Abstract
The structure and function of the sarcomere of striated muscle is well studied but the steps of sarcomere assembly and maintenance remain under-characterized. With the aid of chaperones and factors of the protein quality control system, muscle proteins can be folded and assembled into the contractile apparatus of the sarcomere. When sarcomere assembly is incomplete or the sarcomere becomes damaged, suites of chaperones and maintenance factors respond to repair the sarcomere. Here we show evidence of the importance of the M-line proteins, specifically myomesin, in the monitoring of sarcomere assembly and integrity in previously characterized zebrafish muscle mutants. We show that myomesin is one of the last proteins to be incorporated into the assembling sarcomere, and that in skeletal muscle, its incorporation requires connections with both titin and myosin. In diseased zebrafish sarcomeres, myomesin1a shows an early increase of gene expression, hours before chaperones respond to damaged muscle. We found that myomesin expression is also more specific to sarcomere damage than muscle creatine kinase, and our results and others support the use of myomesin assays as an early, specific, method of detecting muscle damage.
Introduction
Muscle tissue is composed of bundles of myofibrils that are assembled from tandem repeats of the contractile unit, the sarcomere [1–3]; a complex structure of contracting and relaxing sliding filaments. Hundreds of components are involved in building the sarcomere [1–5], which can be roughly grouped into contractile and structural proteins [2–4,6–14], and proteins involved in the assembly and maintenance of the sarcomere (such as chaperones) but not necessarily present in the assembled complex [11,15–23]. The architecture, composition and function of the mature sarcomere is largely well characterized but the steps involved in assembling this complex structure are still largely unresolved. In particular, the structure of the part of the sarcomere known as the M-line remains poorly understood. The M-line anchors parts of the contractile sarcomere (myosin and titn) and contains proteins such as myomesin and obscurin. Titin is the most extensively studied of the M-line proteins and mutations in titin’s C-terminus lead to limb girdle muscular dystrophies, tibial and Salih congenital muscular dystrophies [9,24,25]. Although a mutation in the dimerization domain of myomesin 1 leads to hypertrophic cardiomyopathy in humans, no other mutations have been described in M-line proteins, including myomesin 2 and 3 [26]. The zebrafish experimental system allows us to address these questions in vivo as fish with ultimately fatal myopathies can still survive to the end of embryogenesis, as heart function is not required for these stages of development. Another advantage is that transparent embryos develop externally allowing us to visualize the defective sarcomere as the organism grows [27–31].
In addition to binding terminal domains of titin and myosin, the M-line is composed of three major structural proteins, obscurin, obscurin-like 1, and myomesin (Fig 1); although each protein may have different isoforms in different muscle types and/or at different developmental stages [6,32–34]. Titin, myomesin and myosin form a network at the center of the sarcomere to anchor thick filaments within the A-band. Myomesin, obscurin and obscurin-like 1 connect thick filament bundles throughout the sarcomere and equalize the contractile force exerted by the thick filaments during contraction [6,35]. Myosin must make attachments with the M-line to be correctly positioned within the sarcomere for contraction. Titin must also attach to the M-line to provide an elastic “spring” between the Z-disc and M-line [7,8,36–38]. Additionally, it seems that myosin and titin connections are needed to also hold the M-line together [9,37]. The N-terminus of myomesin attaches to the tails of the myosin thick filaments while the C-terminus allows for dimerization of myomesin proteins within the M-line [39]. The C-terminus of titin makes contact with myomesin and loosely holds several myomesin proteins together [17,36,37]. All of these contacts are critical to the M-line and disruption of one leads to degeneration of the sarcomere [9,17,36]. If one component of the M-line is not present, or all of the above connections cannot be made, the A-band ultimately collapses leading to muscle paralysis [11,20,22,23,40,41].
Fig 1. The sarcomere proteins of the M-line and their physical interactions.
The M-line of the sarcomere diagraming the protein interactions between myosin thick filaments. The physical protein interactions through the M-line between antiparallel thick filaments are demonstrated by solid lines for one connection and faded dotted lines for the other connection between antiparallel thick filaments. Myosin and titin are incorporated into the M-line around the same time. This work suggests that myomesin is added next and requires both titin and myosin to be present for myomesin to be incorporated. Titin and myomesin together recruit obscurin, or obscurin-like 1, to the M-line [9,42].
Myomesin is not required for myosin to be targeted or incorporated into the sarcomere [43], but myosin is required for myomesin incorporation to the M-line, therefore defects in myosin assembly into the sarcomere (such as due to lack of myosin chaperones) may indirectly abolish myomesin incorporation [43,44]. Titin is also required to maintain myomesin in the M-line as observed in animals with C-terminal titin mutations, myomesin is absent from the sarcomere. Recently, myomesin 3 has been proposed to be a more sensitive serum biomarker of muscle damage than creatine kinase [45]. We hypothesize that myomesin incorporation into the sarcomere is essential to maintain muscle health and that the M-band is sensitive to any disruptions in this process. If this hypothesis is correct, we expect myomesin would be one of the last sarcomere proteins to incorporate as an “integrity check” that sarcomere formation is normal. We would also expect that myomesin mRNA expression would increase in response to either auto-regulation or transcriptional activation as part of a repair pathway due to the absence of an M-line structural protein.
Here we show that myomesin is one of the last proteins to appear in the developing sarcomere, and myomesin fails to incorporate in the skeletal sarcomeres of the zebrafish myosin chaperone mutants still heart and steif, and the zebrafish titin mutant, herzschlag. We also observed a strong increase in myomesin1a (myom1a) expression at the stage of thick filament assembly in these mutants. This dramatic increase in myom1a expression signifies a myomesin-dependent response pathway to sarcomere damage at the earliest stages of muscle disease, as this expression is detectable earlier than myosin chaperone expression. These results support the use of myomesin as an early detection method of sarcomere damage in striated muscle disease.
Materials and methods
Ethics statement
All procedures/methods were performed following the guidelines stated by the Canadian Council for Animal Care and the protocols approved by the Animal Care and Use Committee of the University of Alberta (Fish Research License: 14–0101 FR).
Zebrafish husbandry and strain maintenance
All adult zebrafish were bred and maintained according to standard procedures [46], and kept at 28°C on a day/night cycle of 14 hours light/10 hours dark. Adults were housed in a cycled-water aquatic facility and fed twice daily with brine shrimp. Embryos were raised at 28°C in standard embryo medium [46] for up to 3 days prior to fixation.
The mutants still heart (referred to as smyd1btm123a) and herzschlag (referred to as titin2tg287) were initially identified by the Nusslein-Volhard lab from an ENU mutagenesis screen and further characterized by our lab [22,40,47,48]. The unc45bsb60 mutants (steif), which were also generated from a ENU mutagenesis screen, were a generous gift from Dr. Uwe Strahle [19]. All mutant lines were maintained as adult heterozygotes in a recycling aquatics facility [46]. Embryos were collected from the crossing of two adult heterozygotes and maintained at 28.5°C in zebrafish embryo media before collection for experimental procedures.
