Abstract
Objective
To determine whether REM sleep without atonia (RSWA) during polysomnography (PSG) predicts phenoconversion in patients with idiopathic REM sleep behavior disorder (iRBD), a prodromal feature of a neurodegenerative disease.
Methods
We analyzed RSWA in 60 patients with iRBD, including manual phasic, tonic, and any muscle activity in the submentalis and anterior tibialis muscles and the automated REM atonia index in the submentals. We identified patients who developed parkinsonism or mild cognitive impairment (MCI) during at least 3 years of follow-up after PSG. Kaplan-Meier analysis was performed and receiver operator curves were calculated to determine RSWA cutoffs predicting faster phenoconversion.
Results
Twenty-six (43%) patients developed parkinsonism (n = 17) or MCI (n = 9). Phenoconverters were older at iRBD diagnosis (p = 0.02). Median time to phenoconversion was 3.9 ± 2.5 years. iRBD phenoconverters had significantly more RSWA at diagnosis. Phenoconversion risk from iRBD diagnosis was 20% and 35% at 3 and 5 years, respectively, with greater risk in patients with iRBD with >46.4% any combined RSWA, which increased further to 30% and 55% at 3 and 5 years for patients >65 years of age at diagnosis.
Conclusions
Patients with iRBD with higher amounts of polysomnographic RSWA had a greater risk of developing Parkinson disease or MCI. Patients with older age and higher RSWA amounts had more rapid phenoconversion than younger patients with RBD. Our study suggests that RSWA is a potential biomarker for risk stratification of iRBD phenoconversion that could facilitate prognostication for patients with iRBD.
Classification of evidence
This study provides Class II evidence that for patients with iRBD, increased RSWA correlates with increased risk for developing parkinsonism or MCI.
Idiopathic REM sleep behavior disorder (iRBD) is increasingly recognized as an early manifestation of a progressive synucleinopathy neurodegenerative disease, frequently presenting several years before the onset of parkinsonism or cognitive impairment.1 Therefore, RBD has garnered significant interest as a target for the development and implementation of neuroprotective therapies to stop or slow disease progression. The rate of phenoconversion over 5 years is ≈35%, while the 10- to 25-year risk is ≈41% to 90.9%.1–6 However, the rate of progression from RBD to a clinically defined neurodegenerative syndrome is variable, and few predictors of phenoconversion risk have been identified. Age >65 years at RBD diagnosis, the presence of hyposmia or color-vision abnormalities, the absence of antidepressant use at RBD diagnosis, and the presence of constipation, orthostasis, or motor slowing are associated with a more rapid rate (<3 years) of phenoconversion.4 In fact, the combination of several of these variables (in particular, the combination of color-vision, olfactory, and motor deficits at RBD diagnosis) has been shown to have a 65% risk of phenoconversion within 3 years.4 Another proposed but controversial marker for phenoconversion is sleepiness as measured by the Epworth Sleepiness Scale at RBD diagnosis, although findings have been inconsistent.7,8 Additional predictive biomarkers for phenoconversion in RBD, especially markers that enable individualization of risk profiling, are still a significant remaining gap.
REM sleep without atonia (RSWA) is the neurophysiologic substrate of RBD.9,10 Several methods have been developed to quantify RSWA to diagnose RBD more accurately and to aid in the prediction of future neurodegenerative disease.11–13 In general, 2 types of muscle activity are analyzed. Phasic muscle activity is of short duration (0.1–14.9 seconds) and high amplitude (>4 times the background EMG), whereas tonic muscle activity is defined as muscle activity >2 times the background EMG voltage and lasting greater than half of a sleep epoch.11–13 Phasic muscle activity is analyzed on a 3-second mini-epoch basis, and tonic activity is analyzed on the basis of a 30-second epoch (i.e., tonic muscle activity must last for >15 seconds to be scored positively as present).
The amount of tonic RSWA has been shown to be predictive of the development of future Parkinson disease (PD), but not dementia, in previous studies of patients with iRBD using a 10-second definition for positive tonic muscle activity scoring (i.e., muscle activity lasting longer than half of a 20-second epoch duration).14 On the other hand, phasic muscle activity has not been shown to be predictive of phenoconversion.2,14 In another study, the same group reported that patients with PD with higher amounts of tonic RSWA predicted the development of dementia, regardless of whether the patient had clinical RBD.15 Higher tonic REM muscle activity of patients with iRBD has been associated with increased risk of phenoconversion to a defined neurodegenerative disease (diagnosis not specified) in univariate analysis, which loses significance when adjusted for age at diagnosis and sex.4 These studies used a 10-second definition of tonic muscle activity rather than 15 seconds, which is more commonly used across different centers. More recently, a large multicenter-aggregate RSWA analysis found that increased muscle activity predicted phenoconversion.16 One study using 3-second mini-epochs for phasic muscle activity and a 15-second duration of tonic muscle activity did not find significant RSWA differences between patients with iRBD who phenoconverted and those who remained disease-free, although there was nonstatistically significant higher phasic submentalis (SM) muscle activity in those who phenoconverted.2 Given the conflicting results from previous studies, we aimed to determine whether the amount, muscle distribution, and type of RSWA at iRBD diagnosis are predictive of the development of a clinically defined neurodegenerative disease.
