An adhesion-based developmental mechanism triggers global brain circuit organization.
Abstract
Interhemispheric synaptic connections, a prominent feature in animal nervous systems for the rapid exchange and integration of neuronal information, can appear quite suddenly during brain evolution, raising the question about the underlying developmental mechanism. Here, we show in the Drosophila olfactory system that the induction of a bilateral sensory map, an evolutionary novelty in dipteran flies, is mediated by a unique type of commissural pioneer interneurons (cPINs) via the localized activity of the cell adhesion molecule Neuroglian. Differential Neuroglian signaling in cPINs not only prepatterns the olfactory contralateral tracts but also prevents the targeting of ingrowing sensory axons to their ipsilateral synaptic partners. These results identified a sensitive cellular interaction to switch the sequential assembly of diverse neuron types from a unilateral to a bilateral brain circuit organization.
INTRODUCTION
Coherent integration of perception, cognition, and behavior between the two brain hemispheres is mediated by commissural axons forming precise connections between homotopic bilateral synaptic areas (1, 2). The formation of bilateral circuits requires not only a variety of guidance cues and cellular interactions but also the precise regulation of ipsilateral versus contralateral synaptic target recognition (3, 4). Although a diverse set of conserved signaling pathways that control the guidance of commissural neurons at the CNS midline is well characterized (5, 6), we know very little about the coordinated regulation of multiple neural components into a bilateral brain circuit. During brain evolution, novel commissural tracts seem to appear rather rapidly (2). A prominent example for a structural novelty in the nervous system is the emergence of the corpus callosum in placental mammals, according to T. H. Huxley, “the greatest leap anywhere made by Nature in her brainwork” (7). Comparative studies suggest an initial change in axonal interactions for establishing novel interhemispheric connections, but the underlying modification in the developmental program, which causes global brain circuit remodeling, remains obscure (1, 8). In this study, we describe how the activity of a single class of commissural interneurons in the Drosophila olfactory system induces a switch from unilateral to bilateral neural circuit organization.
Olfactory systems are characterized by the precise segregation of sensory neuron projections into distinct synaptic glomerular units, in which odor information is relayed to matching classes of projection neurons (PNs) (9, 10). With a multitude of sensory class-specific synaptic glomeruli, interconnected by various types of modulatory interneurons, vertebrates and insects share main structural and functional features in olfactory map organization (9–11). Although primary sensory representation in most olfactory systems is strictly unilateral, flies have evolved a unique bilateral olfactory map in which olfactory sensory neurons (ORNs) target, in addition to the ipsilateral glomerulus, a homotopic synaptic region on the contralateral hemisphere via a commissural extension (Fig. 1, A and B) (12). As sensory neurons in mosquitoes only connect to the unilateral olfactory brain hemisphere (13), direct bilateral connections may support increased sensitivity and navigation accuracy in fast-flying Diptera with rather short antennae (14, 15).
Fig. 1. Neuroglian is required in bilateral sensory map formation.
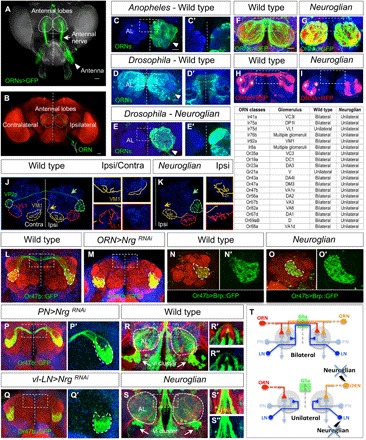
(A and B) Adult olfactory system of Drosophila. (A) Sensory neurons from the antenna (white arrowhead) project via the antennal nerves (white arrow) to the bilateral antennal lobes (ALs; dashed circles). (B) Schematic of a single ORN connecting to a synaptic glomerulus at the ipsilateral AL and a homotopic glomerulus in the contralateral hemisphere (neuropil marker N-cadherin in red). Scale bars, 100 (A) and 50 μm (B). (C to E) Unilateral and bilateral olfactory map organizations in Diptera. Unilateral antennal backfill revealed a strict ipsilateral representation of ORN afferents in mosquitoes (C). In contrast, in higher Brachyceran like Drosophila (D), most ORNs project in a bilateral fashion, as indicated by a large commissural tract and labeling of the contralateral AL. In contrast to wild type, Drosophila carrying a mutation in the cell adhesion molecule Nrg displays a strict unilateral afferent innervation (E). (F to I) Labeling of different bilateral ORN populations in Drosophila wild type (F and H) and nrg mutants (G and I) identified not only the complete absence of the antennal commissure (F and G) but also the precise ipsilateral targeting and class-specific ORN axon convergence [asterisks in (H) and (I)]. (J and K) nrg mutants show a specific loss of bilateral ORN connectivity. (J) Wild type projections from a single olfactory sensillum (ac1) containing two bilateral (Ir92a and Ir31, yellow and green, respectively; arrows indicate contralateral projections) and one unilateral (Ir75d, red) ORN classes. Note the higher degree of synaptic arborization within the ipsilateral glomerulus (left insets) compared to the contralateral target side (right insets). (K) In nrg mutant, bilateral ORN axons show a normal level of ipsilateral arborization but fail to extend any contralateral process (yellow/green arrows, contralateral AL not shown). No changes in the connectivity of the unilateral ORN class can be detected. The table summarizes a systematic analysis of 19 ORN classes in nrg mutants, showing a complete switch of all bilateral into unilateral ORNs but no effect on unilateral ORN classes (100%; n ≥ 8 for wild type and nrg mutant). (L and M) The targeted Nrg RNAi in projecting ORNs (n = 16) uncovers a cell-autonomous function in sensory neurons visualized by the unilateral connectivity (Or47b, green). (N and O) Compared to wild type (N and N′), loss of Nrg (O, O′) has no effect on the presynaptic differentiation at the ipsilateral target side as indicated by the localization of Bruchpilot (Brp) protein. Green, Brp::GFP; red, neuropil marker N-cadherin. (P and Q) Targeted RNAi of Nrg in different cell types of the developing olfactory system. Removal of Nrg from PNs (n = 10) does not influence bilateral ORN (green) connectivity (P and P′). In contrast, loss of Nrg in a cluster of ventro-lateral interneurons (vl-LNs) (n = 8) leads to a complete switch into unilateral ORN circuitry (Q and Q′). (R and S) In the adult olfactory system, a vl cluster (white arrows) of LNs displays, in addition to a broad ipsilateral arborization within the AL, a distinct commissural projection (inset R′ and R″). In nrg mutant, ipsilateral dendritic arborizations seem unaffected, whereas the contralateral LN tract is missing (inset S′ and S″). Green, LNs; red, all neurons labeled by anti-Nrg; blue, neuropil marker N-cadherin. (T) Schematics illustrating sensory map connectivity in the Drosophila olfactory system. Within each pair of homotopic glomeruli, bilateral sensory input (red and orange ORNs) onto unilateral PNs is modified by different classes of bilateral LNs. Loss of Nrg in bilateral ORNs and LNs (but not PNs or midline glial cells) leads to a switch of the bilateral into a unilateral sensory representation. Dashed vertical white lines indicate the midline, commissure position is highlighted by white rectangles, and dotted circles show glomerulus boundaries. Scale bars, 20 μm for all images of adult ALs.
Here, we show that the bilateral sensory map in the Drosophila olfactory system is completely reverted into a unilateral circuit in mutants of the cell adhesion molecule Neuroglian. We could localize Neuroglian activity in a small cluster of contralaterally projecting interneurons, which not only pioneer the commissural sensory tract but also interfere with synaptic partner recognition of these sensory neurons on the ipsilateral target region. As olfactory circuit assembly relies on defined hierarchy of cell type interactions, these findings offer a rather simple mechanism to switch a complete developmental program from the ipsilateral to the contralateral hemisphere.
RESULTS
Loss of Neuroglian switches bilateral to unilateral sensory neuron innervation
To visualize sensory map organization in the olfactory system within Diptera, we performed unilateral labeling of the antennal nerve and determined unilateral versus bilateral projection patterns in the antennal lobes (ALs) of multiple Nematocera and Brachycera species (fig. S1). The bilateral sensory representation is absent in most Nematoceran species like Culicidae and Simuliidae and becomes prominent in basal Brachycera flies like Bombyliidae and Dolichopodidae. In all Schizophora species analyzed, including Drosophilidae and Calliphoridae, most sensory neurons display interhemispheric connection (Fig. 1, C and D, and fig. S1), therefore providing an excellent experimental system to determine the genetic regulation of bilateral neural circuit formation. In a candidate gene approach in Drosophila using unilateral antennal labeling of mutants combined with targeted RNA interference (RNAi) in projecting ORNs, loss of the immunoglobulin (Ig) family member protein Neuroglian (Nrg) leads to a striking and highly penetrant connectivity phenotype: Axons of bilateral ORNs project only to their ipsilateral target glomerulus with a complete absence of a contralateral connection, thereby switching the olfactory sensory map back to the unilateral organization (Fig. 1, E, F to I, L, and M). A systematic analysis of multiple ORN classes in the nrg mutant background showed that most bilateral neuron classes display a precise ipsilateral connectivity pattern similar to unilateral ORN classes in wild type (Fig. 1, table, and fig. S2). Terminal arborization remains confined within glomerular boundaries (Fig. 1, J and K), and no changes in presynaptic density (Fig. 1, N and O) of ORN axons within the ipsilateral hemisphere can be observed in nrg mutants. These data indicate a crucial developmental step in olfactory system formation to determine a unilateral versus bilateral state of circuit organization.