In situ hybridization and immunostaining
In situ hybridization were performed using whole-mount zebrafish embryos fixed overnight in 4% paraformaldehyde at 4°C. Antisense RNA probes were synthesized by in vitro transcription using T7 RNA polymerase. Probes used were synthesized from the 3’ untranslated region of myom1a (GenBank:AL929096; FWD- CACAGAGAGCCAATACAAC and REV- TCCTCTACATCAGCATCTC) and ckmb (GenBank:CR855332; FWD- GTGCCATCATGACTTTTCC and REV- GGTGAATACAACACAGCTAGG). For immunostaining, embryos were staged morphologically and fixed in 4% paraformaldehyde overnight at 4°C. The following day, embryos were transferred into PBST and allowed to permeabilize for 2 days at 4°C before beginning antibody staining. Embryos were washed 3 x 15 mins in PBST before permeabilization in ice-cold acetone at -20°C (30 mins– 24hpf; 45 mins– 32hpf; 1 hour– 48hpf; no acetone for heart immunostaining). Embryos were washed 4 x 15 mins in PBST and then incubated in 5% goat serum+PBST for 1 hour at room temperature. After goat serum incubation, embryos were incubated with primary antibody, myomesin (mMacB4, AB_760349, DSHB), slow myosin (F59, AB_528373, DSHB) or titin (T11, AB_2211848, Thermofisher Scientific) in 5% goat serum+PBST overnight at 4°C. The following day, primary antibody solution was removed and embryos were washed 4 x 15 mins in PBST. Following PBST washes, 5% secondary antibody (Alexa Fluor 488 goat anti-mouse, AB_2534069, Thermofisher Scientific) +1% phalloidin (Alexa Fluor 568, Thermofisher Scientific) in PBST was added to the embryos and incubated overnight at 4°C. The following day, secondary was removed and the embryos were washed 3 x 10 mins at room temperature. Immunofluorescence was imaged on a Nikon C2 Confocal Microscope.
RNA extractions and qPCR
RNA extractions and cDNA preparation were carried out as previously described [22] (myom1a qPCR primers: FWD- AGGTTGCTATAGCCAATGTGAT and REV- GCATTAAGCAAGATATATCAGCAGAC).
Tricaine drug treatment
The paralysis of wild-type, smyd1btm123a, unc45bsb60 and titin2tg287 mutants was carried out using Tricaine or MS-222 (Sigma Aldrich). Stock solutions of Tricaine were prepared by adding 400 mg tricaine to 98 ml of distilled water in low light. Stock solutions were vortexed until mixed thoroughly and then frozen until needed. Working solutions were prepared by adding 4.2 ml of Tricaine stock solution to 50 ml of Zebrafish Embryo Medium (ZEM). 25 ml of Tricaine working solution were added to each plate of wild-type or mutant zebrafish embryos. The solution was drained and fresh working Tricaine solutions added to each plate every 2 hours starting at 18hpf until 32hpf when embryos were fixed for immunostaining. All stages of the Tricaine drug treatment were performed under low light conditions.
Galanthamine drug treatment
The stimulation of constant contractions in wild-type fish was performed by using Galanthamine or Nivalin (Enzo Life Sciences, ALX-550-336-M050). Galanthamine stock was prepared by adding 14.4 mg galanthamine to 50 ml of zebrafish embryo media to a final working concentration of 1 mM. 25 ml of working galanthamine solution were added to each experimental plate of wild-type zebrafish embryos at 28hpf. Control wild-type embryo plates had fresh ZEM added to their plates at 28hpf. Control and galanthamine treated embryos were allowed to grow at 28.5°C until 48hpf when they were fixed for immunostaining or flash frozen for RNA extraction.
Raw data can be found at 10.6084/m9.figshare.9913700
Results
Myomesin is one of the last structural components organized into the sarcomere of both heart and skeletal muscle
Somitogenesis in zebrafish begins at 10.3 hours post fertilization (hpf) and slow muscle fibers start to differentiate at 14hpf [30,49–51]. Fast fiber development begins at 19hpf, after slow fibers have begun migration to the somite periphery [50]. By 24hpf, the assembly of the contractile sarcomere is nearly complete in both slow and fast muscle tissue with the M-line as one of the last regions to finish [1–4,52]. Based on immunostaining, myomesin is incorporated into the sarcomere by at least 30hpf, but earlier stages were not examined [40]. To determine if myomesin protein is likely to be incorporated into the sarcomere earlier than 30hpf, we examined the expression of myom1a transcript along discrete stages of zebrafish myogenesis. myom1a transcript is detected at 14hpf (coincident with slow muscle development), 19hpf (when fast muscle development initiates) and continues to be expressed throughout later stages of muscle development (Fig 2). Since myom1a expression is detected by 14hpf, we examined early stages (24hpf) of sarcomere assembly and found detectable myomesin immunostaining in the sarcomeres of slow fibers and barely detectable in fast fibers (Fig 3). However at 36hpf, myomesin incorporation is easily observable in the slanted fast fibers of zebrafish tail muscle. Compared with slow myosin and titin striations at 24hpf, myomesin striations are very narrow and only observable in the first 12 somites, suggesting that in comparison to other sarcomere components myosin, actin and titin, myomesin is incorporated last (Fig 3) [22].
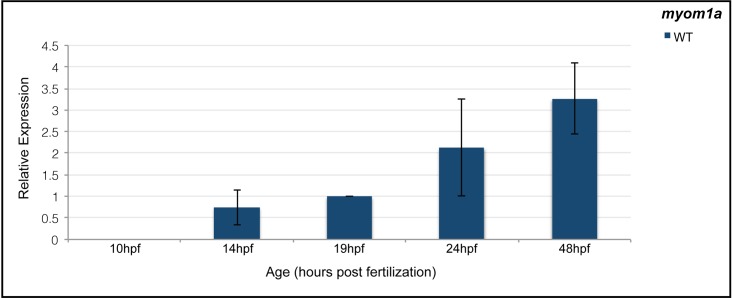
Fig 2. Myomesin 1a is expressed at specific stages of myogenesis.
qPCR analysis of myom1a expression at 10, 14, 19, 24 and 48 hpf in wild-type embryos demonstrated that myom1a is not expressed until some time between 10–14 hpf (slow muscle development) and increases throughout myogenesis.
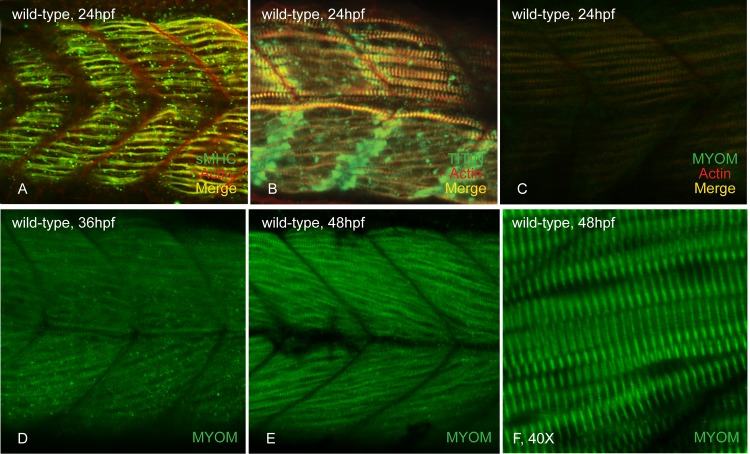
Fig 3. Myomesin is incorporated into skeletal muscle around 24 hpf.