Methods
We identified 60 patients with a diagnosis of iRBD from the Mayo Clinic Center for Sleep Medicine polysomnography (PSG) database who were diagnosed between January 1, 2008, and January 30, 2013. Sixty patients were previously shown to be sufficient to differentiate between phenoconverters and nonphenoconverters.14 Inclusion criteria included diagnosis of RBD meeting International Classification of Sleep Disorders, 2nd edition criteria without evidence of parkinsonism or cognitive impairment at diagnosis (based on clinical history and neurologic examination) and a minimum of 3 years of clinical follow-up.17 Primary indication for sleep referral did not affect inclusion as long as patients ultimately met International Classification of Sleep Disorders, 2nd edition criteria for RBD. Patients were excluded if they met diagnostic criteria for PD, dementia with Lewy bodies, or multiple system atrophy at RBD diagnosis; if they had an REM apnea-hypopnea index of ≥15 per hour; or if they had analyzable REM sleep time <5 minutes.11,18 In patients who underwent split-night studies, the therapeutic positive airway pressure trial was analyzed to minimize sleep-disordered breathing.18 We reviewed medical records to confirm initial diagnosis, development of neurodegenerative disease at follow-up, and other relevant clinical and demographic variables. Because this is a retrospective analysis, patients had follow-up determined at the discretion of their treating neurologist. Patients were diagnosed with PD, mild cognitive impairment, dementia with Lewy bodies, or multiple system atrophy on the basis of the treating neurologist’s evaluation at the time of phenoconversion if they met published clinical criteria for their respective diagnosis.19–21
Analysis of REM sleep muscle activity
Polysomnography recording and RSWA scoring methods were as previously reported with the scorer (S.J.M.) being blinded to the final diagnosis.11,18 EMG channels, including SM and anterior tibialis (AT) muscles, were analyzed for all patients, which are recorded in all patients in our clinical sleep laboratory. In our laboratory during this study period, the extensor digitorum communis (EDC) was recorded selectively in patients with probable dream-enactment behavior and was analyzed as available (n = 46). Phasic (muscle activity duration between 0.1 and 14.9 seconds, with an amplitude of >4 times background EMG) and any muscle activity were calculated separately for SM, AT, and EDC muscles and in the combined SM + AT muscles as performed by our and other previously published groups.2,5,11–13,18,22 Phasic muscle activity–burst durations were directly measured and averaged. All 3-second mini-epochs containing breathing-related or spontaneous arousals were excluded from analysis, minimizing the effect of obstructive sleep apnea on RSWA, regardless of apnea-hypopnea index.2,11,18 Tonic muscle activity (>15 seconds of muscle activity that was double the background EMG voltage, or voltage ≥10 µV) was also scored in the SM.11,13,18 The automated REM atonia index (RAI) in the SM muscle was determined with HypnoLab sleep-scoring software as an additional confirmatory method to the visual quantitative analyses.22 Before RAI analysis, 30-second epochs containing a breathing-related artifact or arousal were excluded, and the SM signal was notch filtered at 60 Hz and rectified.11,22
Statistical analysis
Clinical, demographic, and PSG data are presented as means, SDs, and frequencies. Quantitative variables were analyzed with Wilcoxon rank-sum tests, and χ2 tests were used to analyze categorical variables with JMP statistical software (version 12. SAS Institute Inc, Cary, NC). Phenoconversion risk was estimated with Kaplan-Meier analysis with right censoring of patients who did not phenoconvert. Phenoconversion risk was estimated from the time of RBD symptom onset (i.e., from the historical time of first reported dream-enactment behaviors) and from iRBD diagnosis (i.e., at the time of PSG). To determine the predictive value of RSWA for phenoconversion, Cox proportional hazards test was performed with adjustment for age at RBD diagnosis and sex. Receiver operating characteristic (ROC) curves were calculated for all RSWA types for each muscle and muscle combination. Area under the curve (AUC) was calculated for each analysis, and cutoff diagnostic threshold values were chosen that yielded optimal specificity and sensitivity distinguishing phenoconverters from nonphenoconverters. Predictive markers of phenoconversion were classified in a binary fashion (i.e., above or below the specified cutoff) on the basis of ROC curves with optimal sensitivity and specificity, similar to previous studies.4 Because 2 primary muscles of interest were used to distinguish phenoconverters from nonphenoconverters, the SM and AT, Bonferroni-corrected significance was set at an α < 0.025, without additional correction for confirmatory RAI analysis or pilot EDC analysis.