Non-autonomous function of Neuroglian in bilateral ORN connectivity
Besides ORNs, Nrg is expressed in various central neurons and glial cells (16) in the developing and adult olfactory system (fig. S3), raising the question about the underlying cellular interactions for bilateral circuit formation. We therefore extended the targeted Nrg RNAi approach using a collection of developmentally expressed Gal4 lines for different olfactory cell types. In contrast to a previous study (16), interfering with Nrg function in various olfactory glia types (via repo-Gal4 and 442-Gal4) did not affect the wild type connectivity pattern of bilateral ORNs (fig. S4). Similarly, developmental knockdown of Nrg in PNs (via GH146-Gal4), the main synaptic partner neurons of ORNs, has no effect on sensory axon targeting (Fig. 1P). However, loss of Nrg in a specific cluster of ventro-lateral (vl) interneurons (via OK107-Gal4 and OK371-Gal4) results in a unilateral ORN connectivity phenotype (compare Fig. 1, Q and M). In the adult olfactory system, the vl cluster contains different types of unilateral and bilateral interneurons with specific neural arborization patterns (Fig. 1R) (17, 18). nrg mutant vl-cluster neurons fail to develop a contralateral projection but show no change of arborization within the ipsilateral hemisphere (Fig. 1, S and T).
Commissural pioneer interneurons prepattern bilateral ORN projections
Pioneer sensory axons in the Drosophila olfactory system show high Nrg expression and segregate into a lateral and medial pathway while extending dorsally toward the midline of the developing AL (Fig. 2A and fig. S3). By the time of sensory neuron ingrowth, vl-cluster interneurons have established restricted neural arborizations within the AL and a prominent midline commissure (Fig. 2B). To determine the neuronal diversity of commissural interneurons critical for bilateral circuit organization, we analyzed a collection of expression lines (Fig. 2, C to G) (19) and identified a small population of commissural pioneer interneurons (cPINs), which, upon Nrg knockdown, results in a unilateral ORN projection phenotype (fig. S5). Similarly, targeted ablation of cPINs before ORN innervation leads to a unilateral sensory map (fig. S6). Further developmental and clonal characterization revealed two main morphological cPIN classes, which prefigure the early sensory projections: Most cPINs (8 to 10 neurons) extend along the dorso-lateral pathway and form a restricted commissural tract at the dorsal AL (“lateral cPINs”; Fig. 2, C and F). A smaller subset of cPINs (two to four neurons) projects via the medial AL on the ipsilateral and contralateral hemisphere (“medial cPINs”; Fig. 2, C and G). The neural arborizations of lateral and medial cPINs remain separated in nrg mutants, but both classes of interneuron fail to extend a commissural tract (Fig. 2, D and E).
Fig. 2. Domain-specific organization of cPINs.
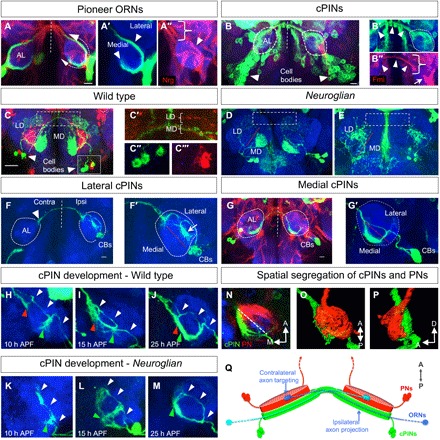
(A and A″) At about 20 hours after pupa formation (APF), Nrg-positive [arrowhead in (A″)] ORN pioneer axons enter the AL and segregate into a lateral and a medial fascicle [arrows in (A) and arrowheads in (A′)], which extend toward the dorsal midline of Nrg-positive fibers [bracket in (A″)]. (B and B″) At the time of ORN axon arrival, a vl cluster (cell bodies indicated by arrowheads) of cPINs has developed localized ipsilateral dendritic arborizations [dashed circles in (B′)] and broad contralateral projection at the dorsal AL [arrowheads in (B′) and (B″)]. ORN axons [arrow in (B″)] and the commissural tract of cPINs [bracket in (B″)] can be identified by their strong expression of the cell adhesion molecule Flamingo [Fmi in (B″)]. (C and C‴) On the basis of the spatial segregation of their dendritic fields in the adult AL, two main classes of cPINs, the lateral and the medial, can be recognized [lateral/medial domain (LD/MD)]. The cell bodies of both cPIN classes are in close proximity [inset, (C″) and (C‴)], but their commissural tracts in the dorsal AL remain separated [dashed rectangles in (C) and brackets in (C′)]. (D and E) In nrg mutants, the dendritic field of each cPIN class in the ipsilateral AL (lateral/medial domain) remains correctly positioned, but the contralateral projection is missing [dashed rectangles in (D) and (E)]. (F and G) Wild type organization of cPIN classes. Before ORN axon arrival, dendritic fields of the cPIN classes segregate in the ipsilateral and contralateral AL and both lateral and medial cPINs have distinct projection patterns. (F and F′) Lateral cPINs have a distinct ipsilateral dendritic field [arrow in (F′)] and a strong commissural tract, which terminate at the dorsal edge of the contralateral AL [arrowhead in (F)]. In contrast, medial cPINs have a thin commissural tract, which extend to ventral region of the contralateral AL (G and G′). CBs, cell bodies. Development of cPIN in wild type (H to J) and nrg mutants (K to M). With the beginning of metamorphosis, cPINs start to extend to the dorsal AL midline, and ventral extensions of the ipsilateral dendritic arborizations become visible. Following the initiation of the ipsilateral dendritic field [green arrowhead in (H)], cPINs project a pioneer commissural track across the dorsal midline [red arrowhead, showing contralateral axon, in (H)], which grows along the medial surface of the contralateral AL (I) to merge with the ipsilateral dendritic arborization at the time of ORN axon arrival (J). In nrg mutants, no changes can be observed for initiation of the dendritic field [green arrowhead in (K)] and the dorsal extension of cPINs within the ipsilateral AL [white arrowheads in (K)]. However, the dorsal process loops back, extends ventrally, and “self-merges” with the ventral ipsilateral processes (L), subsequently forming the appropriate dendritic field in the medial AL region (M). (N) Dendritic fields of cPINs (green) and PNs (red) are spatially segregated within the early AL, with only cPIN localized at the ORN axon entry side in the posterior AL. (O and P) Anterior and lateral view of the AL, respectively. (Q) Model of lateralized ORN axon projection and targeting: ORN axons enter the ipsilateral AL at the posterior domain and are guided by cPINs toward the dorsal ML. With the contralateral hemisphere, ORN axons switch to the anterior domain to recognize their corresponding PN target neurons. Dashed vertical white lines indicate midline, developing AL is indicated by white dashed circles, and lateral and medial domains are indicated by red dashed lines. Scale bars, 10 μm for all images of pupal and 20 μm for adult ALs (C to E).