At 24 hpf, slow myosin (A) and titin (B) are incorporated and easily visible in the slow muscle fibers of wild-type embryos. Myomesin striations are observed in the parallel slow fibers of caudal somites (C). At 36 hpf, myomesin staining is seen in the developing fast fibers (D) and these striations become more organized and sharp as myogenesis continues at 48 hpf (E&F).
The zebrafish heart begins beating at ~22hpf and continues through the process of heart looping, where additional cells are added and take on a cardiomyocyte fate [28,53]. We wanted to determine when myomesin incorporates into cardiac sarcomeres to identify a timeline for myomesin-related cardiomyopathies. Due to the fragility and size of the heart, we were not able to analyze hearts younger than 28hpf. However, there was no detectable organization of myomesin into striations within the cardiac sarcomeres at 28hpf (Fig 4). Using immunostaining, we show that myomesin is organized into striations in the hearts of 32hpf wild-type embryos (Fig 4). We hypothesize that as one of the last structures to develop during myofibrillogenesis, the M-band serves as a monitor of sarcomere integrity.
Fig 4. Myomesin is organized into cardiac sarcomeres between 28 and 32hpf.
At 28hpf, we could not detect any incorporated myomesin into the sarcomeres of wild-type embryo hearts (A-C). However at 32hpf, myomesin striations are easily visualized in the hearts of wild-type embryos (D-F; white arrowheads in F’).
Myomesin is not present in the M-line of diseased/damaged sarcomeres
As one test of this hypothesis, we examined the sarcomeres of zebrafish mutant in genes encoding myosin co-chaperones, unc45b and smyd1b, and a titin2 mutant, herzschlag. Still heart, a smyd1b mutant, lacks fast myosin incorporation [22] while steif, an unc45b mutant, lacks both fast and slow myosin incorporation [11,19]. Herzschlag mutants, that are predicted to lack the Titin2 C-terminal domain [40], also show a lack of myomesin staining, suggesting the C-terminus of Titin2 is required for myomesin incorporation. These mutants allow us to examine myomesin in conditions where contacts with myosin thick filaments and titin are disrupted. At 48hpf, myomesin is not incorporated into the fast muscle of still heart (Fig 5F & 5G) and either fast or slow muscle in steif and herzschlag mutants (Fig 5). To determine if this loss of myomesin localization in the M-band is due to a loss of connection with myosin and not due to an attempt of the sarcomeres to contract that dislocates myomesin, we anaesthetized wild-type, and the muscle mutant embryos beginning at a time before contractions start (18hpf) to 32hpf and stained for myomesin in the sarcomeres. Anaesthetized still heart, steif and herzschlag mutant embryos did not have myomesin in their M-bands when compared to anaesthetized wild-type embryos (Fig 6), suggesting that it is the lack of myosin in the sarcomere that results in a lack of myomesin incorporation. These results are consistent with a model that without myosin and/or titin connections, myomesin is absent from the sarcomere.
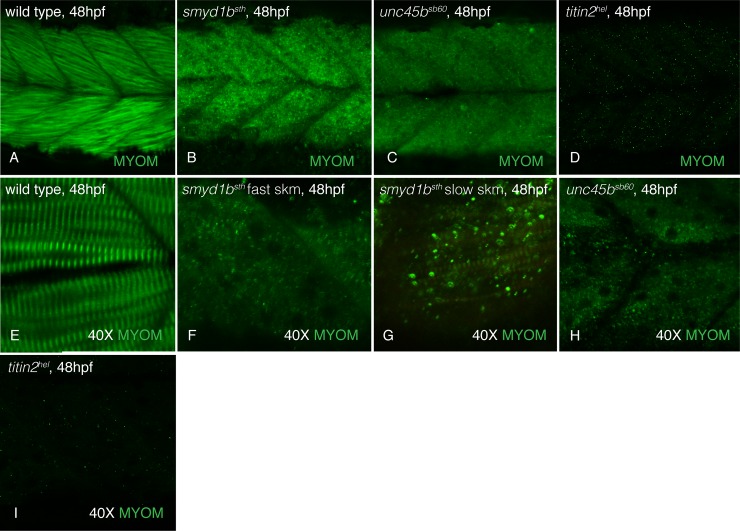
Fig 5. Myomesin is absent in thick filament and titin mutant sarcomeres.
At 48hpf, myomesin is incorporated into the slow (perpendicular fibers) and fast (slanted fibers) muscle of wild-type siblings (A&E) while disorganized in smyd1btm123a (B&F), unc45bsb60 (C&H) and titin2tg287 (D&I) embryos. Increased magnification (40X) of muscle tissue demonstrates the sharp repeating striations of incorporated myomesin in wild-type siblings (E). Smyd1btm123a fast muscle (F; striations are visible in smyd1btm123a slow muscle, G), unc45bsb60 (H) and titin2tg287 (I) muscle lack myomesin striations even at 40X magnification.
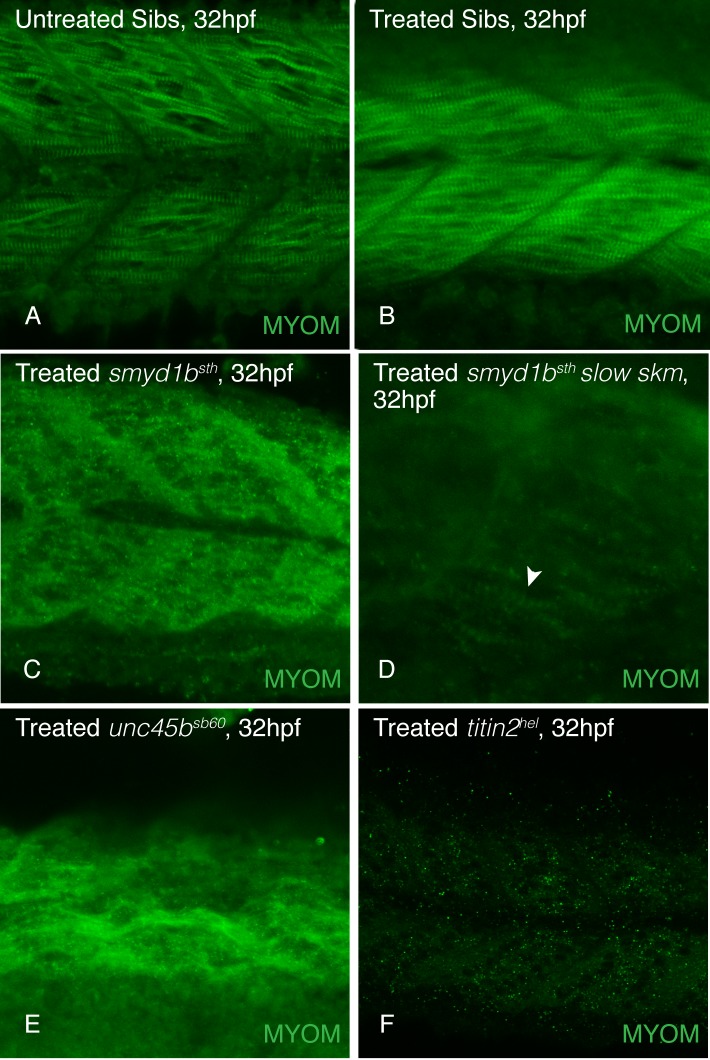
Fig 6. Myosin and titin are required for myomesin incorporation into the sarcomere.