Standard protocol approvals, registrations, and patient consents
The Mayo Clinic Institutional Review Board approved this study, and participating patients (or their legally authorized representatives) provided written consent to use their medical information.
Data sharing
All relevant data have been shared and published in this article.
Results
Demographics
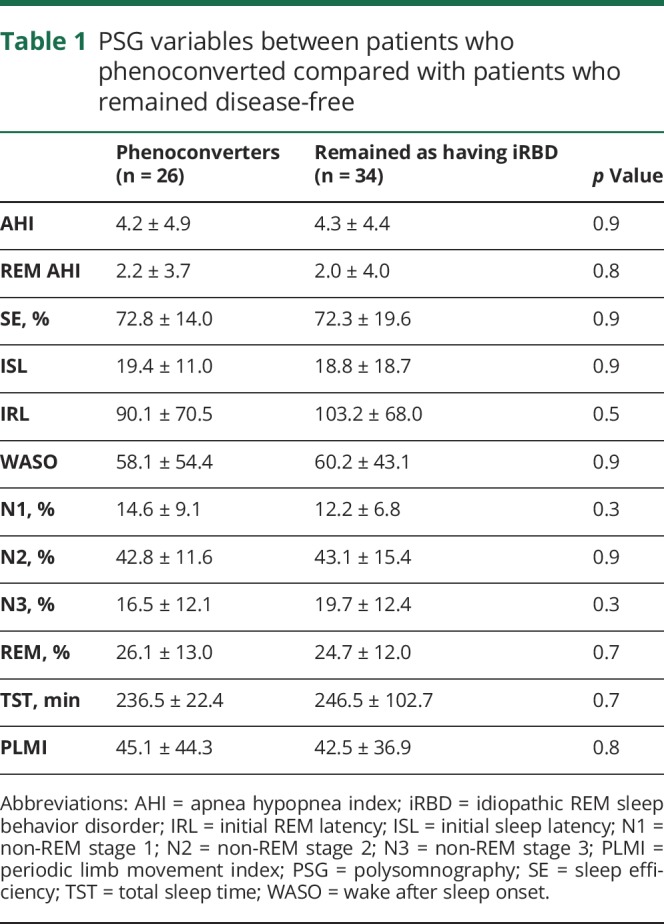
Mean age at RBD diagnosis for the entire cohort was 66.7 ± 9.1 years, with 14 (23%) women and 46 (77%) men. Duration of follow-up was 5.2 ± 2.4 years, with 26 (43%) patients developing parkinsonism (17 of 26) or cognitive impairment (9 of 26). Patients who phenoconverted were older at RBD diagnosis (p = 0.02) and dream-enactment symptom onset (p = 0.02). Twenty-nine (48%) patients were taking antidepressants at the time of PSG. Thirty-three (55%) patients were referred for sleep consultation due to RBD symptoms, 17 (28%) for sleep apnea, 7 (12%) for excessive daytime sleepiness, and 3 (5%) for other reasons (insomnia and restless legs). There was no difference in antidepressant use, sex, sleepiness assessed by the Epworth Sleepiness Scale, cancer diagnosis, gout, obstructive sleep apnea, or restless leg syndrome between patient groups. Average age at onset of parkinsonism or cognitive impairment was 70.5 ± 8.0 years, with an average of 6.6 ± 7.3 years between the onset of dream-enactment behaviors and the development of neurologic symptoms. There were no differences in conventional PSG variables between phenoconverters and nonconverters (table 1).
Table 1.
