Abstract
Corynebacterium ulcerans infection is emerging in humans. We conducted phylogenetic analyses of C. ulcerans and C. diptheriae, which revealed diverse diphtheria toxin in C. ulcerans. Diphtheria toxin diversification could decrease effectiveness of diphtheria toxoid vaccine and diphtheria antitoxin for preventing and treating illnesses caused by this bacterium.
Keywords: Diphtheria toxin, Corynebacterium ulcerans, diversity, amino acid sequence, bacteria, Japan
Corynebacterium ulcerans is a rod-shaped, aerobic, gram-positive bacterium closely related to C. diphtheria. Some strains of C. ulcerans can produce diphtheria toxin, which causes respiratory diphtheria in humans and animals. Reports of human infections with C. ulcerans have increased during the past 20 years, and C. ulcerans is a recognized emerging human pathogen (1). Humans can contract toxin-producing C. ulcerans from companion animals (2,3). Human death can occur if appropriate treatment is delayed (4). Non–toxin-producing C. diphtheriae and C. ulcerans can convert to toxin-producing strains through a process of lysogeny with diphtheria toxin gene–carrying corynebacteriophages (5–7). Although increased coverage of the diphtheria toxoid vaccine has reduced the frequency of C. diphtheriae infections, reports of C. ulcerans infections in humans are increasing.
A report evaluating the differences in the amino acid sequences of the diphtheria toxins in C. diphtheriae and C. ulcerans used only limited data, comparing 1 strain of C. diphtheriae against 2 strains of C. ulcerans (8), leaving the differences among the toxins of these 2 species unclear. Others have conducted bacterial genome analyses and deposited several genomic sequences of C. diphtheriae and C. ulcerans strains into a public database. We collected amino acid sequences of the diphtheria toxin and the nucleic acid sequences of the 16S rRNA gene of 6 C. diphtheriae strains and 6 C. ulcerans strains from the National Center for Biotechnology Information genome database (https://www.ncbi.nlm.nih.gov/genome). Then, we performed phylogenetic analyses by using MEGA 7.0 (https://www.megasoftware.net).
We found that the 16S rRNA gene sequences divided into separate C. diphtheriae and C. ulcerans strains with some sequence variability among the strains in each species (Figure, panel A). The amino acid sequences of the toxins also divided into separate clades for each species. However, we noted that C. diphtheriae strains were identical, but C. ulcerans strains were diverse (Figure, panel B), suggesting that C. ulcerans tends to acquire mutations more frequently than C. diphtheriae. Two possible explanations for this phenomenon are that C. ulcerans is maintained by various animals, increasing its diversity compared with C. diphtheria, which is believed to infect only humans; or that C. ulcerans has a phage-independent pathway to acquire the diphtheria toxin–encoding gene, as reported (9).
Figure.
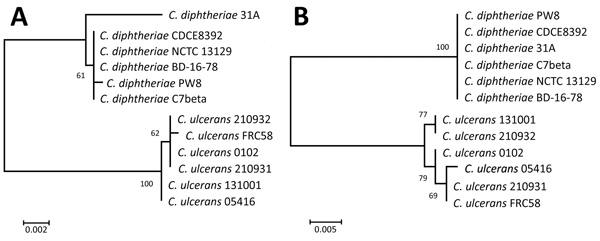
Phylogenetic analysis of the 16S rRNA gene sequences (A) and amino acid sequences (B) of diphtheria toxin genes of 6 Coynebacterium ulcerans strains and 6 C. ulcerans strains. All strains had the diphtheria toxin gene; whole-genome analysis data are available from the National Center for Biotechnology Information database (https://www.ncbi.nlm.nih.gov/genome). We generated phylogenetic trees by using the maximum-likelihood method in MEGA 7.0 (https://www.megasoftware.net). 16S rRNA gene sequences were analyzed by the Hasegawa-Kishino-Yano model with 1,000 bootstrap replications; amino acid sequences were analyzed by the Whelan and Goldman model with 100 bootstrap replications. Scale bars indicate substitutions per site.
