Abstract
STUDY QUESTION
Can mice serve as a translational model to investigate the reproductive effects of testosterone (T) therapy commonly used by transgender men?
SUMMARY ANSWER
T enanthate subcutaneous injections at 0.45 mg twice weekly can be used in the postpubertal C57BL/6N female mouse to investigate the reproductive effects of T therapy given to transgender men.
WHAT IS KNOWN ALREADY
Most models of T treatment in female mice involve prenatal or prepubertal administration, which are not applicable to transgender men who often begin T therapy after puberty. Studies that have looked at the impact of postpubertal T treatment in female mice have generally not investigated reproductive outcomes.
STUDY DESIGN, SIZE, DURATION
A total of 20 C57BL/6N female mice were used for this study. Study groups (n = 5 mice per group) included sesame oil vehicle controls and three doses of T enanthate (0.225, 0.45 and 0.90 mg). Mice were injected subcutaneously twice weekly for 6 weeks.
PARTICIPANTS/MATERIALS, SETTING, METHODS
Daily vaginal cytology was performed prior to initiation of treatment to confirm that all mice were cycling. At 8–9 weeks of age, therapy with subcutaneous T enanthate (0.225, 0.45 or 0.90 mg) or the vehicle control was begun. T therapy continued for 6 weeks, at which point mice were sacrificed and compared to control mice sacrificed during diestrus/metestrus. Data collected included daily vaginal cytology, weekly and terminal reproductive hormone levels, terminal body/organ weights/measurements, ovarian follicular distribution/morphology and corpora lutea counts.
MAIN RESULTS AND THE ROLE OF CHANCE
Of the mice treated with 0.90 mg T enanthate, two of five mice experienced vaginal prolapse, so this group was excluded from further analysis. T enanthate administration twice weekly at 0.225 or 0.45 mg resulted in cessation of cyclicity and persistent diestrus. One of five mice at the 0.225-mg dose resumed cycling after 2.5 weeks of T therapy. As compared to controls, T-treated mice had sustained elevated T levels and luteinizing hormone (LH) suppression in the terminal blood sample. T-treated mice demonstrated increases in clitoral area and atretic cyst-like late antral follicles (0.45 mg only) as compared to controls. No reduction in primordial, primary, secondary or total antral follicle counts was detected in T-treated mice as compared to controls, and T-treated mice demonstrated an absence of corpora lutea.
LIMITATIONS, REASONS FOR CAUTION
Mouse models can provide us with relevant key findings for further exploration but may not perfectly mirror human reproductive physiology.
WIDER IMPLICATIONS OF THE FINDINGS
To our knowledge, this report describes the first mouse model mimicking T therapy given to transgender men that facilitates analysis of reproductive changes. This model allows for future studies comparing duration and reversibility of T-induced changes, on the reproductive and other systems. It supports a role for T therapy in suppressing the hypothalamic–pituitary–gonadal axis in adult female mice as evidenced by LH suppression, persistent diestrus and absence of corpora lutea. The increase in atretic cyst-like late antral follicles aligns with the increased prevalence of polycystic ovary morphology seen in case series of transgender men treated with T therapy. The results also suggest that T therapy does not deplete the ovarian reserve.
STUDY FUNDING/COMPETING INTEREST(S)
This work was supported by the American Society for Reproductive Medicine/Society of Reproductive Endocrinology and Infertility Grant and NIH R01-HD098233 to M.B.M. and University of Michigan Office of Research funding (U058227). H.M.K. was supported by the Career Training in Reproductive Biology and Medical Scientist Training Program T32 NIH Training Grants (T32-HD079342, T32-GM07863) as well as the Cellular and Molecular Biology Program. The University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core is supported by the Eunice Kennedy Shriver NICHD/NIH (NCTRI) Grant P50-HD28934. E.E.M. consults for Allergan. No other authors have competing interests.
Keywords: testosterone, gender-affirming, hormone therapy, transgender, mouse model, postpubertal, ovary, acyclicity
Introduction
The gender identity of transgender individuals does not align with their sex assigned at birth. In the United States, there are an estimated 1.4 million transgender adults (0.6%) (Flores et al., 2016). Transgender people may seek cross-sex hormone therapy and/or surgery to develop physical characteristics of their affirmed gender. Masculinizing hormone therapy for transgender men typically involves parenteral (intramuscular or subcutaneous) or transdermal testosterone (T). Levels of T are monitored to ensure that they are within the range of cisgender (or non-transgender) men (Hembree et al., 2017). Gender-affirming T therapy typically causes cessation of menses, body fat redistribution, clitoral enlargement, voice deepening and increased body hair in a more masculine distribution (Coleman et al., 2011).
As the effect of masculinizing T therapy on reproduction is largely unknown, national and international medical organizations recommend counseling about fertility preservation prior to starting T therapy (Coleman et al., 2011; Ethics Committee of the American Society for Reproductive Medicine, 2015; Hembree et al., 2017). Unfortunately, oocyte or embryo cryopreservation strategies are expensive, time-consuming and physically invasive. As such, many transgender men do not preserve gametes prior to starting T but may later express interest in carrying a pregnancy or using their gametes in a gestational carrier (De Roo et al., 2016;Wierckx et al., 2012). There is minimal data on fertility after T therapy. One published survey included 21 transgender men who self-reported pregnancy and live birth using their own oocytes after prior T therapy, but these results cannot be generalized as they screened for individuals with successful births (Light et al., 2014).
