DOI] [PubMed] [Google Scholar]
- Cox R. M., & Alexander G. C. (1995). The Abbreviated Profile of Hearing Aid Benefit. Ear and Hearing, 16(2), 176–186. [DOI] [PubMed] [Google Scholar]
- Davis A., McMahon C. M., Pichora-Fuller K. M., Russ S., Lin F., Olusanya B. O., … Tremblay K. L. (2016). Aging and hearing health: The life-course approach. Gerontologist, 56(Suppl. 2), S256–S267. https://doi.org/10.1093/geront/gnw033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Champlain A. F. (2010). A primer on classical test theory and item response theory for assessments in medical education. Medical Education, 44(1), 109–117. https://doi.org/10.1111/j.1365-2923.2009.03425.x [DOI] [PubMed] [Google Scholar]
- Gatehouse S., & Noble W. (2004). The Speech, Spatial and Qualities of Hearing Scale (SSQ). International Journal of Audiology, 43(2), 85–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hays R. D., Morales L. S., & Reise S. P. (2000). Item response theory and health outcomes measurement in the 21st century. Medical Care, 38(9, Suppl.), II28–II42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinderink J. B., Krabbe P. F., & Van Den Broek P. (2000). Development and application of a health-related quality-of-life instrument for adults with cochlear implants: The Nijmegen Cochlear Implant Questionnaire. Otolaryngology—Head & Neck Surgery, 123(6), 756–765. https://doi.org/10.1067/mhn.2000.108203 [DOI] [PubMed] [Google Scholar]
- Hughes S. E., Hutchings H. A., Rapport F. L., McMahon C. M., & Boisvert I. (2018). Social connectedness and perceived listening effort in adult cochlear implant users: A grounded theory to establish content validity for a new patient-reported outcome measure. Ear and Hearing, 39(5), 922–934. https://doi.org/10.1097/AUD.0000000000000553 [DOI] [PubMed] [Google Scholar]
- Kelley T., Ebel R., & Linacre J. M. (2002). Item discrimination indices. Rasch Measurement Transactions, 16(3), 883–884. [Google Scholar]
- Linacre J. (1994). Sample size and item calibration stability. Rasch Measurement Transactions, 7(4), 328. [Google Scholar]
- Linacre J. (2002). Optimizing rating scale category effectiveness. Journal of Applied Measurement, 3(1), 85–106. [PubMed] [Google Scholar]
- Linacre J. (2016). Rasch measurement computer program: User's guide. Retrieved from http://www.winsteps.com/winman/principalcomponents.htm
- MacCallum R. C., Widaman K. F., Zhang S., & Hong S. (1999). Sample size in factor analysis. Psychological Methods, 4(1), 84–99. [Google Scholar]
- McRackan T. R., Bauschard M., Hatch J. L., Franko-Tobin E., Droghini H. R., Nguyen S. A., & Dubno J. R. (2018). Meta-analysis of quality-of-life improvement after cochlear implantation and associations with speech recognition abilities. The Laryngoscope, 128(4), 982–990. https://doi.org/10.1002/lary.26738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRackan T. R., Bauschard M., Hatch J. L., Franko-Tobin E., Droghini H. R., Velozo C. A., … Dubno J. R. (2018). Meta-analysis of cochlear implantation outcomes evaluated with general health-related patient-reported outcome measures. Otology and Neurotology, 39(1), 29–36. https://doi.org/10.1097/MAO.0000000000001620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRackan T. R., Hand B. N., Velozo C. A., & Dubno J. R. (2019). Association of demographic and hearing-related factors with cochlear implant-related quality of life. JAMA Otolaryngology—Head & Neck Surgery, 145, 422–430. https://doi.org/10.1001/jamaoto.2019.0055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRackan T. R., Hand B. N., Velozo C. A., Dubno J. R., & Cochlear Implant Quality of Life Development Consortium. (2019). Development of the Cochlear Implant Quality of Life item bank. Ear and Hearing, 40(4), 1016–1024. https://doi.org/10.1097/AUD.0000000000000684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRackan T. R., Velozo C. A., Holcomb M. A., Camposeo E. L., Hatch J. L., Meyer T. A., … Dubno J. R. (2017). Use of adult patient focus groups to develop the initial item bank for a cochlear implant quality-of-life instrument. JAMA Otolaryngology—Head & Neck Surgery, 143(10), 975–982. https://doi.org/10.1001/jamaoto.2017.1182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mick P., Kawachi I., & Lin F. R. (2014). The association between hearing loss and social isolation in older adults. Otolaryngology—Head & Neck Surgery, 150(3), 378–384. https://doi.org/10.1177/0194599813518021 [DOI] [PubMed] [Google Scholar]