Embryos treated with tricaine to inhibit movement and contraction of skeletal sarcomeres from 18 to 32 hpf showed myomesin incorporates normally in untreated (A) and treated (B) wild-type siblings. Myomesin incorporation is absent in tricaine treated smyd1btm123a fast muscle (C; myomesin striations are visible in slow muscle (white arrowhead), D) unc45bsb60 (E), and titin2tg287 (F) mutant embryos.
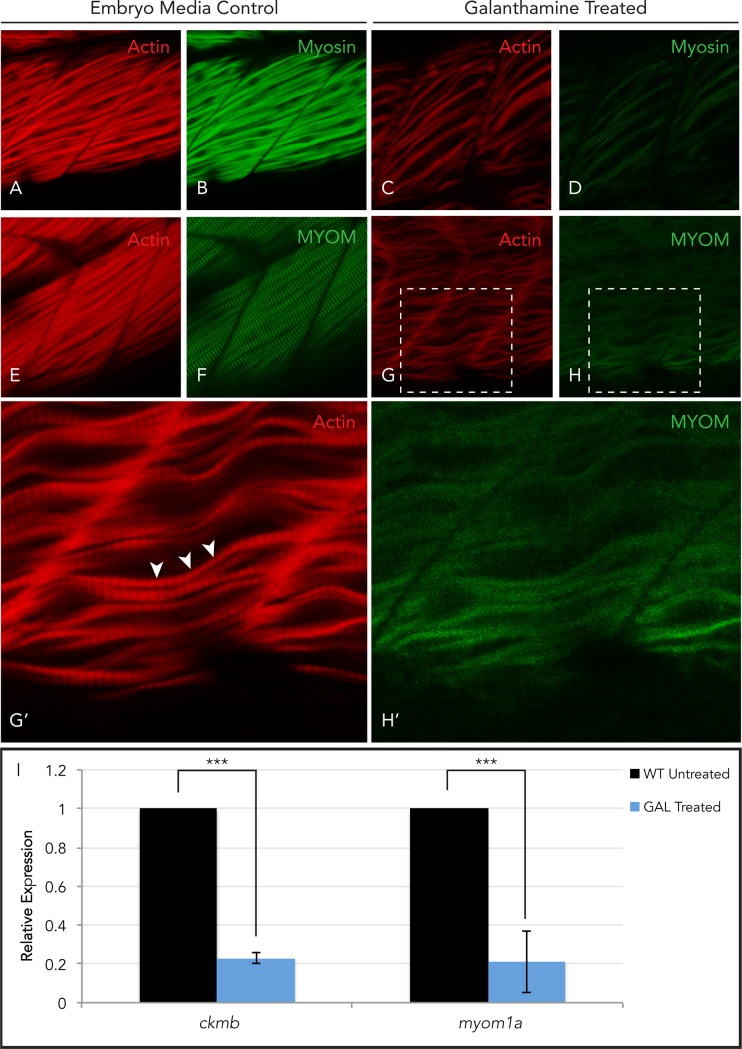
We have shown that myomesin cannot localize to the M-band of diseased sarcomeres but we also wanted to test if myomesin would be absent from damaged muscle that assembled normally. To test this hypothesis, we chemically treated wild-type zebrafish embryos with galanthamine (GAL), a well-known inhibitor of acetylcholinesterase [54,55]. Galanthamine causes hypercontractility in zebrafish skeletal muscle leading to contraction-induced degeneration or dystrophy [54,56]. Wild-type embryos were treated at 28hpf (after skeletal sarcomere assembly) to 48hpf and analyzed for myomesin staining in the sarcomeres. GAL-treated wild-type embryos displayed disorganized myofibers with evident actin and myosin striations but myomesin striations were absent when compared to embryo media control embryos (Fig 7). This suggests that in normally developed muscle that undergoes significant cellular damage, such as in muscular dystrophies, myomesin is absent from the sarcomere and that this occurs even when myosin is still present.
Fig 7. Myomesin is absent in damaged skeletal muscle.
Zebrafish embryos treated with galanthamine (GAL) to induce muscle damage by hypercontraction from 28 to 48hpf demonstrated actin and myosin striations in both untreated (A&B) and treated (C&D) wild-type embryos; although GAL treated embryos exhibited fiber disorganization. Myomesin striations are visible in untreated wild-type embryos (E&F) while absent in GAL treated embryo muscle (G&H) even though actin striations, indicative of intact sarcomeres, are present (G’, white arrowheads; H’). qPCR analysis of ckmb and myom1a expression in galanthamine treated and untreated wild-type embryos demonstrated a significant decrease in the expression of both M-band genes at 48hpf (I). (Error bars are standard deviation. Expression is normalized using ef1a and wild-type at 48hpf).
Since we did not observe myomesin in the M-line of GAL-treated embryos, we wanted to determine if the expression of myom1a was affected by drug-induced hyper-contractility. After 20 hours of galanthamine treatment, myom1a expression was significantly down-regulated in drug-induced damaged muscle (Fig 7I). When compared to another M-band localized protein, muscle creatine kinase b, which is used as a biomarker for damaged muscle, we observed a significant reduction in ckmb expression in GAL-treated embryos at 48hpf (Fig 7I). This would suggest the damage caused by hypercontraction activates a transcriptional feedback that reduces the expression and organization of M-band components. These transcriptional changes may provide insight into the mechanism behind hypertrophic cardiomyopathy disease progression.
Myomesin responds to defects in sarcomere assembly before other markers of muscle damage
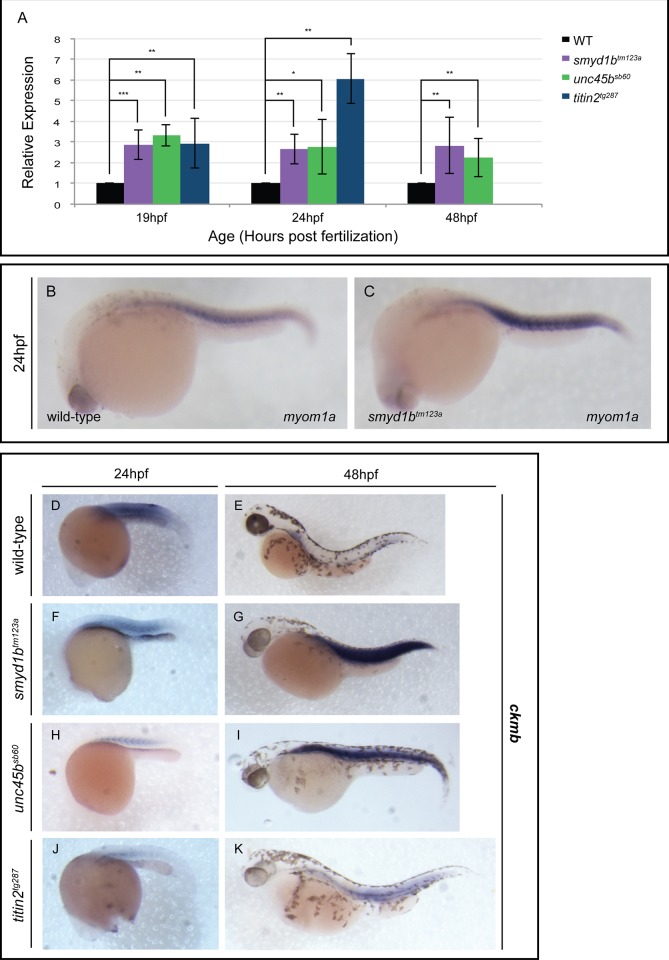
If the M band acts as a monitor of sarcomere integrity, we hypothesize that sarcomeres that cannot incorporate, or dislodge myomesin, will display a transcriptional response similar to that of other muscle damage response pathways. The chaperones hsp90a1, unc45b and smyd1b are transcriptionally up-regulated in response to misfolded myosins at 24 and 48hpf in still heart and steif mutants [11,19,22]. This response seems to be due to a threshold level of misfolded myosins that cause Hsp90a1-bound Hsf1 to translocate to the nucleus to induce a chaperone response [57]. Our model predicts that if myomesin cannot make appropriate interactions with proteins in the M-band, it fails to incorporate, and we would see a positive feedback on myom1a transcription to help repair the defective sarcomere assembly. We show that the expression of myom1a is significantly up-regulated at 19hpf and older stages in still heart, steif and herzschlag mutants (Fig 8A–8C), an increase that is not observed in chaperone expression until 24hpf [22].