PSG variables between patients who phenoconverted compared with patients who remained disease-free
RSWA analysis
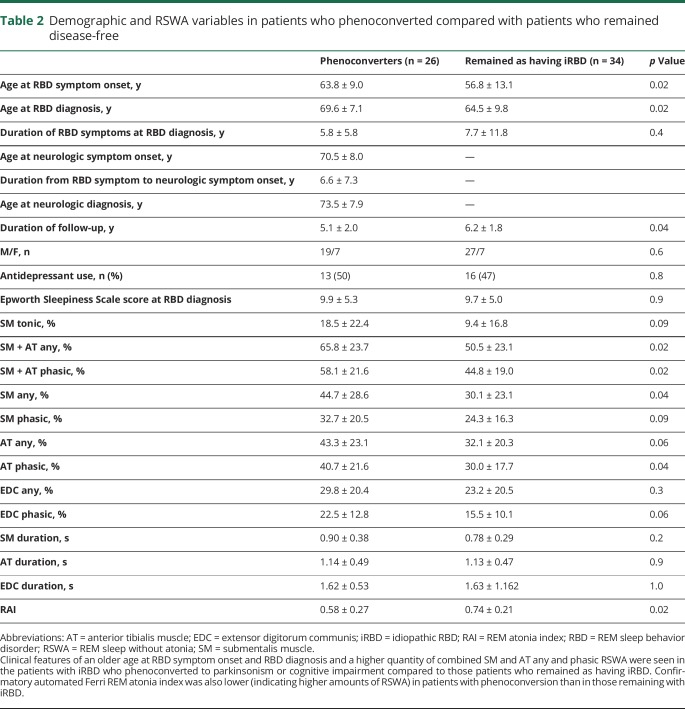
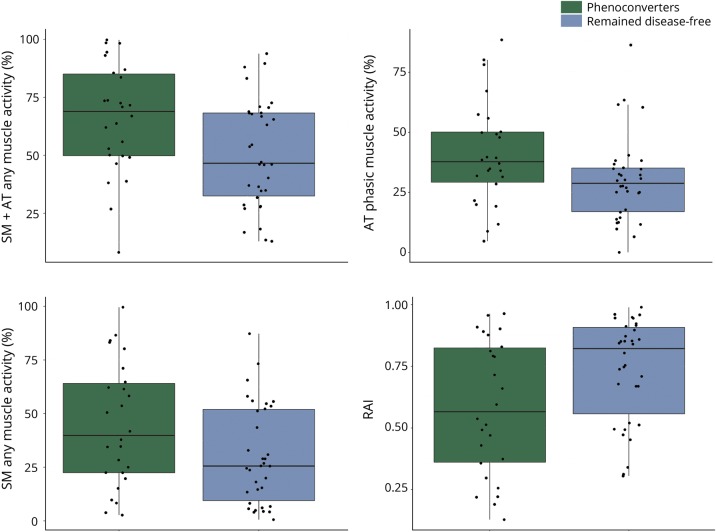
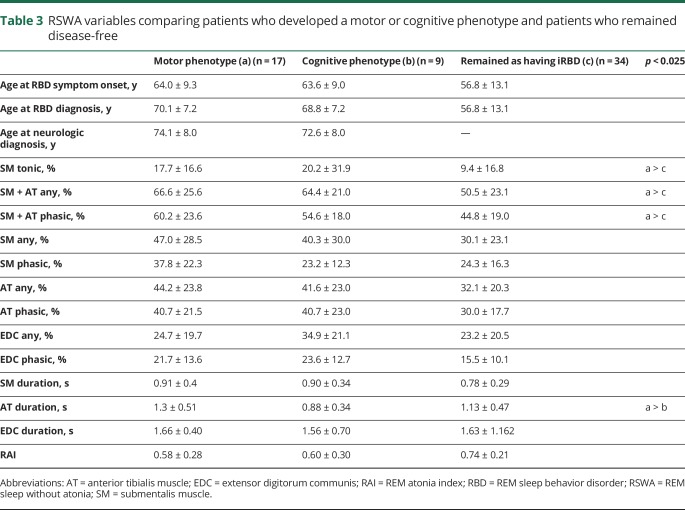
Those who phenoconverted to either parkinsonism or cognitive impairment had higher SM + AT any and phasic muscle activity and lower RAI (indicating higher amounts of REM sleep atonia loss and higher RSWA) at RBD diagnosis compared with those who remained disease-free (p < 0.025) (table 2 and figure 1). Phenoconverters who developed a motor phenotype had higher SM tonic, SM + AT phasic, and any muscle activity compared with those who remained disease-free (p < 0.025) (table 3). However, in contrast to the analysis above for combined phenoconversion outcome of parkinsonism or cognitive impairment, there were no statistically significant differences in RSWA between patients who developed a cognitive phenotype and those who remained with iRBD in a comparison between subgroups (iRBD vs parkinsonism vs cognitive impairment) (table 3). When those who developed a motor phenotype were compared with those who developed a cognitive phenotype, the only statistically significant difference in RSWA metrics was a longer AT duration in those who developed a motor phenotype. SM + AT any was the most sensitive and specific for phenoconversion with a cutoff of 46.4% (85% sensitive, 50% specific, AUC 0.69). Automated analysis with an RAI of 0.66 was 58% sensitive and 74% specific (AUC 0.68) for phenoconverters. ROC analysis of other RSWA variables was less sensitive and specific than SM + AT any and RAI.
Table 2.
Demographic and RSWA variables in patients who phenoconverted compared with patients who remained disease-free
Figure 1. Boxplots of REM sleep without atonia comparisons between phenoconverters and patients who remained disease-free.
AT = anterior tibialis muscle; RAI = REM atonia index; SM = submentalis muscle.
Table 3.