Studies of T-exposed ovaries in transgender men at the time of gender-affirming surgery suggest that T induces an ovarian phenotype similar to polycystic ovarian morphology. This does not imply that T therapy causes the multifactorial polycystic ovary syndrome (PCOS), as PCOS by definition excludes exogenous T therapy (The Rotterdam ESHRE/ASRM-Sponsored PCOS consensus workshop group, 2004). Furthermore, the milder hyperandrogenemia and elevated pulsatile luteinizing hormone (LH) of PCOS may not parallel the hormonal milieu of transgender men. In T-treated ovaries of transgender men, multiple studies report increased tunica albuginea collagenization (Amirikia et al., 1986; Chadha et al., 1994; Futterweit and Deligdisch, 1986; Ikeda et al., 2013; Pache et al., 1991; Spinder et al., 1989), stromal hyperplasia (Chadha et al., 1994; Futterweit and Deligdisch, 1986; Grynberg et al., 2010; Ikeda et al., 2013; Pache et al., 1991; Spinder et al., 1989) and increased luteinization of stromal cells (Futterweit and Deligdisch, 1986; Spinder et al., 1989; Pache et al., 1991; Chadha et al., 1994; Ikeda et al., 2013). Reports of follicular changes were more heterogeneous and included multiple cystic follicles (Chadha et al., 1994; Futterweit and Deligdisch, 1986; Miller et al., 1986; Pache et al., 1991; Spinder et al., 1989), multifollicular ovaries (Loverro et al., 2016) and antral follicle count >12 follicles per ovary (Grynberg et al., 2010), although other studies report similar antral follicle counts between transgender men and controls (Ikeda et al., 2013; Caanen et al., 2017). Limitations to these studies include variation in clinical treatment and higher reported rates (15–58%) of PCOS in transgender men prior to T therapy (Baba et al., 2007; Mueller et al., 2008; Becerra-Fernández et al., 2014). In sum, there is uncertainty around T-induced reproductive outcomes and almost no data on any potential reversibility if T is paused for reproductive purposes. Given the ethical limitations of conducting these studies in humans, animal models provide a potential alternative strategy that allows for control of dosage, age, timing, appropriate controls and studies around future fertility. Unfortunately, the existing animal models of androgen administration to female animals either do not adequately parallel the long-term, postpubertal administration of T given to transgender men or are not compatible with studying reproductive capacity (Goetz et al., 2017; Padmanabhan and Veiga-Lopez, 2013; Walters et al., 2012). The objective of this study was to establish a mouse model mimicking T therapy given to transgender men in which the reproductive effects of masculinizing T therapy can be investigated.
Materials and Methods
Ethical approval
Animal studies were performed in accordance with the protocol approved by the Institutional Animal Care & Use Committee (IACUC) at the University of Michigan (PRO00007618).
Experimental design
Twenty C57BL/6NHsd female mice (Envigo, Indianapolis, IN, USA) were used for this study. All mice were housed in ventilated cages in groups of five in a non-barrier facility (photoperiod 12 h light and 12 h dark) with free access to food and water at the University of Michigan, Ann Arbor. Mice were 8–9 weeks old and 19 ± 1 g (mean ± SD) at the time of their first injections. Mice received twice-weekly mid-back 100-μL subcutaneous injections (Monday a.m. and Thursday p.m.) of T enanthate in sesame oil (n = 5 mice/dose) or sesame-oil-only control (n = 5 mice). T enanthate at 0.225, 0.45 or 0.90 mg per dose was diluted from the stock solution (200 mg/mL, dissolved in sesame oil, Hikma Pharmaceuticals, Portugal). Due to vaginal prolapse in two of five mice treated with 0.90 mg T enanthate, all analyses were performed on mice in the 0.225- and 0.45-mg groups. Control mice treatment and analysis was performed prior to T-treated mice to prevent incidental exposure to T. Sesame oil was sterile-filtered prior to injection (USP/NF grade, Welch, Holme & Clark Co., Inc., Newark, NJ, USA). After 6 weeks of T or vehicle injections, mice were sacrificed and organs harvested for histology.
Vaginal cytology
Daily vaginal cytology was performed for at least 2–3 cycles prior to T injection to confirm that the mice were postpubertal and cycling, and subsequently was performed throughout the 6 weeks of T therapy (Nelson et al., 1990). Estrous cycle staging was based on the distribution of leukocytes, cornified epithelial cells and nucleated epithelial cells (Cora et al., 2015).
Blood collection and hormone analysis
Lateral tail vein blood was collected weekly at the midpoint between doses (Wednesday a.m.) with collection volumes up to, but not exceeding, 0.5% of body weight (~75 μL). Terminal blood was collected 2 days after the final T injection via cardiac puncture while under isoflurane anesthesia. Blood samples were kept at 4°C overnight and centrifuged for 10 min (8100g) and the collected serum stored at −20°C. Peptide hormone analysis for LH, follicle stimulating hormone (FSH) and anti-Müllerian hormone (AMH) was performed at the Ligand Assay and Analysis Core Facility, University of Virginia Center for Research in Reproduction. The reportable range for the LH Mouse & Rat in house protocol RIA was 0.04–75.0 ng/mL. The reportable range for the FSH Mouse & Rat in house protocol RIA was 1.6–56.0 ng/mL. The reportable range for the AMH Mouse & Rat ANSH ELISA was 3.36–215 ng/mL. Steroid hormone analysis (testosterone and estradiol) was performed using liquid chromatography tandem mass spectrometry (LC-MS/MS) in the Auchus Laboratory at the University of Michigan. The limit of detection was 20 pg/mL, and the limit of quantification was 50 pg/mL for both testosterone and estradiol. Instrumentation included an Agilent 6495A triple quadrupole mass spectrometer coupled to an Agilent Infinity 1260 and Infinity II 1290 liquid chromatography system.