Fig 8. Myomesin1a is significantly upregulated at early stages of myogenesis in muscle mutants.
qPCR analysis at 19, 24 and 48 hpf revealed a statistically significant up-regulation of myom1a expression in smyd1btm123a, unc45bsb60 and titin2tg287 mutants relative to wild-type embryos at identical stages (A). At 24 hpf, in situ hybridization demonstrated somite-restricted myom1a expression in wild-type siblings (B), which is increased in smyd1btm123a embryos (C). At 24 hpf, wild-type (D) and smyd1btm123a (E) embryos display similar levels of muscle creatine kinase b (ckmb) expression in their skeletal muscle. Compared to wild-type embryos at 24 hpf (D), unc45bsb60 (H) and titin2tg287 (J) show a significant decrease in ckmb expression. At 48 hpf, smyd1btm123a (G), unc45bsb60 (I) and titin2tg287 (K) embryos demonstrated increased ckmb expression in their skeletal muscle when compared to wild-type siblings (E). (Error bars are standard deviation. Expression is normalized using ef1a and wild-type at corresponding stages).
One of the most well-known and used biomarkers for sarcomere damage is muscle creatine kinase [58]. Unlike myomesin 3, creatine kinase serum levels increase from normal exercise or diseased muscle while myom3 is specific to sarcomere damage [45]. Since myom1a shows increased expression early in congenital myopathies, we wanted to test whether myom1a is also a specific biomarker for sarcomere damage when compared to muscle creatine kinase in zebrafish at a tissue level. When compared to myom1a, which shows increased expression starting at 19hpf and onwards, muscle creatine kinase b showed increased expression at 48hpf but not at 24hpf (Fig 8D–8K), suggesting muscle creatine kinase is either less specific to muscle disease than Myomesin 1a or that muscle creatine kinase expression is regulated by a separate pathway activated in later stages of sarcomere damage. This supports the model proposed by Rouillon and colleagues [45] that myomesin assays could detect muscle disease earlier than current markers, but expands this assay to myopathies beyond muscular dystrophies. However, our expression analysis of myom1a and ckmb in hypercontractile muscle displayed an opposite trend for both genes, which had decreased expression after 20 hours of galanthamine treatment (Fig 7). This corroborates the changes in myomesin expression observed in rats with hypertrophic cardiomyopathy with increased activity of their cardiac sarcomeres [59].
Discussion
We hypothesized that the M line acts as a monitor of sarcomere integrity. In support of our hypothesis, we show that myomesin is one of the last proteins to incorporate into the developing sarcomere and that this incorporation requires connections with myosin and Titin2. In diseased sarcomeres, myomesin is missing and a positive feedback myomesin transcriptional response is observed as early as 19hpf. As this response occurs earlier than known markers of muscle damage, chaperones and the clinically used, creatine kinase, we and others support myomesin assays as a early and specific marker of muscle damage [26,36,45,59–62].
Myomesin makes physical contact with myosin heavy chains, titin and obscurin and is required to distribute contractile forces across thick filaments [35–37,39,42]. We focused on the effect that an absence of myosin or the C-terminus of titin has on myomesin. It could be that the loss of one or both of these protein interactions allows myomesin to translocate to the cytoplasm or nucleus [63,64]. Support for this displacement of myomesin comes from the lack of detectable myomesin incorporated into diseased/damaged sarcomeres (Figs 5 & 7) and the high concentration of myomesin 3 protein in the sera of Duchenne muscular dystrophy, Limb-girdle muscular dystrophy and dilated cardiomyopathy patients and animal models [45,60–62,65]. We showed that the absence of myosin or the C-terminus of titin can prevent myomesin from incorporating into the sarcomere and that it is not paralysis or contractions in a defective sarcomere that displace myomesin in these fish (Fig 6). In addition to the absence of myomesin in improperly developed sarcomeres, we demonstrated that myomesin is absent from the sarcomere when muscle undergoes significant cellular damage (Fig 7). Despite our galanthamine treatment being shorter than the typical 3 day treatment, the effects of GAL-induced hypercontraction were evident in the treated embryos, suggesting myomesin translocates from the sarcomere in the early stages of muscle wasting and degeneration. While this study focused on myom1a at the tissue level, future work should focus on assaying the sera of embryonic higher vertebrate models for myomesin 1(a) in drug-treated or dystrophic animals.
Myomesin1a mRNA expression is increased much earlier than the myosin chaperones in the smyd1btm123a mutant, defective in thick filament assembly (Fig 8). Myom1a expression is also increased in titin2tg287 mutants before sarcomere damage is detectable. This early up-regulation in myom1a gene transcription could suggest that myomesin1a is part of a sarcomere integrity/repair pathway. Several other integrity monitors exist in the M-band such as myomasp/LRCC39 and Murf2, which isolate Serum Response Factor (SRF) to the sarcomere in healthy muscle [66,67]. Myomasp physically interacts with myosin, myomesin and SRF while Murf2 binds SRF and titin, both regulating SRF-mediated transcriptionduring normal sarcomere assembly and function [67,68]. If myosin is damaged, or absent, myomasp cooperates withSRF to activate SRF target genes such as myosin heavy chains, myomesin and smyd1b, suggesting the activation of these genes is to aid in rebuilding or repairing the sarcomere [68–71]. If titin is absent, damaged or inactive, MuRF2 translocates to the nucleus to degrade nuclear-localized SRF, which then supresses the SRF transcriptional pathway. The SRF-mediated response pathway could explain the early increase of myom1a gene transcription that we observed in myosin and titin mutants where myompasp may activate the SRF signalling pathway in conjunction with the activation of the Misfolded Myosin Response [19,22,40,57,68]. If the increased myomesin1a expression were secondary to the SRF-mediated response pathway, it would be essential to test the SRF response in a myom1a mutant where, hypothetically, myosin and titin should remain intact at the M-line but Myomasp would be incapable of making all of the necessary protein connections. This would provide an explanation for the results seen in human Dilated Cardiomyopathy (DCM) patients, which have defects in sarcomere structure or force sensing mechanisms in their cardiac muscle, who display significantly increased expression of the EH-myom1a transcript and immunostaining of EH-MYOM1a in their heart tissue [36,60].