RSWA variables comparing patients who developed a motor or cognitive phenotype and patients who remained disease-free
Survival analysis
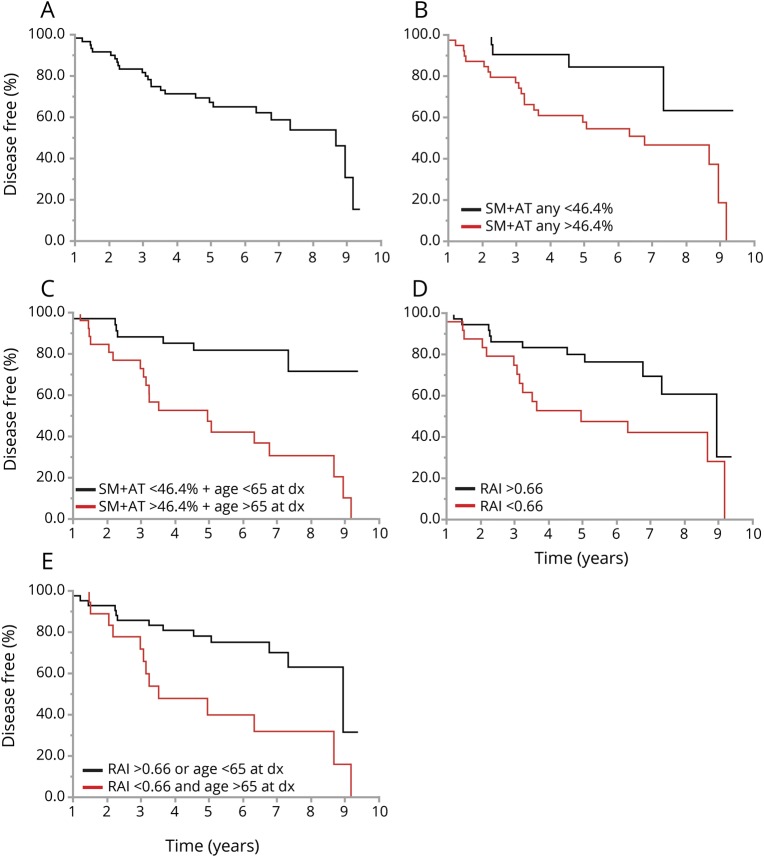
Phenoconversion risk at 3 years from iRBD diagnosis was 20%, which increased to 35% at 5 years (figure 2A). Patients with SM + AT any >46.4% had a 25% 3-year risk and a 45% 5-year risk of phenoconversion with a hazard ratio of 2.75 (p = 0.04) (figure 2B). Phenoconversion risk increased to 30% and 55% at 3 and 5 years, respectively, with patients having combined SM + AT any of >46.4% and an age >65 years at RBD diagnosis (p = 0.001) (figure 2C). Patients with an RAI of <0.66 had a 27% and 55% risk of phenoconversion at 3 and 5 years, respectively (figure 2D), which increased to 30% and 60% at 3 and 5 years, respectively, when an RAI of <0.66 and age >65 years at RBD diagnosis were combined (figure 2E). Phenoconversion rate from RBD symptom onset was 28% at 10 years, 45% at 15 years, and 67% at 19 years. Patients with dream-enactment behaviors starting after age 55 years had a faster rate of phenoconversion compared with those who developed dream-enactment behaviors before age 55 years (10-year risk from dream-enactment behavior onset 39% vs 5%), with a hazard ratio of 12.0 when adjusted for sex and antidepressant use (p < 0.001). There was also a more rapid phenoconversion rate when RBD was identified as a secondary complaint rather than the primary reason for sleep evaluation, with a hazard ratio of 2.97 when adjusted for age at diagnosis and sex (p = 0.009). There was no difference in phenoconversion rate between motor and cognitive phenotypes, patients taking antidepressants at PSG, presence/absence of injuries, or treatment of RBD symptoms with melatonin or clonazepam.
Figure 2. Survival analysis showing predictive markers of neurodegeneration in idiopathic RBD.
(A) Time from REM sleep behavior disorder (RBD) diagnosis (dx) to phenoconversion for entire cohort. (B) Time from RBD diagnosis to phenoconversion in patients with submentalis muscle (SM) + anterior tibialis (AT) any REM sleep without atonia (RSWA) >46.4% at diagnosis. (C) Time from RBD diagnosis to phenoconversion in patients who had SM + AT any RSWA >46.4% and were >65 years old at diagnosis. (D) Time from RBD diagnosis to phenoconversion in patients with REM atonia index (RAI) <0.66 at diagnosis. (E) Time from RBD diagnosis to phenoconversion in patients who had RAI <0.66 and were >65 years old at diagnosis.
Discussion
Higher amounts of RSWA at RBD diagnosis were predictive of more rapid phenoconversion to a defined neurodegenerative disease. Interestingly, this appeared to be driven largely by those who developed parkinsonism rather than cognitive impairment, similar to the findings of a previous study.14 The significance of this finding may be related to the fact that RSWA is a motor phenomenon; however, it may also be due to the relatively small number of patients who developed cognitive impairment (15% of all patients) compared with parkinsonism (28% of all patients). It is also possible that the remaining patients with iRBD will ultimately develop cognitive rather than motor impairment.