Body weights and measures
Mice were weighed weekly prior to blood collection. The uterus and liver were weighed prior to fixation on the day of sacrifice. Ovary collection aimed to preserve extraovarian structures (e.g. rete ovarii), and so weights were not collected. External mouse clitoral structures were imaged while the mice were supine and anesthetized, and clitoral length and width were measured in ImageJ.
Histological analysis
Ovaries were fixed in Bouin’s fixative. Samples were processed at the Histology Core in the Microscopy & Image Analysis Laboratory at the University of Michigan. After processing, samples were embedded in paraffin and serially sectioned at 5 μm with five sections per slide, and every other slide was stained with hematoxylin and eosin.
Follicle distribution analysis
Every 10th section was analyzed for the presence of primordial, primary and secondary follicles at ×20 magnification using a light microscope (DM1000, Leica, Germany). Counting was performed while blinded to the experimental group. Follicle distribution was recorded as follicle type per ovary and was averaged between both ovaries. Images of every 10th section were taken at ×5 magnification and used for counting total numbers of corpora lutea and antral follicles. Images for an entire ovary were examined alongside each other to prevent overcounting of corpora lutea and antral follicles across sections. Primordial follicles were defined as an oocyte surrounded by a single layer of squamous granulosa cells, primary follicles as an oocyte surrounded by a single layer of cuboidal granulosa cells and secondary follicles as an oocyte surrounded by two or more layers of granulosa cells. Primordial and primary follicles were counted when their nucleus was visible, and secondary follicles were counted when their nucleolus was present to prevent overcounting. Corpora lutea were identified as discrete round structures with increased pink cytoplasmic staining with hematoxylin and eosin. Antral follicles were identified by the presence of an antral cavity. Atretic cyst-like late antral follicles were defined as a fluid-filled cyst, with an oocyte lacking connection to granulosa cells, and an attenuated granulosa cell layer (adapted from Caldwell et al., 2014).
Statistical analysis
Data were analyzed in GraphPad Prism 7 with unit of analysis of a single mouse. Parametric and non-parametric testing was based on results of the Shapiro–Wilk normality test. Non-parametric testing included Mann–Whitney and Kruskal–Wallis with Dunn’s multiple comparisons test. Parametric testing included Welch’s t-test and ordinary one-way analysis of variance (ANOVA) with Bonferroni’s multiple comparisons test. For analysis, non-detectable hormone levels below the limit of detection were treated as the value set for the limit of quantification. P < 0.05 was considered to be statistically significant.
Results
T enanthate caused persistent diestrus in postpubertal adult female mice
Control mice progressed through the estrous cycle, demonstrating metestrus (M), diestrus (D), proestrus (P) and estrus (E) on vaginal cytology (Fig. 1A). In contrast, T-treated mice demonstrated persistent diestrus starting ~3–4 days after beginning T injections (Fig. 1B). Figure 1C highlights two representative control mice who continued to cycle during the pretreatment period and 6 weeks of injections. This contrasts with two representative T-treated mice who demonstrated cyclicity prior to starting T treatment, after which point consistent diestrus was observed (Fig. 1D). In Fig. 1E, 100% of the control mice continued to cycle throughout the 6 weeks of injections, while 100% of the T-treated mice at 0.45 mg twice weekly stopped cycling (Fig. 1E). For mice treated with 0.225 mg twice weekly, one mouse (20%) demonstrated some cyclic changes in vaginal cytology starting at 2.5 weeks of T treatment (Fig. 1E).
Figure 1.
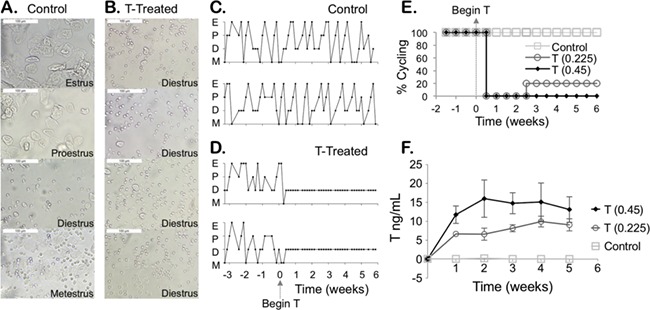
Longitudinal hormonal and cyclic profile. (A) Control mice injected with the sesame oil vehicle progress through the estrous cycle: metestrus (M), diestrus (D), proestrus (P) and estrus (E), while mice treated with testosterone (T) show persistent diestrus as determined by the presence of round leukocytes (B) (scale 100 μm). Cyclicity of two representative control mice (C) versus two T-treated mice (D) for several cycles prior to starting injections and then during 6 weeks of T or vehicle injections. (E) Percent cyclicity for mice after starting T treatment at time 0 (control n = 5, T 0.45 mg n = 5, T 0.225 mg n = 5). (F) Longitudinal T levels for mice over 6 weeks of treatment with injections twice per week (mean ± SD).