Further analysis is required to complete our understanding of the signaling pathways involved with myomesin and muscular dystrophies. The data presented here supports the lack of myomesin in dystrophic muscle (Fig 5)[62] with myom1a transcription significantly upregulated (Fig 8), suggesting post-transcriptional regulation of myomesin. Our titin2 mutant lacks a C-terminus after 19hpf, which means MuRF2 and associated factors cannot localize to the kinase domain of titin2 [67]. Whether this affects the SRF-mediated signaling pathway or other transcriptional regulators of sarcomere components is unknown.
Taken together, myomesin1a is a promising early detection marker for diseased or damaged sarcomeres and is part of a specific early detection pathway (Fig 8D–8G). Recent research has started to look at serum and urine levels of titin as a biomarker for muscle disease, supporting the movement of early and specific disease detection to structural components of the sarcomere [72–75]. This work in combination with research of myomesin paralogs, supports the use of myomesin assays as early detection methods of muscle damage in myopathy patients as well as a measure of the effectiveness of treatments in muscle disease.
Acknowledgments
We would like to express thanks to Dr. Andrew Waskiewicz for allowing us use of his zebrafish aquatic facility. We would like to thank ZIRC for the wild-type AB zebrafish line that is maintained in our fish facility. Some of the antisera used here was obtained from the Developmental Studies Hybridoma Bank, (DSHB) created by the NICHD of the NIH and maintained at The University of Iowa, Department of Biology, Iowa City, IA 52242.
Data Availability
All in situ and immunofluorescent files are available from the 10.6084/m9.figshare.9913700 database.
Funding Statement
This work was supported by a Discovery Grant from the Natural Sciences and Engineering Research Council of Canada, www.nserc-crsng.gc.ca, #RGPIN-2018-06684 to DBP. CC holds an Alexander Graham Bell- Canada Graduate Scholarship from NSERC. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Sanger JW, Kang S, Siebrands CC, Freeman N, Du A, Wang J, et al. How to build a myofibril. Journal of Muscle Research and Cell Motility. 2005. pp. 343–354. 10.1007/s10974-005-9016-7 [DOI] [PubMed] [Google Scholar]
- 2.Sanger JW, Wang J, Holloway B, Du A, Sanger JM. Myofibrillogenesis in skeletal muscle cells in zebrafish. Cell Motil Cytoskeleton. 2009;66: 556–566. 10.1002/cm.20365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sanger JW, Wang J, Fan Y, White J, Sanger JM. Assembly and dynamics of myofibrils. Journal of Biomedicine and Biotechnology. 2010. 10.1155/2010/858606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rhee D, Sanger JM, Sanger JW. The premyofibril: Evidence for its role in myofibrillogenesis. Cell Motil Cytoskeleton. 1994;28: 1–24. 10.1002/cm.970280102 [DOI] [PubMed] [Google Scholar]
- 5.Sanger JM, Sanger JW. The dynamic Z bands of striated muscle cells. Science Signaling. 2008. 10.1126/scisignal.132pe37 [DOI] [PubMed] [Google Scholar]
- 6.Gautel M, Djinović-Carugo K. The sarcomeric cytoskeleton: From molecules to motion. Journal of Experimental Biology. 2016. 10.1242/jeb.124941 [DOI] [PubMed] [Google Scholar]
- 7.Linke WA, Rudy DE, Centner T, Gautel M, Witt C, Labeit S, et al. I-band titin in cardiac muscle is a three-element molecular spring and is critical for maintaining thin filament structure. J Cell Biol. 1999. 10.1083/jcb.146.3.631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maruyama K. Connectin/titin, giant elastic protein of muscle. FASEB Journal. 1997. [DOI] [PubMed] [Google Scholar]
- 9.Fukuzawa A, Lange S, Holt M, Vihola A, Carmignac V, Ferreiro A, et al. Interactions with titin and myomesin target obscurin and obscurin-like 1 to the M-band—Implications for hereditary myopathies. J Cell Sci. 2008. 10.1242/jcs.028019 [DOI] [PubMed] [Google Scholar]
- 10.Choi CK, Vicente-Manzanares M, Zareno J, Whitmore LA, Mogilner A, Horwitz AR. Actin and α-actinin orchestrate the assembly and maturation of nascent adhesions in a myosin II motor-independent manner. Nat Cell Biol. 2008. 10.1038/ncb1763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wohlgemuth SL, Crawford BD, Pilgrim DB. The myosin co-chaperone UNC-45 is required for skeletal and cardiac muscle function in zebrafish. Dev Biol. 2007;303: 483–492. 10.1016/j.ydbio.2006.11.027 [DOI] [PubMed] [Google Scholar]
- 12.De Deyne PG. Formation of sarcomeres in developing myotubes: Role of mechanical stretch and contractile activation. Am J Physiol—Cell Physiol. 2000. [DOI] [PubMed] [Google Scholar]
- 13.Stout AL, Wang J, Sanger JM, Sanger JW. Tracking changes in Z-band organization during myofibrillogenesis with FRET imaging. Cell Motil Cytoskeleton. 2008. 10.1002/cm.20265 [DOI] [PubMed] [Google Scholar]
- 14.Wang J, Shaner N, Mittal B, Zhou Q, Chen J, Sanger JM, et al. Dynamics of Z-band based proteins in developing skeletal muscle cells. Cell Motil Cytoskeleton. 2005. 10.1002/cm.20063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.JM B CC B, I O, HF E. unc-45 mutations in Caenorhabditis elegans implicate a CRO1/She4p-like domain in myosin assembly. J Cell Biol. 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barral JM, Broadley SA, Schaffar G, Hartl FU. Roles of molecular chaperones in protein misfolding diseases. Seminars in Cell and Developmental Biology. 2004. 10.1016/j.semcdb.2003.12.010 [DOI] [PubMed] [Google Scholar]
- 17.Bernick EP, Zhang PJ, Du S. Knockdown and overexpression of Unc-45b result in defective myofibril organization in skeletal muscles of zebrafish embryos. BMC Cell Biol. 2010;11 10.1186/1471-2121-11-70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shao J Du, Li H, Bian Y, Zhong Y. Heat-shock protein 90α1 is required for organized myofibril assembly in skeletal muscles of zebrafish embryos. Proc Natl Acad Sci U S A. 2008. 10.1073/pnas.0707330105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Etard C, Behra M, Fischer N, Hutcheson D, Geisler R, Strähle U. The UCS factor Steif/Unc-45b interacts with the heat shock protein Hsp90a during myofibrillogenesis. Dev Biol. 2007;308: 133–143. 10.1016/j.ydbio.2007.05.014 [DOI] [PubMed] [Google Scholar]