For our overall cohort, we found patients with iRBD to have a 20% phenoconversion rate at 3 years and 35% rate at 5 years from RBD diagnosis, similar to the rates previously reported.2–5,23 We also found a rate of phenoconversion from RBD symptom onset that was nearly identical to that reported in previous studies.3,14
While the amounts of quantitative RSWA overlapped between the subgroups of patients with iRBD who did or did not phenoconvert, those with an RSWA threshold >46.4% combined SM + AT any muscle activity at time of diagnosis had a more rapid phenoconversion than those who did not meet this cutoff (45% vs 16% at 5 years from RBD diagnosis). This finding remained significant when adjusted for age at RBD diagnosis and sex, suggesting RSWA is a biomarker for phenoconversion, providing a further objective measure to aid short-term prognostication for more severe overt motor or cognitive deficits for patients, their families, and clinicians. However, when an SM + AT RSWA cutoff of >46.4% is combined with an age at diagnosis of >65 years, the rate of phenoconversion at 5 years from RBD diagnosis increased to 55%, suggesting this combination as a more sensitive means to identify phenoconverters in individual patients. Previous studies have shown the amount of tonic RSWA at diagnosis to predict phenoconversion, although not after adjustment for age and sex.4 Elevated tonic RSWA was seen in patients who developed parkinsonism compared with those who remained disease-free with iRBD, but this difference in amount of tonic RSWA between groups was not statistically significant. The difference between our study and the previous studies is likely secondary to different definitions of tonic RSWA used (i.e., 10 vs 15 seconds), which may have increased the amount of tonic RSWA scored in the previous studies of the Montreal group due to shorter duration criteria for tonic muscle activity. Similar results were seen for an RAI of <0.66, especially when combined with an age >65 years at diagnosis, providing a more rapid method to risk-stratify patients rather than laborious manual RSWA quantification.
We found that younger age at onset, particularly dream-enactment behaviors before age 55 years, was associated with a slower rate of phenoconversion. Furthermore, we also found that patients diagnosed with RBD as a secondary complaint (i.e., those discovered to have RBD while being evaluated for sleep-disordered breathing or insomnia) had a more rapid phenoconversion rate compared with patients whose primary complaint was aggressive and injurious nocturnal behaviors. The reason may be that aggressive, injurious dream-enactment behaviors lead to earlier diagnosis and therefore to a longer duration between RBD onset and phenoconversion than would have been identified with more subtle behaviors. Alternatively, patients with symptomatic RBD, i.e., RBD associated with PD or dementia with Lewy bodies, are less likely to have injurious dream-enactment behaviors than patients with iRBD.24 In addition, there is anecdotal evidence that RBD may “burn out” over time, and dream enactment becomes less violent as patients begin phenoconverting, which may partly explain the more rapid rate of phenoconversion in those with RBD identified as a secondary complaint. Interestingly, antidepressant use, which has been associated with younger-onset RBD in prior studies, did not affect the phenoconversion rate in our study.25 Finally, RSWA may change throughout the disease course of RBD. For example, early in the course of iRBD disease, phasic RSWA may be predominant, resulting in more aggressive brief movements and causing more overt, violent, clinically manifest dream-enactment behaviors despite a relatively limited overall amount of RSWA during REM sleep. On the other hand, tonic RSWA may be predominant later in the disease course, becoming even more strongly associated with clinical symptoms of PD or multiple system atrophy and possibly leading to higher amounts of RSWA but less clinically obvious dream-enactment behaviors. Further longitudinal cohort studies of the natural history of RBD with sequential PSG analyses are necessary to confirm or refute these hypothesized temporal characteristics of RSWA evolution.