T enanthate induced elevated serum T levels
Levels of T were measured every week over the 6-week period for T-treated and control mice. T levels (ng/mL, mean ± SD) in T-treated female mice (<0.05) were comparable to controls (0.1 ± 0.1) prior to starting T (Week 0). Levels were significantly different in subsequent weeks following T injections between control mice (Week 1: 0.10 ± 0.07, Week 2: 0.2 ± 0.3, Week 3: 0.08 ± 0.02, Week 4: 0.07 ± 0.05, Week 5: 0.06 ± 0.01) and T-treated mice at twice weekly T doses of 0.225 mg (Week 1: 7 ± 1, Week 2: 7 ± 4, Week 3: 8 ± 2, Week 4: 10 ± 3, Week 5: 9 ± 4) and 0.45 mg (Week 1: 12 ± 2, Week 2: 16 ± 5, Week 3: 15 ± 3, Week 4: 15 ± 5, Week 5: 13 ± 3) (Fig. 1F).
T enanthate suppressed LH, minimally changed FSH and increased AMH
Terminal hormones were collected 2 days following the final T injection. Terminal LH levels (ng/mL, mean ± SD) in T-treated mice were suppressed (0.225 mg at 0.045 ± 0.005, 0.45 mg at 0.05 ± 0.02) as compared to control mice (0.4 ± 0.2) in diestrus/metestrus (Fig. 2A, P < 0.05). Terminal FSH levels (ng/mL, mean ± SD) were not significantly different for T-treated mice at the 0.225-mg dose (5.1 ± 0.6), although they were statistically significantly higher in T-treated mice at the 0.45-mg dose (5.4 ± 0.5, P < 0.05) when compared to the controls (4 ± 1) (Fig. 2B). Of note, these FSH levels were lower than the FSH levels observed for two control mice sacrificed in proestrus/estrus, which were not included in the analysis (Fig. 2B, open circles). AMH levels (ng/mL, mean ± SD) were significantly increased in T-treated mice at both doses (0.225 mg 76 ± 15, 0.45 mg 82 ± 11) versus control mice (50 ± 8) (Fig. 2C, P < 0.05).
Figure 2.
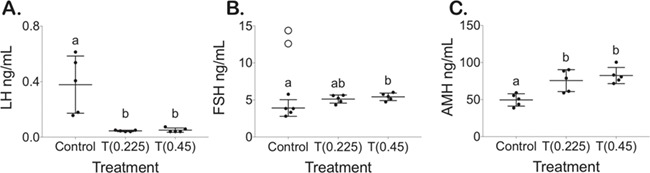
Terminal hormone levels. (A) LH, (B) FSH with open circles for mice in proestrus/estrus not included in calculations and (C) anti-Müllerian hormone (AMH) levels for control and mice treated with T after 6 weeks of T enanthate at 0.225 and 0.45 mg twice per week (mean ± SD).
T enanthate increased uterine weight and clitoral area
There was no significant difference between the increase in body weight (g) for T-treated mice (0.225 mg 3.4 ± 1.1 g, 0.45 mg 4.0 ± 0.7 g) versus control mice (3.2 ± 0.8 g) over the 6-week period (Fig. 3A). T enanthate also did not significantly increase terminal liver weight (g) (normalized to terminal average of 22.5 g mouse) after 6 weeks in T-treated mice (0.225 mg 1.11 ± 0.05 g, 0.45 mg 1.07 ± 0.08 g) as compared to control mice (1.02 ± 0.08 g) (Fig. 3B). Terminal uterine weight (mg) (normalized to terminal average of 22.5 g mouse) was significantly elevated in T-treated mice at 0.225 mg (160 ± 49) as compared to control mice (85 ± 18) (Fig. 3C, P < 0.05). Normalized uterine weight in T-treated mice at 0.45 mg (142 ± 40) was not significantly different from 0.225-mg T-treated mice or controls (Fig. 3C). T-treated mice demonstrated a significantly enlarged clitoral area (0.225 mg 12.2 ± 1.7 mm2, 0.45 mg 11.2 ± 1.6 mm2) (Fig. 3D and F—white triangle) as compared to controls (5.5 ± 0.8 mm2) (Fig. 3D and E—white triangle) (P < 0.05).
Figure 3.
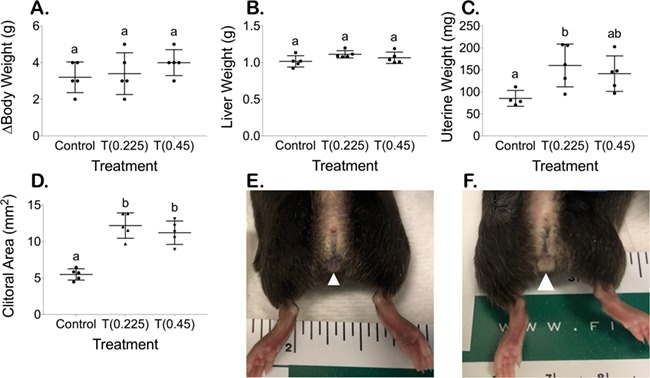
Terminal body measurements (mean ± SD). (A) Increase in body weight over 6 weeks, (B) terminal liver weight (normalized to terminal average of 22.5 g mouse), (C) terminal uterine weight (normalized to terminal average of 22.5 g mouse) and (D) terminal clitoral area. Increased clitoral size between (E) controls and (F) mice treated with T, with white arrowhead pointing to clitoral structure.