During wild type development, outgrowing cPINs start to project at late third instar larval stage to establish ipsilateral arbors and a commissural extension, which converge on the contralateral side (Fig. 2, H to J, and fig. S7). In nrg mutants, the formation of ipsilateral processes is not affected, but the contralateral extensions become rerouted and merge with the ipsilateral arborizations (Fig. 2, K to M). Co-labeling of cPINs and unilateral PNs revealed a spatial segregation of their neuronal fields by the time of pioneer ORN arrival. Here, the entry side of pioneer ORN axons at the posterior AL is covered by cPIN arborizations and devoid of PN dendrites, which are enriched at the anterior AL region. These distinct AL domains for extending ORN axons, a posterior projection domain and an anterior targeting domain, support an instructive role of cPINs in bilateral sensory neuron innervation by preventing ipsilateral axon-target interaction via spatial segregation from the synaptic area (Fig. 2, N to Q).
Neuroglian suppresses ipsilateral ORN axon targeting
To induce interhemispheric circuit organization, a likely cellular scenario would be the formation of a novel contralateral branch following the default developmental program of unilateral axon targeting (Fig. 3A, 1). However, the comparison of initial axon targeting of unilateral and bilateral pioneer neurons in wild type and nrg mutants points toward an alternative developmental strategy to coordinate bilateral innervation (Fig. 3A, 2). In wild type, axons of atonal (ato)–positive pioneer ORNs form a solid fiber track (Fig. 3B), which extends beyond the ipsilateral target region (Fig. 3C). After sending a commissural process across the dorsal midline (Fig. 3D), glomeruli are induced via bilateral axon convergence (Fig. 3E). In contrast, loss of Nrg results in a strong accumulation of pioneer axons at the ipsilateral prospective target side (Fig. 3, F and G). During the period of contralateral axon projection in wild type, nrg mutants show an accelerated glomerular convergence (Fig. 3H) but no obvious differences in glomerulus maturation (Fig. 3I). Single-cell analysis revealed a dynamic growth cone morphology with a dense array of filopodia all along the AL surface (Fig. 3, J to M). Here again, no signs of filopodia enrichment at the putative target region can be detected. During ipsilateral extension, single axons form the same amounts of filopodia in the central and dorsal AL domains (Fig. 3, L and P). By the time of contralateral axon projection, the number of ipsilateral filopodia reduces in the dorsal area and processes at the central synaptic target region become stabilized (Fig. 3, M and Q). In contrast, axons of unilateral ORN display a restricted field of filopodia during initial targeting and axon convergence (Fig. 3, N to Q). In the subsequent period of glomerulus assembly, nrg mutants show an increased axonal restriction at the unilateral presynaptic region (Fig. 3, S and U) compared to the bilateral innervation in wild type (Fig. 3, R and T). These results show that the transient suppression of ipsilateral target site recognition defines a key event in bilateral map formation. Here, ipsilateral ORN-cPIN interaction shifts the primary ORN-PN recognition program to the contralateral hemisphere followed by ipsilateral axon targeting. The functional coupling of delayed axon targeting and contralateral growth by cPINs ensures an organized assembly of bilateral sensory circuits.
Fig. 3. Sensory neurons bypass their ipsilateral target.