- 20.Geach TJ, Zimmerman LB. Paralysis and delayed Z-disc formation in the Xenopus tropicalis unc45b mutant dicky ticker. BMC Dev Biol. 2010;10 10.1186/1471-213X-10-75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Just S, Meder B, Berger IM, Etard C, Trano N, Patzel E, et al. The myosin-interacting protein SMYD1 is essential for sarcomere organization. J Cell Sci. 2011;124: 3127–3136. 10.1242/jcs.084772 [DOI] [PubMed] [Google Scholar]
- 22.Prill K, Reid PW, Wohlgemuth SL, Pilgrim DB. Still heart encodes a structural HMT, SMYD1b, with chaperone-like function during fast muscle sarcomere assembly. PLoS One. 2015. 10.1371/journal.pone.0142528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li H, Zhong Y, Wang Z, Gao J, Xu J, Chu W, et al. Smyd1b is required for skeletal and cardiac muscle function in zebrafish. Mol Biol Cell. 2013;24: 3511–3521. 10.1091/mbc.E13-06-0352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kirschner J, Bönnemann CG. The Congenital and Limb-Girdle Muscular Dystrophies: Sharpening the Focus, Blurring the Boundaries. Archives of Neurology. 2004. 10.1001/archneur.61.2.189 [DOI] [PubMed] [Google Scholar]
- 25.Salih MAM. A novel form of familial congenital muscular dystrophy in two adolescents. Neuropediatrics. 1998. 10.1055/s-2007-973579 [DOI] [PubMed] [Google Scholar]
- 26.Siegert R, Perrot A, Keller S, Behlke J, Michalewska-Włudarczyk A, Wycisk A, et al. A myomesin mutation associated with hypertrophic cardiomyopathy deteriorates dimerisation properties. Biochem Biophys Res Commun. 2011. 10.1016/j.bbrc.2011.01.056 [DOI] [PubMed] [Google Scholar]
- 27.Abdelilah S, Mountcastle-Shah E, Harvey M, Solnica-Krezel L, Schier AF, Stemple DL, et al. Mutations affecting neural survival in the zebrafish Danio rerio. Development. 1996. [DOI] [PubMed] [Google Scholar]
- 28.Bakkers J. Zebrafish as a model to study cardiac development and human cardiac disease. Cardiovascular Research. 2011. 10.1093/cvr/cvr098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Detrich HW, Westerfield M, Zon LI. Overview of the zebrafish system. Methods in Cell Biology. 1999. [DOI] [PubMed] [Google Scholar]
- 30.CB K, WW B, SR K, B U, TF S. Stages of embryonic development of the zebrafish. Dev Dyn. 1995. [DOI] [PubMed] [Google Scholar]
- 31.Liu J, Stainier DYR. Zebrafish in the study of early cardiac development. Circulation Research. 2012. 10.1161/CIRCRESAHA.111.246504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Agarkova I, Auerbach D, Ehler E, Perriard JC. A novel marker for vertebrate embryonic heart, the EH-myomesin isoform. J Biol Chem. 2000. 10.1074/jbc.275.14.10256 [DOI] [PubMed] [Google Scholar]
- 33.Schoenauer R, Lange S, Hirschy A, Ehler E, Perriard JC, Agarkova I. Myomesin 3, a Novel Structural Component of the M-band in Striated Muscle. J Mol Biol. 2008. 10.1016/j.jmb.2007.11.048 [DOI] [PubMed] [Google Scholar]
- 34.Greaser ML, Guo W, Bharmal SJ, Esbona K. Titin diversityalternative splicing gone wild. Journal of Biomedicine and Biotechnology. 2010. 10.1155/2010/753675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bertoncini P, Schoenauer R, Agarkova I, Hegner M, Perriard JC, Güntherodt HJ. Study of the mechanical properties of myomesin proteins using dynamic force spectroscopy. J Mol Biol. 2005. 10.1016/j.jmb.2005.03.040 [DOI] [PubMed] [Google Scholar]
- 36.Schoenauer R, Bertoncini P, Machaidze G, Aebi U, Perriard JC, Hegner M, et al. Myomesin is a molecular spring with adaptable elasticity. J Mol Biol. 2005. 10.1016/j.jmb.2005.03.055 [DOI] [PubMed] [Google Scholar]
- 37.Agarkova I, Perriard JC. The M-band: An elastic web that crosslinks thick filaments in the center of the sarcomere. Trends in Cell Biology. 2005. 10.1016/j.tcb.2005.07.001 [DOI] [PubMed] [Google Scholar]
- 38.Benian GM, Mayans O. Titin and obscurin: Giants holding hands and discovery of a new Ig domain subset. Journal of Molecular Biology. 2015. 10.1016/j.jmb.2014.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lange S, Himmel M, Auerbach D, Agarkova I, Hayess K, Fürst DO, et al. Dimerisation of myomesin: Implications for the structure of the sarcomeric M-band. J Mol Biol. 2005. 10.1016/j.jmb.2004.10.040 [DOI] [PubMed] [Google Scholar]
- 40.Myhre JL, Hills JA, Prill K, Wohlgemuth SL, Pilgrim DB. The titin A-band rod domain is dispensable for initial thick filament assembly in zebrafish. Dev Biol. 2014;387: 93–108. 10.1016/j.ydbio.2013.12.020 [DOI] [PubMed] [Google Scholar]
- 41.Li H, Xu J, Bian YH, Rotllant P, Shen T, Chu W, et al. Smyd1b_tv1, a key regulator of sarcomere assembly, is localized on the M-Line of skeletal muscle fibers. PLoS One. 2011. 10.1371/journal.pone.0028524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pernigo S, Fukuzawa A, Beedle AEM, Holt M, Round A, Pandini A, et al. Binding of Myomesin to Obscurin-Like-1 at the Muscle M-Band Provides a Strategy for Isoform-Specific Mechanical Protection. Structure. 2017. 10.1016/j.str.2016.11.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu J, Gao J, Li J, Xue L, Clark KJ, Ekker SC, et al. Functional Analysis of Slow Myosin Heavy Chain 1 and Myomesin-3 in Sarcomere Organization in Zebrafish Embryonic Slow Muscles. J Genet Genomics. 2012. 10.1016/j.jgg.2012.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Codina M, Li J, Gutiérrez J, Kao JPY, Du SJ. Loss of Smyhc1 or Hsp90α1 function results in different effects on myofibril organization in skeletal muscles of zebrafish embryos. PLoS One. 2010;5 10.1371/journal.pone.0008416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rouillon J, Poupiot J, Zocevic A, Amor F, Léger T, Garcia C, et al. Serum proteomic profiling reveals fragments of MYOM3 as potential biomarkers for monitoring the outcome of therapeutic interventions in muscular dystrophies. Hum Mol Genet. 2015;24: 4916–4932. 10.1093/hmg/ddv214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Westerfield M. The Zebrafish Book A Guide for the Laboratory Use of Zebrafish (Danio rerio), 5th Edition Univ Oregon Press Eugene; 2007. [Google Scholar]
- 47.Granato M, Van Eeden FJM, Schach U, Trowe T, Brand M, Furutani-Seiki M, et al. Genes controlling and mediating locomotion behavior of the zebrafish embryo and larva. Development. 1996;123: 399–413. [DOI] [PubMed] [Google Scholar]
- 48.Chen JN, Haffter P, Odenthal J, Vogelsang E, Brand M, Van Eeden FJM, et al. Mutations affecting the cardiovascular system and other internal organs in zebrafish. Development. 1996;123: 293–302. [DOI] [PubMed] [Google Scholar]