T enanthate minimally changed the preantral follicle distribution
T-treated mice at 0.225 mg had a significant increase in the number of primordial follicles (0.225 mg 135 ± 41) as compared to controls (71 ± 15) (Fig. 4A, P < 0.05), while 0.45 mg (0.45 mg 99 ± 9) did not differ significantly from controls or T-treated mice at 0.225 mg (Fig. 4A). No differences were detected in primary follicle counts (0.225 mg 24 ± 3, 0.45 mg 20 ± 4) as compared to control mice (24 ± 6) (Fig. 4B) or for secondary follicle counts (0.225 mg 17 ± 3, 0.45 mg 16 ± 4) as compared to control mice (19 ± 3) (Fig. 4C).
Figure 4.
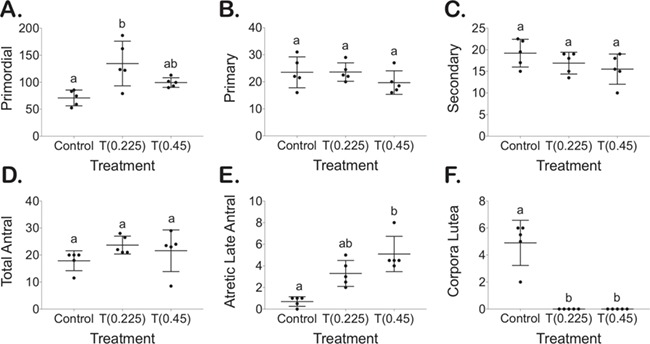
Follicle counts for every 10th section for primordial (A), primary (B), secondary (C), total antral (D) and atretic cyst-like late antral (E) follicles as well as corpora lutea (F).
T enanthate increased atretic cyst-like late antral follicles and prevented corpora lutea formation
Representative histology for whole ovaries, antral follicles and atretic cyst-like late antral follicles is shown in Fig. 5. T-treated mice did not demonstrate differences in total antral follicle counts (0.225 mg 24 ± 3, 0.45 mg 22 ± 8) as compared to controls (18 ± 4) (Fig. 4D). T-treated mice at 0.45 mg demonstrated increased atretic cyst-like late antral follicles (5 ± 2) when compared to control mice (0.7 ± 0.4) (Fig. 4E, P < 0.05). T-treated mice had an absence of corpora lutea (0.225 mg 0 ± 0, 0.45 mg 0 ± 0), which was significantly different than controls (5 ± 2) (Fig. 4F, P < 0.05).
Figure 5.
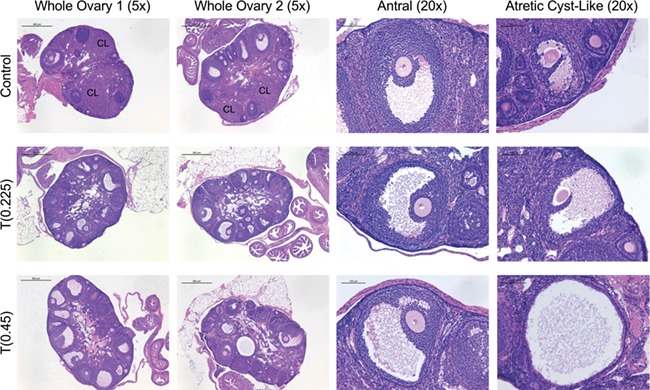
Perturbed histology in mice treated with T. Hematoxylin and eosin-stained control ovaries with corpora lutea (row 1) and T-treated ovaries at 0.225 mg (Row 2) and 0.45 mg (Row 3) after 6 weeks of treatment (Columns 1 and 2, ×5, scale 500 μm). Antral follicles with oocytes surrounded by several layers of cumulus granulosa cells from control (Row 1) and T-treated mice at 0.225 mg (Row 2) and 0.45 mg (Row 3) doses (Column 3, ×20, scale 100 μm). Atretic cyst-like late antral follicles from control (Row 1) and T-treated mice at 0.225 mg (Row 2) and 0.45 mg (Row 3) (Column 4, ×20, scale 100 μm).
Discussion
The above described mouse model mimics several reproductive perturbations observed in transgender men on T therapy (Table I) and therefore can likely be used for preliminary investigation of the reproductive effects of T therapy given to transgender men. Due to vaginal prolapse observed in the 0.90-mg group, and breakthrough cyclicity observed in the 0.225-mg group, we propose that 0.45 mg T enanthate injected subcutaneously twice weekly is the ideal dose for the model. We selected subcutaneous T administration to parallel the parenteral (intramuscular and subcutaneous) administration methods commonly used for masculinizing hormone therapy in transgender men (Hembree et al., 2017). Implants, such as those made from silastic tubing, are also commonly used for androgen administration in animal models (Walters et al., 2012) and may warrant future study in mouse models of masculinizing hormone therapy. Additionally, histological analysis suggests similar numbers of primordial, primary and secondary follicles between control and T-treated mice. These results suggest that T treatment may not affect overall ovarian reserve, and there is likely a pool of normal primordial follicles to recruit once the more advanced, abnormal-appearing follicles have been cleared.