Our study has several limitations. As a retrospective, convenience-sample study from a tertiary care referral center, our findings may not be generalizable to patients with RBD seen in community sleep centers and may be subject to referral and sampling bias. Furthermore, in this retrospective analysis, prodromal markers such as olfactory testing, color-vision testing, and autonomic questionnaires were not yet being done routinely at our center. Our prospective iRBD Patient Registry began in 2016; this study involved patients seen before its creation. Patients who phenoconverted or had concerns of developing symptoms may have been more likely to follow up, possibly leading to selection bias; however, follow-up duration in nonconverters was longer than in those who phenoconverted. Because of the retrospective nature of our study, we are unable to account for current disease status unless patients received follow-up care, although of the individuals remaining disease-free, only 15 had not been seen at our institution within the last year. Thus, our phenoconversion rate may be an underestimation. As a clinically based study, we did not have neuropsychological testing to determine cognitive status uniformly in all participants, potentially leading to ascertainment bias. Similarly, EDC muscle recording was selective and not systematically performed, so we were unable to evaluate this muscle uniformly throughout the study population. Because some investigators have reported that arm muscle RSWA evaluation may improve the diagnosis of RBD, it is possible that recording EDC or other arm muscles such as the flexor digitorum superficialis is superior for determining prognosis for phenoconversion.12,13 Finally, while comorbid sleep-disordered breathing is known to worsen the frequency and severity of iRBD, we excluded all 3-second mini-epochs containing breathing-related or spontaneous arousals from analysis, minimizing the effect of obstructive sleep apnea on RSWA, regardless of the apnea-hypopnea index.2,11,18 Therefore, we feel that the inclusion of patients with moderate sleep apnea in our study both is a naturalistic representation of typical patients with iRBD and likely had minimal effect on our RSWA analysis. Future prospective cohort studies analyzing patients with iRBD with serial neuropsychological, motor, autonomic, and PSG (including arm muscle EMG recordings) will be necessary to determine RSWA thresholds that best distinguish prognosis for phenoconversion and risk for evolving a specific synucleinopathy disease subtype.
Similar to previous studies, we found that patients with higher RSWA at iRBD diagnosis have a substantial risk of developing a clinically overt neurodegenerative syndrome, particularly if they develop a motor syndrome such as PD. Increased RSWA at iRBD diagnosis appears to be predictive of more rapid phenoconversion. The rate of phenoconversion increases when patients diagnosed at age >65 years have increased SM + AT RSWA at RBD diagnosis. These data suggest that quantitative RSWA analysis deserves further study as a biomarker for risk stratification for phenoconversion in iRBD. Future prospective trials of RSWA analysis in patients with iRBD with systematic follow-up evaluating prodromal neurodegenerative disease markers are needed to further risk-stratify patients.
Acknowledgment
The authors thank Ms. Lea Dacy for her assistance with manuscript formatting and submission.
Glossary
- AT
anterior tibialis
- AUC
area under the curve
- EDC
extensor digitorum communis
- iRBD
idiopathic REM sleep behavior disorder
- PD
Parkinson disease
- PSG
polysomnography
- RAI
REM atonia index
- RBD
REM sleep behavior disorder
- ROC
receiver operating characteristic
- RSWA
REM sleep without atonia
- SM
submentalis
Appendix. Authors
Footnotes
Class of Evidence: NPub.org/coe
CME Course: NPub.org/cmelist
Study funding
Funded by the Parkinson's Disease Foundation–American Parkinson Disease Association Summer Student Fellowship PDF-APDA-SFW-1656 and Mayo Clinic Alzheimer's Disease Research Center Grant Award from the National Institute on Aging (P50 AG016574), and the National Center for Research Resources and the National Center for Advancing Translational Sciences, NIH, through grant 1 UL1 RR024150-01. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or Parkinson's Disease Foundation–American Parkinson Disease Association.
Disclosure
S. McCarter reports that he received grant funding from a Parkinson's Disease Foundation–American Parkinson Disease Association Summer Student Fellowship (PDF-APDA-SFW-1656). D. Sandness, A. McCarter, J. Feemster, L. Teigen, and P. Timm report no disclosures relevant to the manuscript. B. Boeve reports that he is an investigator in clinical trials sponsored by Axovant and GE Healthcare. He receives royalties from the publication of Behavioral Neurology of Dementia (Cambridge Medicine, 2017). He receives research support from grants U01 AG045390, U54 NS092089, P50 AG016574, UO1 AG006786, RO1 AG015866, RO1 AG032306, and RO1 AG041797; the Mangurian Foundation; and the Little Family Foundation. M. Silber reports no disclosures relevant to the manuscript. E. St. Louis reports that he receives research support from the Mayo Clinic Center for Translational Science Activities, supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences, NIH, through grant 1 UL1 RR024150-01; from the Mayo Clinic Alzheimer's Disease Research Center Grant Award from the National Institute on Aging (P50 AG016574); from the Michael J. Fox Foundation; and from Sunovion, Inc. He has also served as a consultant for Axovant, Inc but receives no personal fees. Go to Neurology.org/N for full disclosures.