- Mokkink L. B., Terwee C. B., Patrick D. L., Alonso J., Stratford P. W., Knol D. L., … de Vet H. C. (2010). The COSMIN checklist for assessing the methodological quality of studies on measurement properties of health status measurement instruments: An international Delphi study. Quality of Life Research, 19(4), 539–549. https://doi.org/10.1007/s11136-010-9606-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman C. W., Weinstein B. E., Jacobson G. P., & Hug G. A. (1990). The Hearing Handicap Inventory for Adults: Psychometric adequacy and audiometric correlates. Ear and Hearing, 11(6), 430–433. [DOI] [PubMed] [Google Scholar]
- Nirmalasari O., Mamo S. K., Nieman C. L., Simpson A., Zimmerman J., Nowrangi M. A., … Oh E. S. (2017). Age-related hearing loss in older adults with cognitive impairment. International Psychogeriatrics, 29(1), 115–121. https://doi.org/10.1017/S1041610216001459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilkonis P. A., Choi S. W., Reise S. P., Stover A. M., Riley W. T., Cella D., & PROMIS Cooperative Group. (2011). Item banks for measuring emotional distress from the Patient-Reported Outcomes Measurement Information System (PROMIS®): Depression, anxiety, and anger. Assessment, 18(3), 263–283. https://doi.org/10.1177/1073191111411667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter S. R., Whitcomb M. E., & Weitzer W. H. (2004). Multiple surveys of students and survey fatigue. New Directions for Institutional Research, 2004(121), 63–73. [Google Scholar]
- Prieto L., Alonso J., & Lamarca R. (2003). Classical test theory versus rasch analysis for quality of life questionnaire reduction. Health Quality of Life Outcomes, 1, 27 https://doi.org/10.1186/1477-7525-1-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patient-Reported Outcome Measurement Information System. (2013). Instrument Development and Validation Scientific Standards (Version 2.0). Retrieved from http://www.healthmeasures.net/images/PROMIS/PROMISStandards_Vers2.0_Final.pdf
- Reeve B. B., Hays R. D., Bjorner J. B., Cook K. F., Crane P. K., Teresi J. A., … PROMIS Cooperative Group. (2007). Psychometric evaluation and calibration of health-related quality of life item banks: Plans for the Patient-Reported Outcomes Measurement Information System (PROMIS). Medical Care, 45(5, Suppl. 1), S22–S31. https://doi.org/10.1097/01.mlr.0000250483.85507.04 [DOI] [PubMed] [Google Scholar]
- Rose M., Bjorner J. B., Becker J., Fries J. F., & Ware J. E. (2008). Evaluation of a preliminary physical function item bank supported the expected advantages of the Patient-Reported Outcomes Measurement Information System (PROMIS). Journal of Clinical Epidemiology, 61(1), 17–33. [DOI] [PubMed] [Google Scholar]
- Rosseel Y., Byrnes J., Vanbrabant L., Savalei V., Merkle E., Hallquist M., & Rhemtulla M. (2017). Package ‘lavaan.’ Retrieved from https://cran.r-project.org/web/packages/lavaan/lavaan.pdf
- Tabachnick B. G., & Fidell L. S. (2013). Using multivariate statistics (6th ed.). Boston, MA: Pearson Education. [Google Scholar]
- Ventry I. M., & Weinstein B. E. (1982). The hearing handicap inventory for the elderly: A new tool. Ear and Hearing, 3(3), 128–134. [DOI] [PubMed] [Google Scholar]
- Wright B., & Linacre J. (1994). Reasonable mean-square fit values. Rasch Measurement Transactions, 8(3), 370. [Google Scholar]
- Zekveld A. A., Kramer S. E., & Festen J. M. (2010). Pupil response as an indication of effortful listening: The influence of sentence intelligibility. Ear and Hearing, 31(4), 480–490. https://doi.org/10.1097/AUD.0b013e3181d4f251 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.