Table I.
Comparison of reproductive changes seen in transgender men given T therapy and in our mouse model of postpubertal T therapy.
| Reproductive effects of postpubertal T therapy | Transgender men with T therapy | Mouse model with T therapy |
|---|---|---|
| Acyclicity | ✓a | ✓ |
| T in male range | ✓b | ✓ |
| LH reduction | ✓c | ✓ |
| Ovarian phenotype similar to polycystic ovary morphology | ✓d | ✓ |
Citations: a(Meyer et al., 1986; Grimstad et al., 2019), b(Hembree et al., 2017), c(Spinder et al., 1989; Wierckx et al., 2014), d(Futterweit and Deligdisch, 1986; Spinder et al., 1989). T, testosterone
Prior animal models have demonstrated changes in ovarian architecture and reproductive function in response to androgen administration, usually in the context of PCOS. In PCOS models, however, androgen treatment is typically initiated prenatally or in the prepubertal period (Walters et al., 2012; Caldwell et al., 2014; van Houten and Visser, 2014), which does not directly translate to postpubertal gender-affirming hormone therapy. The historical paradigm for sex-steroid-induced changes held that organizational (permanent) changes were possible during development and activational (transient) changes took place during adulthood. This framework has likely limited research around changes induced by long-term sex steroid administration in adults because of the assumption that persistent changes would not occur (Arnold and Breedlove, 1985). Of the studies that have examined postpubertal androgen administration on reproduction, one study notes that adult mice treated with dihydrotestosterone (DHT) stopped cycling and had reduced fertility compared to controls (Ma et al., 2017), but DHT is not used for masculinizing therapy in transgender men. Another study treated adult female mice with T for 1 week and demonstrated reduced mature oocyte production from superovulation; however, the short treatment duration limits generalizability (Yang et al., 2015). A model has been recently proposed to mimic cross-sex T therapy; however, mice were ovariectomized prior to T initiation, preventing analysis of reproductive changes (Goetz et al., 2017). Several studies on malarial susceptibility have utilized adult female mice treated with 0.90 mg T twice weekly (Benten et al., 1997; Delić et al., 2010), but did not investigate reproductive changes. Finally, although the description of the ovarian phenotype following T therapy varies across the limited number of studies in transgender men, most studies point to a polycystic ovary morphology-like presentation, including reports of multiple cystic follicles similar to the increase in atretic cyst-like late antral follicles seen in our model (Futterweit and Deligdisch, 1986; Spinder et al., 1989).
Utilizing a mouse model of T therapy in transgender men has limitations and may not perfectly mirror human physiology. In considering development of an animal model to mimic masculinizing T therapy, genealogically, non-human primates are optimal, but they are cost-prohibitive, and their long reproductive life span and gestational cycle limits their utility, particularly when studying effects on offspring (van Houten and Visser, 2014). Sheep models have similar limitations (Padmanabhan and Veiga-Lopez, 2013). Although polyovular, rodents have been used extensively in studies of fertility and ovarian function and are frequently used to model PCOS (Walters et al., 2012), and rodent models can be capitalized upon prior to validating key findings in non-human primates for human translation. Mice are easy to handle and maintain, have a well-characterized reproductive cycle, are relatively inexpensive and have a short generation time and accelerated life span (van Houten and Visser, 2014). Importantly, the follicles in the mouse ovary closely resemble those in the human ovary, and many of the genes expressed in ovarian follicles are highly conserved in mice and humans (Walters et al., 2012). Although they differ from humans in that their ovarian differentiation occurs postnatally (Smith et al., 2014), this difference should not be relevant to studies of postpubertal ovarian function that are not examining in utero insults.
In summary, we have reported the first mouse model mimicking T therapy given to transgender men that allows for investigation of reproductive outcomes. This model is simple and inexpensive to establish, can be used in any mouse laboratory and allows for investigation of reproductive phenotype with T cessation and comparative fertility in controlled manner that is unethical to study in humans. Furthermore, this model can provide a tool for researchers studying the effects of masculinizing T therapy on other aspects of reproduction, other organ systems and transgenerational effects. Furthering the understanding of reproductive changes during masculinizing T therapy, and the reversibility of any observed changes, will hopefully lead to improved counseling for transgender men considering fertility preservation or family building in the future.
Acknowledgements
The authors thank Richard Auchus, MD, PhD, at the University of Michigan for performing steroid hormone analyses and the University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core for performing peptide hormone analyses.
Authors’ roles
H.M.K. designed the study, acquired and interpreted the data and drafted the article. E.S.C. contributed to the study design, data acquisition and article review. A.D. contributed to the study design, data acquisition and article review. E.E.M. contributed to the study design and article review. V.P. designed the study and contributed to the data interpretation and article review. A.S. designed the study and contributed to the data interpretation and article review. M.B.M. conceived and designed the study and contributed to the data interpretation and article review.
Funding
American Society for Reproductive Medicine/Society of Reproductive Endocrinology and Infertility Grant; National Institutes of Health (R01-HD098233 to M.B.M.); University of Michigan Office of Research funding (U058227); Career Training in Reproductive Biology and Medical Scientist Training Program T32 NIH Training Grants (T32-HD079342, T32-GM07863 to H.M.K.); Cellular and Molecular Biology Program (to H.M.K.). The University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core is supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development/National Institutes of Health (National Centers for Translational Research in Reproduction and Infertility) Grant (P50-HD28934).