(A) Two alternative developmental pathways to switch from unilateral to bilateral circuit assemblies: (1) Following the ipsilateral ORN targeting to glomerulus-specific PNs, contralateral innervation is induced via a commissural branch (blue) across the midline (ML). (2) Direct contralateral projection via suppression of ipsilateral targeting followed by the induction of an ipsilateral synaptic collateral (blue). (B to I) Axon growth analysis of a single pioneer ORN class (Ir92a), which targets a ventral medial glomerulus (VM1; see Fig. 1). In wild type, pioneer Ir92a ORN axon enters the AL around 20 hours APF (B) and extends along the medial pathway to the dorsal AL with no signs of accumulation at the putative target region in the ventro-medial AL region (C) (white dashed lines). Following midline crossing and extension to the contralateral target region [red line in (D)], ORN axons converge within the next 20 hours into spatially restricted synaptic glomeruli (E). (F to I) In nrg mutants, ORN axons reach the AL within the temporal period of wild type axons. In contrast to the smooth ipsilateral extension of pioneer axons in wild type, loss of nrg leads to an instant accumulation of pioneer axons at the prospective ventral target region [arrowhead in (F)]. During the period of wild type dorsal extension and contralateral projection (25 to 30 hours APF), nrg mutant pioneer axons converge prematurely at the target region [arrowheads in (G) and (H)], with no differences during the following period of glomerulus maturation (I). (J to Q) Single-cell analysis of pioneer axon branch dynamics. During the period of ipsilateral growth, individual axons of bilateral ORNs induce a large number of lateral processes all along the medial AL neuropil [ventral, central, and dorsal area in (L)] with no enrichment at the prospective target region [TR; red dashed lines in (J), high magnification in (J′), and quantification in (P)]. Following the contralateral projection, the number of ipsilateral filopodia reduces at the dorsal AL and restricts to the prospective ventro-medial target region [(K) and (M); quantification in (Q)]. In contrast to bilateral ORNs, axons of ingrowing unilateral ORNs aggregate at the prospective ventral target region, with filopodia extending into multiple directions [(N) and (O); quantifications in (P) and (Q)]. (R to U) Similarly to the sequence of axon projection, the presynaptic differentiation following contralateral projections, as indicated by the localization of Bruchpilot-GFP, is more restricted in nrg mutants compared to wild type (R and S), but similar pattern of synaptic maturation is observed during glomerulus assembly (T and U). Scale bars, 10 μm for all images of pupal ALs.
Differential Neuroglian signaling mediates hierarchical neuron interactions in bilateral circuit formation
Neuron type–specific interference with Nrg function revealed not only a cell-autonomous role in cPINs and ORNs but also a strict hierarchy in their cellular interactions [Fig. 4, B to M, summarized in (A)]. The removal of Nrg from cPINs triggers unilateral targeting of all bilateral ORN classes (Fig. 4, F and G), whereas the knockdown of Nrg in ORNs has no effect on the bilateral organization of cPINs (Fig. 4, H and K). Similarly, axons of amos-positive follower ORNs rely on Nrg expression in cPINs and atonal-positive pioneer sensory neurons (Fig. 4J) but not vice versa (Fig. 4, K and L), indicating a defined sequence of interneuronal interactions, which correlates with the specific window of axon growth (Fig. 4A and fig.S8). Furthermore, Contralaterally projecting Serotonin-immunoreactive Deutocerebral neurons (CSD) (20), which innervate the AL after sensory neurons, depend on Nrg specifically for the olfactory commissure but not for the development of an evolutionary more ancient protocerebral commissure (fig. S9) (20). cPINs subsequently develop into glutamatergic inhibitory interneurons of the adult olfactory system (21), indicating that an efficient bilateral odor representation requires the combined segregation of different types of modulatory interneurons along with sensory neurons.
Fig. 4. Neuroglian-dependent hierarchical interactions coordinate sensory map development.

(A) Analysis of cell type–specific Nrg RNA interference (RNAi) to define autonomous and non-autonomous functions of sequentially ingrowing cPINs (blue), pioneer (red), and follower (green) ORNs, as well as serotonergic CSD neurons (magenta). The table summarizes the resulting connectivity phenotypes. (E to G) Removal of Nrg in developing cPINs does not only switch bilateral into unilateral interneurons (E) but also disrupt the bilateral projection of pioneer and follower ORNs [(F) and (G), respectively, compared to wild type (B to D)]. (H to J) Down-regulation of Nrg in pioneer neurons interferes with their bilateral projection (I) and the projections of follower neurons (J) but has no effect on bilateral cPIN development (H). (K to M) Restricted removal of Nrg in late-projecting bilateral ORN classes (“followers”) transforms them into a unilateral projection type (M) but leaves the bilateral organization of cPINs (K) and pioneer ORNs (L) unaffected, demonstrating a strict temporal hierarchy of Nrg-mediated interneuronal interactions in bilateral circuit formation. (N to R) Analysis of Nrg domain requirement for two classes of cPINs (N and O) and two classes of bilateral ORN types, pioneer ORNs (P), and follower ORNs (Q). Removal of the consensus sequence for Ankyrin signaling (∆FIGQY) strongly affects the bilateral projection of follower ORNs (Q′) but not pioneer ORNs (P′) and cPINs (N′ and O′). The combined deletion of Ankyrin and PDZ binding domains (∆C) switches all bilateral ORNs into a unilateral connectivity pattern (P″ and Q") but does not interfere with bilateral cPIN development (N″ and O″). The deletion of the Moesin-binding domain (∆FERM) leads to a complete unilateral connectivity pattern for all four classes of bilateral olfactory neurons (N‴ to Q‴). (R) Schematics illustrate domain organization of Nrg and the connectivity phenotype of the deletion mutants (top) and their quantification (bottom). Scale bars, 20 μm for all images of adult ALs. For the list of genotypes used in this study, see table S1.