References
- 1.St Louis EK, Boeve BF. REM sleep behavior disorder: diagnosis, clinical implications, and future directions. Mayo Clin Proc 2017;92:1723–1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iranzo A, Molinuevo JL, Santamaría J, et al. Rapid-eye-movement sleep behaviour disorder as an early marker for a neurodegenerative disorder: a descriptive study. Lancet Neurol 2006;5:572–577. [DOI] [PubMed] [Google Scholar]
- 3.Iranzo A, Tolosa E, Gelpi E, et al. Neurodegenerative disease status and post-mortem pathology in idiopathic rapid-eye-movement sleep behaviour disorder: an observational cohort study. Lancet Neurol 2013;12:443–453. [DOI] [PubMed] [Google Scholar]
- 4.Postuma RB, Gagnon JF, Bertrand JA, Génier Marchand D, Montplaisir JY. Parkinson risk in idiopathic REM sleep behavior disorder: preparing for neuroprotective trials. Neurology 2015;84:1104–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Postuma RB, Gagnon JF, Vendette M, Fantini ML, Massicotte-Marquez J, Montplaisir J. Quantifying the risk of neurodegenerative disease in idiopathic REM sleep behavior disorder. Neurology 2009;72:1296–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schenck CH, Boeve BF, Mahowald MW. Delayed emergence of a parkinsonian disorder or dementia in 81% of older men initially diagnosed with idiopathic rapid eye movement sleep behavior disorder: a 16-year update on a previously reported series. Sleep Med 2013;14:744–748. [DOI] [PubMed] [Google Scholar]
- 7.Arnulf I, Neutel D, Herlin B, et al. Sleepiness in idiopathic REM sleep behavior disorder and Parkinson disease. Sleep 2015;38:1529–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fereshtehnejad SM, Montplaisir JY, Pelletier A, Gagnon JF, Berg D, Postuma RB. Validation of the MDS research criteria for prodromal Parkinson's disease: longitudinal assessment in a REM sleep behavior disorder (RBD) cohort. Mov Disord 2017;32:865873. [DOI] [PubMed] [Google Scholar]
- 9.Boeve BF. REM sleep behavior disorder: updated review of the core features, the REM Sleep Behavior Disorder-Neurodegenerative Disease Association, evolving concepts, controversies, and future directions. Ann NY Acad Sci 2010;1184:15–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McCarter SJ, St Louis EK, Boeve BF. REM sleep behavior disorder and REM sleep without atonia as an early manifestation of degenerative neurological disease. Curr Neurol Neurosci Rep 2012;12:182–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCarter SJ, St Louis EK, Duwell EJ, et al. Diagnostic thresholds for quantitative REM sleep phasic burst duration, phasic and tonic muscle activity, and REM atonia index in REM sleep behavior disorder with and without comorbid obstructive sleep apnea. Sleep 2014;37:1649–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frauscher B, Iranzo A, Gaig C, et al. Normative EMG values during REM sleep for the diagnosis of REM sleep behavior disorder. Sleep 2012;35:835–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iranzo A, Frauscher B, Santos H, et al. Usefulness of the SINBAR electromyographic montage to detect the motor and vocal manifestations occurring in REM sleep behavior disorder. Sleep Med 2011;12:284–288. [DOI] [PubMed] [Google Scholar]
- 14.Postuma RB, Gagnon JF, Rompré S, Montplaisir JY. Severity of REM atonia loss in idiopathic REM sleep behavior disorder predicts Parkinson disease. Neurology 2010;74:239–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Postuma RB, Bertrand JA, Montplaisir J, et al. Rapid eye movement sleep behavior disorder and risk of dementia in Parkinson's disease: a prospective study. Mov Disord 2012;27:720–726. [DOI] [PubMed] [Google Scholar]
- 16.Postuma RB, Iranzo A, Hu M, et al. Risk and predictors of dementia and parkinsonism in idiopathic REM sleep behaviour disorder: a multicentre study. Brain 2019;142:744–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.International Classification of Sleep Disorders, 2nd ed. Darien, CT: American Academy of Sleep Medicine; 2005. [Google Scholar]
- 18.McCarter SJ, St Louis EK, Sandness DJ, et al. Diagnostic REM sleep muscle activity thresholds in patients with idiopathic REM sleep behavior disorder with and without obstructive sleep apnea. Sleep Med 2017;33:23–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 1992;55:181–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McKeith IG, Dickson DW, Lowe J, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology 2005;65:1863–1872. [DOI] [PubMed] [Google Scholar]
- 21.Gilman S, Wenning GK, Low PA, et al. Second consensus statement on the diagnosis of multiple system atrophy. Neurology 2008;71:670–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferri R, Rundo F, Manconi M, et al. Improved computation of the atonia index in normal controls and patients with REM sleep behavior disorder. Sleep Med 2010;11:947–949. [DOI] [PubMed] [Google Scholar]
- 23.Iranzo A, Fernández-Arcos A, Tolosa E, et al. Neurodegenerative disorder risk in idiopathic REM sleep behavior disorder: study in 174 patients. PLoS One 2014;9:e89741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCarter SJ, St Louis EK, Boswell CL, et al. Factors associated with injury in REM sleep behavior disorder. Sleep Med 2014;15:1332–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Postuma RB, Gagnon JF, Tuineaig M, et al. Antidepressants and REM sleep behavior disorder: isolated side effect or neurodegenerative signal? Sleep 2013;36:1579–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]