Conflict of interest
E.E.M. consults for Allergan. No other authors have competing interests.
References
- Amirikia H, Savoy-Moore RT, Sundareson AS, Moghissi KS. The effects of long-term androgen treatment on the ovary. Fertil Steril 1986;45:202–208. [DOI] [PubMed] [Google Scholar]
- Arnold AP, Breedlove SM. Organizational and activational effects of sex steroids on brain and behavior: a reanalysis. Horm Behav 1985;19:469–498. [DOI] [PubMed] [Google Scholar]
- Baba T, Endo T, Honnma H, Kitajima Y, Hayashi T, Ikeda H, Masumori N, Kamiya H, Moriwaka O, Saito T. Association between polycystic ovary syndrome and female-to-male transsexuality. Hum Reprod 2007;22:1011–1016. [DOI] [PubMed] [Google Scholar]
- Becerra-Fernández A, Pérez-López G, Román MM, Martín-Lazaro JF, Pérez MJL, Araque NA, Rodríguez-Molina JM, Sertucha MCB, Vilas MVA. Prevalence of hyperandrogenism and polycystic ovary syndrome in female to male transsexuals. Endocrinol Nutr 2014;61:351–358. [DOI] [PubMed] [Google Scholar]
- Benten WPM, Ulrich P, Kühn-Velten WN, Vohr HW, Wunderlich F. Testosterone-induced susceptibility to Plasmodium chabaudi malaria: persistence after withdrawal of testosterone. J Endocrinol 1997;153:275–281. [DOI] [PubMed] [Google Scholar]
- Caanen MR, Schouten NE, Kuijper EAM, van Rijswijk J, van den Berg MH, van Dulmen-den Broeder E, Overbeek A, van Leeuwen FE, van Trotsenburg M, Lambalk CB. Effects of long-term exogenous testosterone administration on ovarian morphology, determined by transvaginal (3D) ultrasound in female-to-male transsexuals. Hum Reprod 2017;32:1457–1464. [DOI] [PubMed] [Google Scholar]
- Caldwell ASL, Middleton LJ, Jimenez M, Desai R, McMahon AC, Allan CM, Handelsman DJ, Walters KA. Characterization of reproductive, metabolic, and endocrine features of polycystic ovary syndrome in female hyperandrogenic mouse models. Endocrinology 2014;155:3146–3159. [DOI] [PubMed] [Google Scholar]
- Chadha S, Pache TD, Huikeshoven FJM, Brinkmann AO, van der Kwast TH. Androgen receptor expression in human ovarian and uterine tissue of long term androgen-treated transsexual women. Hum Pathol 1994;25:1198–1204. [DOI] [PubMed] [Google Scholar]
- Coleman E, Bockting W, Botzer M, Cohen-Kettenis P, DeCuypere G, Feldman J, Fraser L, Green J, Knudson G, Meyer WJ et al. Standards of care for the health of transsexual, transgender, and gender-nonconforming people, version 7. Int J Transgenderism 2011;13:165–232. [Google Scholar]
- Cora MC, Kooistra L, Travlos G. Vaginal cytology of the laboratory rat and mouse: review and criteria for the staging of the estrous cycle using stained vaginal smears. Toxicol Pathol 2015;43:776–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delić D, Gailus N, Vohr HW, Dkhil M, Al-Quraishy S, Wunderlich F. Testosterone-induced permanent changes of hepatic gene expression in female mice sustained during Plasmodium chabaudi malaria infection. J Mol Endocrinol 2010;45:379–390. [DOI] [PubMed] [Google Scholar]
- De Roo C, Tilleman K, Tsjoen G, De Sutter P. Fertility options in transgender people. Int Rev Psychiatry 2016;28:112–119. [DOI] [PubMed] [Google Scholar]
- Ethics Committee of the American Society for Reproductive Medicine Access to fertility services by transgender persons: an Ethics Committee opinion. Fertil Steril 2015;104:1111–1115. [DOI] [PubMed] [Google Scholar]
- Flores AR, Herman JL, Gates GJ, Brown TNT. How Many Adults Identify As Transgender in the United States? [Internet]. Williams Inst [Internet] 2016; Available from: https://williamsinstitute.law.ucla.edu/wp-content/uploads/How-Many-Adults-Identify-as-Transgender-in-the-United-States.pdf.