As Nrg-mediated adhesion triggers different types of intracellular signaling pathways (22), we tested if the sequence of bilateral neuronal interactions involves distinct downstream effectors (Fig. 4, N to R). Loss of the PDZ interacting domain has no effect on bilateral map formation (Fig. 4R), whereas the interference with Ankyrin binding disrupts bilateral organization of amos-positive follower ORNs (Fig. 4Q′) but not cPIN and pioneer ORN connectivity (Fig. 4, N′ to P′). The combined deletion of Ankyrin/PDZ interacting domains affects all bilateral ORN classes, suggesting partially redundant function in pioneer ORNs (Fig. 4, P″ and Q″) but not cPINs (Fig. 4, N″ and O″). Bilateral cPIN development is mediated via Moesin interaction, with the complete absence of a contralateral extension following the deletion of the corresponding intracellular domain (Fig. 4, N‴ and O‴). These results revealed distinct effector pathways in the Nrg-mediated hierarchical interactions to coordinate the bilateral assembly of key circuit components.
DISCUSSION
Olfactory coding relies on the precise synaptic matching of distinct classes of sensory to the corresponding set of central PNs. During Drosophila olfactory system development, PN dendrites localize within the olfactory target field according to their final glomerular position before sensory neuron arrival and induce the ORN class-specific axonal convergence by a mostly unknown recognition process (23). In contrast to a unilateral sensory map, in which sensory neurons have to connect to a single array of PN classes at the ipsilateral brain hemisphere, the development of bilateral ORN connectivity has to coordinate the interaction of projecting sensory axons with two mirror-symmetric populations of unilateral PNs.
On the basis of the adult morphology, bilateral sensory neuron innervation in Drosophila has been considered as a sequential process where ipsilateral axon targeting is followed by the extension of a contralateral branch toward the homotopic synaptic PN class (24). Here, we show that bilaterality is established through direct contralateral targeting of sensory axons, followed by the induction of synaptic connections on the ipsilateral hemisphere (Fig. 3A). We identified a distinct class of commissural interneurons, which not only provide a contralateral tract for ingrowing sensory neurons but also prevent their interaction with ipsilateral PNs. On the basis of the spatial segregation of PN and cPIN processes in the early target region, we are proposing a mechanism in which ipsilateral target site recognition of ORNs is suppressed by a cPIN-based projection domain distinct from the PN-defined targeting region (Fig. 2Q). ORN axons exit the antennal nerve encounters cPIN processes at the posterior target region and extend toward and across the dorsal midline to interact with the contralateral PN field. Nrg function allows ORN axons to stay within the projection domain, most likely via a direct interaction with cPIN processes, in which loss of Nrg in cPIN or the removal of cPIN itself results in ipsilateral circuit assembly. A small subset of unilateral ORN classes in Drosophila escapes the cPIN-mediated contralateral guidance mechanisms (fig. S2D). Here, target regions of unilateral ORNs are clustered in distinct vl AL domain, indicating an additional level of domain organization in the target region.
Mutations in the human Nrg homolog, L1CAM, lead to a severe reduction in corpus callosum formation (25). During cortex development, callosal projection neurons build homotopic connections in a sequential manner, in which axons of early-generated neurons pioneer the commissural tract, providing a conceptual framework for corpus callosum evolution (1, 8). With a conserved midline pattern between vertebrates and insects (5, 6), the identification of distinct unilateral and bilateral sensory maps within dipteran species offers a unique opportunity to determine how evolutionary-dynamic Nrg expression could support novel cellular interactions. For example, persisting Nrg-positive larval commissures in close proximity to the developing adult olfactory system (26, 27) could provide a permissive substrate for unilateral cPIN precursors to extend across the midline. A similar mechanism has been proposed for corpus callosum development with novel adhesive interactions of pioneer fibers with more ancient commissures of the cingulate cortex and hippocampus (1, 8). As sequential afferent interaction is a common theme not only among sensory neurons in insect and vertebrate olfactory system development (28–31) but also in corpus callosum formation (1, 8); cPINs could have “hijacked” preexisting Nrg-mediated ORN interactions and thereby shifting unilateral olfactory circuit assembly to the contralateral hemisphere followed by ipsilateral collateral formation. Callosal projection neurons extending to the contralateral hemisphere leave filopodia behind, from which subsequently interstitial collateral branches emerge (32).