Most severe human cases of disease caused by toxigenic C. ulcerans have occurred in unvaccinated or inadequately vaccinated persons. However, a fatal case was reported in a person who received a diphtheria vaccination booster ≈10 years before disease onset (10). Diversification of the C. ulcerans diphtheria toxin gene is of note because accumulation of these gene mutations potentially could lead to decreased effectiveness of the diphtheria toxoid vaccine for prevention and diphtheria antitoxin for treatment of toxigenic C. ulcerans disease.
Acknowledgment
We thank Christopher Carman for his valuable editorial advice on the manuscript.
Biography
Dr. Otsuji is an assistant professor of intensive care medicine at the University of Occupational and Environmental Health Japan, Kitakyushu, Japan. His research interests are critical care and microbiology, including zoonotic infections and microbiota.
Footnotes
Suggested citation for this article: Otsuji K, Fukuda K, Ogawa M, Saito M. Mutation and diversity of diphtheria toxin in Corynebacterium ulcerans. Emerg Infect Dis. 2019 Nov [date cited]. https://doi.org/10.3201/eid2511.181455
References
- 1.Hacker E, Antunes CA, Mattos-Guaraldi AL, Burkovski A, Tauch A. Corynebacterium ulcerans, an emerging human pathogen. Future Microbiol. 2016;11:1191–208. 10.2217/fmb-2016-0085 [DOI] [PubMed] [Google Scholar]
- 2.Katsukawa C, Komiya T, Umeda K, Goto M, Yanai T, Takahashi M, et al. Toxigenic Corynebacterium ulcerans isolated from a hunting dog and its diphtheria toxin antibody titer. Microbiol Immunol. 2016;60:177–86. 10.1111/1348-0421.12364 [DOI] [PubMed] [Google Scholar]
- 3.Katsukawa C, Komiya T, Yamagishi H, Ishii A, Nishino S, Nagahama S, et al. Prevalence of Corynebacterium ulcerans in dogs in Osaka, Japan. J Med Microbiol. 2012;61:266–73. 10.1099/jmm.0.034868-0 [DOI] [PubMed] [Google Scholar]
- 4.Otsuji K, Fukuda K, Endo T, Shimizu S, Harayama N, Ogawa M, et al. The first fatal case of Corynebacterium ulcerans infection in Japan. JMM Case Rep. 2017;4:e005106. 10.1099/jmmcr.0.005106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Freeman VJ. Studies on the virulence of bacteriophage-infected strains of Corynebacterium diphtheriae. J Bacteriol. 1951;61:675–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sekizuka T, Yamamoto A, Komiya T, Kenri T, Takeuchi F, Shibayama K, et al. Corynebacterium ulcerans 0102 carries the gene encoding diphtheria toxin on a prophage different from the C. diphtheriae NCTC 13129 prophage. BMC Microbiol. 2012;12:72. 10.1186/1471-2180-12-72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sangal V, Hoskinsson PA. Corynephages: infections of the infectors. In: Burkovski A, editor. Diphtheria and its etiological agents. Dordrecht (the Netherlands): Springer; 2014. p. 67–82. [Google Scholar]
- 8.Sing A, Hogardt M, Bierschenk S, Heesemann J. Detection of differences in the nucleotide and amino acid sequences of diphtheria toxin from Corynebacterium diphtheriae and Corynebacterium ulcerans causing extrapharyngeal infections. J Clin Microbiol. 2003;41:4848–51. 10.1128/JCM.41.10.4848-4851.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meinel DM, Margos G, Konrad R, Krebs S, Blum H, Sing A. Next generation sequencing analysis of nine Corynebacterium ulcerans isolates reveals zoonotic transmission and a novel putative diphtheria toxin-encoding pathogenicity island. Genome Med. 2014;6:113. 10.1186/s13073-014-0113-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vandentorren S, Guiso N, Badell E, Boisrenoult P, Micaelo M, Troché G, et al. Toxigenic Corynebacterium ulcerans in a fatal human case and her feline contacts, France, March 2014. Euro Surveill. 2014;19:20910. 10.2807/1560-7917.ES2014.19.38.20910 [DOI] [PubMed] [Google Scholar]