- Futterweit W, Deligdisch L. Histopathological effects of exogenously administered testosterone in 19 female to male transsexuals. J Clin Endocrinol Metab 1986;62:16–21. [DOI] [PubMed] [Google Scholar]
- Goetz LG, Mamillapalli R, Devlin MJ, Robbins AE, Majidi-Zolbin M, Taylor HS. Cross-sex testosterone therapy in ovariectomized mice: addition of low-dose estrogen preserves bone architecture. Am J Physiol-Endocrinol Metab 2017;313:E540–E551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimstad FW, Fowler KG, New EP, Ferrando CA, Pollard RR, Chapman G, Gomez-Lobo V, Gray M. Uterine pathology in transmasculine persons on testosterone: a retrospective multicenter case series. Am J Obstet Gynecol 2019;220:257.e1–257.e7. [DOI] [PubMed] [Google Scholar]
- Grynberg M, Fanchin R, Dubost G, Colau JC, Brémont-Weil C, Frydman R, Ayoubi JM. Histology of genital tract and breast tissue after long-term testosterone administration in a female-to-male transsexual population. Reprod Biomed Online 2010;20:553–558. [DOI] [PubMed] [Google Scholar]
- Hembree WC, Cohen-Kettenis PT, Gooren L, Hannema SE, Meyer WJ, Murad MH, Rosenthal SM, Safer JD, Tangpricha V, T’Sjoen GG. Endocrine treatment of gender-dysphoric/gender-incongruent persons: an Endocrine Society* clinical practice guideline. J Clin Endocrinol Metab 2017;102:1–35. [DOI] [PubMed] [Google Scholar]
- Ikeda K, Baba T, Noguchi H, Nagasawa K, Endo T, Kiya T, Saito T. Excessive androgen exposure in female-to-male transsexual persons of reproductive age induces hyperplasia of the ovarian cortex and stroma but not polycystic ovary morphology. Hum Reprod 2013;28:453–461. [DOI] [PubMed] [Google Scholar]
- Light AD, Obedin-Maliver J, Sevelius JM, Kerns JL. Transgender men who experienced pregnancy after female-to-male gender transitioning. Obstet Gynecol 2014;124:1120–1127. [DOI] [PubMed] [Google Scholar]
- Loverro G, Resta L, Dellino M, Edoardo DN, Cascarano MA, Loverro M, Mastrolia SA. Uterine and ovarian changes during testosterone administration in young female-to-male transsexuals. Taiwan J Obstet Gynecol 2016;55:686–691. [DOI] [PubMed] [Google Scholar]
- Ma Y, Andrisse S, Chen Y, Childress S, Xue P, Wang Z, Jones D, Ko C, Divall S, Wu S. Androgen receptor in the ovary theca cells plays a critical role in androgen-induced reproductive dysfunction. Endocrinology 2017;158:98–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer WJ, Webb A, Stuart CA, Finkelstein JW, Lawrence B, Walker PA. Physical and hormonal evaluation of transsexual patients: a longitudinal study. Arch Sex Behav 1986;15:121–138. [DOI] [PubMed] [Google Scholar]
- Miller N, Bédard YC, Cooter NB, Shaul DL. Histological changes in the genital tract in transsexual women following androgen therapy. Histopathology 1986;10:661–669. [DOI] [PubMed] [Google Scholar]
- Mueller A, Gooren LJ, Naton-Schötz S, Cupisti S, Beckmann MW, Dittrich R. Prevalence of polycystic ovary syndrome and hyperandrogenemia in female-to-male transsexuals. J Clin Endocrinol Metab 2008;93:1408–1411. [DOI] [PubMed] [Google Scholar]
- Nelson JF, Karelus K, Felicio LS, Johnson TE. Genetic influences on the timing of puberty in mice. Biol Reprod 1990;42:649–655. [DOI] [PubMed] [Google Scholar]
- Pache TD, Chadha S, Gooren LJ, Hop WC, Jaarsma KW, Dommerholt HB, Fauser BC. Ovarian morphology in long-term androgen-treated female to male transsexuals. A human model for the study of polycystic ovarian syndrome? Histopathology 1991;19:445–452. [DOI] [PubMed] [Google Scholar]
- Padmanabhan V, Veiga-Lopez A. Animal models of the polycystic ovary syndrome phenotype. Steroids 2013;78:734–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P, Wilhelm D, Rodgers RJ. Development of mammalian ovary. J Endocrinol 2014;221:R145–R161. [DOI] [PubMed] [Google Scholar]
- Spinder T, Spijkstra JJ, van den Tweel JG, Burger CW, van Kessel H, Hompes PGA, Gooren LJG. The effects of long term testosterone administration on pulsatile luteinizing hormone secretion and on ovarian histology in eugonadal female to male transsexual subjects. J Clin Endocrinol Metab 1989;69:151–157. [DOI] [PubMed] [Google Scholar]
- The Rotterdam ESHRE/ASRM-Sponsored PCOS consensus workshop group Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod 2004;19:41–47. [DOI] [PubMed] [Google Scholar]
- Houten ELAF, Visser JA. Mouse models to study polycystic ovary syndrome: a possible link between metabolism and ovarian function? Reprod Biol 2014;14:32–43. [DOI] [PubMed] [Google Scholar]
- Walters KA, Allan CM, Handelsman DJ. Rodent models for human polycystic ovary syndrome. Biol Reprod 2012;86:1–12. [DOI] [PubMed] [Google Scholar]
- Wierckx K, van Caenegem E, Pennings G, Elaut E, Dedecker D, Van de Peer F, Weyers S, De Sutter P, T’Sjoen G. Reproductive wish in transsexual men. Hum Reprod 2012;27:483–487. [DOI] [PubMed] [Google Scholar]
- Wierckx K, Van Caenegem E, Schreiner T, Haraldsen I, Fisher A, Toye K, Kaufman JM, T’Sjoen G. Cross-sex hormone therapy in trans persons is safe and effective at short-time follow-up: results from the European network for the investigation of gender incongruence. J Sex Med 2014;11:1999–2011. [DOI] [PubMed] [Google Scholar]
- Yang M, Li J, An Y, Zhang S. Effects of androgen on immunohistochemical localization of androgen receptor and Connexin 43 in mouse ovary. Tissue Cell 2015;47:526–532. [DOI] [PubMed] [Google Scholar]