In an alternative scenario, bilateral cPIN may have appeared de novo and triggered interhemispheric connectivity of sensory neurons. The ventral AL neuroblast lineages generate a highly diverse population of bilateral neurons (18, 26), and a recent study showed how changes in Hox gene expression within these progenitors result in major remodeling of brain circuit organization (33). As the larval olfactory system in higher dipterans displays a unilateral connectivity pattern (34), an adult life style with highly agile flight behaviors seems to be a major determinant for the evolution of bilateral sensory representation. Even unilateral ORN classes have established interglomerular connectivity via a unique type of bilateral PNs (35), indicating a strong requirement for fast interhemispheric communication. The enhanced sensitivity due to a higher degree of sensory convergence in bilateral versus unilateral olfactory circuits is accompanied by a substantial reduction in spatial information. It is tempting to speculate that the bilateral olfactory map became stabilized in the course of dipteran evolution by strengthening directional sensitivity via lateralized synaptic differentiation (36).
How do cPINs cross the midline in the first place? Although a balanced activity of chemoattraction and chemorepulsion has been a broadly accepted mechanism in bilateral circuit development for decades (5, 6), recent studies on Netrin signaling have challenged the concept of long-range guidance also at the midline, and a critical role for cell adhesion has been proposed in both vertebrates (37, 38) and insects (39). In Drosophila, a midline glial structure [transient interhemispheric fibrous ring (TIFR)] has been shown to support ORN axon crossing (16), but interference with Netrin signaling in this region does not prevent bilateral map formation. In addition, the different Robo genes show differential expression in the developing AL, but commissure defects have only been described in gain-of-function studies (40), further supporting an adhesion-based mechanism of midline guidance. Although similarities in brain circuit organization and developmental cell-cell interactions suggest a Nrg-related mechanisms in the formation of bilateral circuits in other animals, a direct experimental proof requires the identification of cPIN neurons and the analysis of Nrg expression patterns. With the rapidly improving technologies of targeted gene knockout and transgene expression outside Drosophila, our results will stimulate future comparative studies, especially within more basal Diptera, to determine how modulation of cell adhesion can trigger brain circuit evolution.
MATERIALS AND METHODS
Methods summary
For developmental analysis of individual ORN axon targeting, wild type single-cell clones were generated using the Flybow (FB) construct (41) in combination with a heat-induced FLPm5 on second chromosome. Flies expressing FB1.1B transgene under the control of R86G11-Gal4 (19) were exposed to single heat shock for 90 min at 37°C to induce transient mFLP5 (41) activity between 0 and 5 hours after pupa formation (APF). Confocal images were processed and analyzed using ImageJ and Imaris 9.2 (Bitplane).
To characterize the neuronal morphology of cPIN, wild type single-cell clones were generated using the hs-FLP and FRT/FLP system (42). Wild type cPINs were labeled with R19H07-Gal4 (19), driving the expression of UAS-mCD8::GFP (42). Second instar larvae were heat-shocked for 20 to 30 min at 37°C. Pupae at the desired stage were dissected. The developmental pattern of pioneer ORNs, their synaptogenesis and LNs, in wild type and Nrg mutant background was analyzed in confocal image stacks of stained pupal and adult brains. R86G11-Gal4 (19) and R20F11-Gal4 (19) driver lines for ORNs and LNs were used, respectively. Nrg mutant hemizygous males and heterozygous females (control) were preselected at third instar and separated in different food vials for both clonal and expression analysis. White pupae (defined as 0 hours APF) were collected for staging at 25°C. Pupae at desired age and adult flies were dissected after eclosion. For developmental analysis, 5 to 10 brains were analyzed at each time point.
Methods
Fly stocks and genetics
Fly stocks and crosses were maintained in standard medium at 25°C, whereas RNAi experiments were performed at 29°C. To discover molecules involved in bilateral sensory map formation, an RNAi screen was conducted against several cell adhesion molecules using pebbeled-Gal4 [a gift from L. Luo (28)]. To perform Nrg RNAi knockdown in ORNs, glia, and AL-associated neurons, the following stocks were obtained from Bloomington Drosophila Stock Center (BDSC) or received as gift: ORN drivers, Sg18.1-Gal4 (43) (BDSC 6405), atonal-Gal4 (a gift from B.A. Hassan), and amos-Gal4 [a gift from T. Chihara (29)]; ORN-specific drivers and markers were provided by B. Dickson and L. Vosshall (44, 45); LN drivers, OK107-Gal4 [BDSC 854 (18)] and OK371-Gal4 [a gift from H. Aberle (17)]; Gal4 and LexA driver lines having expression in subset of LNs were selected on the basis of expression pattern (19) and were obtained from BDSC; glia-specific drivers, repo-Gal4 (BDSC 7415), 442-Gal4 [a gift from T. Préat (16)], PN-specific GH146-Gal4 (46), UAS-NrgRNAi (BDSC 38215), GH146-QF, and QUAS-mtdTomato-3×HA (BDSC 30037). Stocks used for Nrg mutant and intracellular domain deletion constructs (P[acman] constructs) were the following: nrg849 (BDSC 35827), nrg14, and P[acman] constructs were provided by J. Pielage (22). For visualizing axons and synaptic terminals and co-labeling of two distinct cell types, reporters of different binary systems were used: UAS-mCD8::GFP (42), UAS-Brp::GFP (BDSC 36291), UAS-mCherry (BDSC 27392), 10XUAS-mCD8::RFP,13X LexAop2-mCD8::GFP (BDSC 32229), GH146-QF, QUAS-mtdTomato-3xHA (BDSC 30037). For generating single-cell clones, hs-FLPm5 (41) (BDSC 56799 and BDSC 35534) and UAS-Flybow1.1B (41) (BDSC 56803) constructs were used. For cell ablation, the following stocks were used: UAS-DTI (BDSC 25039) and UAS-hid (a gift from J. R. Nambu).