- 49.Devoto SH, Melan??on E, Eisen JS, Westerfield M. Identification of separate slow and fast muscle precursor cells in vivo, prior to somite formation. Development. 1996;122: 3371–3380. [DOI] [PubMed] [Google Scholar]
- 50.Blagden CS, Currie PD, Ingham PW, Hughes SM. Notochord induction of zebrafish slow muscle mediated by sonic hedgehog. Genes Dev. 1997. 10.1101/gad.11.17.2163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Roy S, Wolff C, Ingham PW. The u-boot mutation identifies a Hedgehog-regulated myogenic switch for fiber-type diversification in the zebrafish embryo. Genes Dev. 2001. 10.1101/gad.195801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sparrow JC, Schöck F. The initial steps of myofibril assembly: Integrins pave the way. Nat Rev Mol Cell Biol. 2009;10: 293–298. 10.1038/nrm2634 [DOI] [PubMed] [Google Scholar]
- 53.Yang J, Hartjes KA, Nelson TJ, Xu X. Cessation of contraction induces cardiomyocyte remodeling during zebrafish cardiogenesis. Am J Physiol—Hear Circ Physiol. 2014. 10.1152/ajpheart.00721.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Behra M, Etard C, Cousin X, Strähle U. The use of Zebrafish mutants to identify secondary target effects of acetylcholine esterase inhibitors. Toxicol Sci. 2004. 10.1093/toxsci/kfh020 [DOI] [PubMed] [Google Scholar]
- 55.Sramek JJ, Frackiewicz EJ, Cutler NR. Review of the acetylcholinesterase inhibitor galanthamine. Expert Opin Investig Drugs. 2000. 10.1517/13543784.9.10.2393 [DOI] [PubMed] [Google Scholar]
- 56.Otten C, van der Ven PF, Lewrenz I, Paul S, Steinhagen A, Busch-Nentwich E, et al. Xirp proteins mark injured skeletal muscle in zebrafish. PLoS One. 2012. 10.1371/journal.pone.0031041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Etard C, Armant O, Roostalu U, Gourain V, Ferg M, Strähle U. Loss of function of myosin chaperones triggers Hsf1-mediated transcriptional response in skeletal muscle cells. Genome Biol. 2015. 10.1186/s13059-015-0825-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Baird MF, Graham SM, Baker JS, Bickerstaff GF. Creatine-kinase- and exercise-related muscle damage implications for muscle performance and recovery. Journal of Nutrition and Metabolism. 2012. 10.1155/2012/960363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rozanski A, Takano APC, Kato PN, Soares AG, Lellis-Santos C, Campos JC, et al. M-protein is down-regulated in cardiac hypertrophy driven by thyroid hormone in rats. Mol Endocrinol. 2013. 10.1210/me.2013-1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schoenauer R, Emmert MY, Felley A, Ehler E, Brokopp C, Weber B, et al. EH-myomesin splice isoform is a novel marker for dilated cardiomyopathy. Basic Res Cardiol. 2011. 10.1007/s00395-010-0131-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Murphy S, Brinkmeier H, Krautwald M, Henry M, Meleady P, Ohlendieck K. Proteomic profiling of the dystrophin complex and membrane fraction from dystrophic mdx muscle reveals decreases in the cytolinker desmoglein and increases in the extracellular matrix stabilizers biglycan and fibronectin. J Muscle Res Cell Motil. 2017. 10.1007/s10974-017-9478-4 [DOI] [PubMed] [Google Scholar]
- 62.Murphy S, Dowling P, Zweyer M, Henry M, Meleady P, Mundegar RR, et al. Proteomic profiling of mdx-4cv serum reveals highly elevated levels of the inflammation-induced plasma marker haptoglobin in muscular dystrophy. Int J Mol Med. 2017. 10.3892/ijmm.2017.2952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Reddy KB, Fox JEB, Price MG, Kulkarni S, Gupta S, Das B, et al. Nuclear localization of myomesin-1: Possible functions. J Muscle Res Cell Motil. 2008. 10.1007/s10974-008-9137-x [DOI] [PubMed] [Google Scholar]
- 64.Wang X, Liu X, Wang S, Luan K. Myofibrillogenesis regulator 1 induces hypertrophy by promoting sarcomere organization in neonatal rat cardiomyocytes. Hypertens Res. 2012. 10.1038/hr.2011.228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shakeel M, Irfan M, Khan IA. Rare genetic mutations in Pakistani patients with dilated cardiomyopathy. Gene. 2018. 10.1016/j.gene.2018.06.019 [DOI] [PubMed] [Google Scholar]
- 66.Lange S, Agarkova I, Perriard JC, Ehler E. The sarcomeric M-band during development and in disease. Journal of Muscle Research and Cell Motility. 2005. 10.1007/s10974-005-9019-4 [DOI] [PubMed] [Google Scholar]
- 67.Lange S, Xiang F, Yakovenko A, Vihola A, Hackman P, Rostkova E, et al. Cell biology: The kinase domain of titin controls muscle gene expression and protein turnover. Science (80-). 2005. 10.1126/science.1110463 [DOI] [PubMed] [Google Scholar]
- 68.Will RD, Eden M, Just S, Hansen A, Eder A, Frank D, et al. Myomasp/LRRC39, a heart- and muscle-specific protein, is a novel component of the sarcomeric m-band and is involved in stretch sensing. Circ Res. 2010. 10.1161/CIRCRESAHA.110.222372 [DOI] [PubMed] [Google Scholar]
- 69.Nelson TJ, Balza R, Xiao Q, Misra RP. SRF-dependent gene expression in isolated cardiomyocytes: Regulation of genes involved in cardiac hypertrophy. J Mol Cell Cardiol. 2005. 10.1016/j.yjmcc.2005.05.004 [DOI] [PubMed] [Google Scholar]
- 70.Detrich HW, Westerfield M, Zon LI. The zebrafish: disease models and chemical screens. Methods Cell Biol. 2011. [Google Scholar]
- 71.Li D, Niu Z, Yu W, Qian Y, Wang Q, Li Q, et al. SMYD1, the myogenic activator, is a direct target of serum response factor and myogenin. Nucleic Acids Res. 2009. 10.1093/nar/gkp773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Matsuo M, Awano H, Nishio H. Can urinary titin be used for predicting Duchenne muscular dystrophy? Clinica Chimica Acta. 2019. 10.1016/j.cca.2018.10.045 [DOI] [PubMed] [Google Scholar]
- 73.Awano H, Matsumoto M, Nagai M, Shirakawa T, Maruyama N, Iijima K, et al. Diagnostic and clinical significance of the titin fragment in urine of Duchenne muscular dystrophy patients. Clin Chim Acta. 2018. 10.1016/j.cca.2017.11.024 [DOI] [PubMed] [Google Scholar]
- 74.Matsuo M, Awano H, Maruyama N, Nishio H. Titin fragment in urine: A noninvasive biomarker of muscle degradation. Advances in Clinical Chemistry. 2019. 10.1016/bs.acc.2019.01.001 [DOI] [PubMed] [Google Scholar]
- 75.Awano H, Matsumoto M, Nagai M, Shirakawa T, Maruyama N, Iijima K, et al. Urinary titin reveals persistent proteolysis in Duchenne muscular dystrophy. Neuromuscul Disord. 2017. 10.1016/j.nmd.2017.06.273 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All in situ and immunofluorescent files are available from the 10.6084/m9.figshare.9913700 database.