Immunohistology
Dissection of the larval, pupal, or adult brains was carried out in 1× phosphate-buffered saline (PBS) and fixed in 2% freshly prepared paraformaldehyde (PFA) (prepared in 1× PBS) for 60 min for larval and pupal brains and 90 min for adult brains at room temperature. The fixative was removed, and the brains were washed four times with 0.3% PBTx (Triton X-100 in 1× PBS) for 15 min each at room temperature. Blocking of the samples was performed for 60 min at room temperature in 10% goat serum, prepared in 0.3% PBTx, and incubated with primary antibody overnight at 4°C. Following four times washing, 15 min each, samples were incubated with secondary antibody overnight at 4°C. After four times washing, 15 min each, samples were mounted in VECTASHIELD (Vector Laboratories), an antifade mounting medium for confocal microscopy. Both primary and secondary antibodies were diluted in 10% goat serum. Fluorescent samples were analyzed using a Leica TCS SP5II confocal microscope. To process and analyze images and quantify phenotypes, the open source tool ImageJ, Adobe Photoshop, and Imaris (Bitplane) were used.
Primary antibodies used for this study were rat anti–N-cadherin extracellular domain [DN-Ex no.8, 1:10; Developmental Studies Hybridoma Bank (DSHB)], mouse anti-Flamingo (1:5; DSHB), mouse anti–Neuroglian-180 (BP 104, 1:10; DSHB), rabbit anti-GFP (green fluorescent protein) (1:1000; Invitrogen), and mouse anti-Fasciclin2 (1:5; DSHB). Secondary antibodies used were as follows: goat anti-rabbit Alexa 488 (1:500), goat anti-rat Alexa 568 (1:300), goat anti-rat Alexa 647 (1:500), goat anti-mouse Alexa 568 (1:300), and avidin Alexa 488 (1:40). For general nuclear staining, TOTO-3 (1:5000) was used. All secondary antibodies were obtained from Invitrogen.
Unilateral antennal backfills
All experiments were performed on adult flies. Mosquitoes (Anopheles arabiensis) were provided by International Atomic Energy Agency Laboratories, Seibersdorf Laboratories, Austria. Almost all diptera species were collected within the state of Vienna area, with the exception of Hermetia illucens, which was caught in South Tyrol. Species identity of fly samples was performed by a diptera determination key to reach family level and further specific literature to refine the taxon. In cases where a genus level could not be reached or there were remaining uncertainties, the help of experts on the diptera forum (www.diptera.info) was claimed. Living flies were first anesthetized via CO2 and then inserted into 200- or 1000-μl plastic pipette tips, where parts of the tips have been cut off according to the body size so that the head could stick out of the tip. The flies were immobilized with plasticine. In a petri dish, the loaded pipette tip was placed on a plasticine cube with a small indentation for the fly head. To apply the tracer, a wall was built around the antenna with vaseline, creating a small cavity and leaving only one antenna exposed. In all flies, the right antenna was cut, at the base of the third segment, and completely submerged with a drop of neuronal backfill tracer—2% neurobiotin (Vector Laboratories) diluted in Millipore water. The cavity was then completely covered with vaseline to prevent desiccation, and the petri dish was kept in 4°C for 90 min. Afterward, fly heads were removed and processed with the abovementioned immunohistology process for visualization on the same day (47). DN-cadherin was used as a general neuropil marker, and anti-Nrg was used to label the olfactory commissure.
Flyclear
Three- to 5-day-old adult flies were fixed in 4% PFA at 4°C for 90 min, followed by three times washing with 1× PBS at 4°C for 20 min. Furthermore, the flies were dipped in Solution-1 (48) for 5 days at 37°C. Flies with the complete depigmentation of the compound eyes were further processed and washed with 1× PBS for three times at 25°C. Last, the samples were immersed in Solution-2 (48) for a minimum of 1 day at 25°C and mounted in VECTASHIELD (Vector Laboratories) for imaging using a Leica TCS SP5II confocal microscope.
Cell ablation
For the genetic ablation of cPINs, two different reagents were used. The expression of temperature-sensitive diphtheria toxin (UAS-DTI) was induced directly from Gal4 driver line, and its expression was developmentally controlled by temperature shifts. In the case of UAS-hid, Gal4 expression was controlled via Gal80ts. In both cases, the crosses were raised at 18°C. Late third instar larvae were picked and kept at 29°C to allow transgene expression. The adult flies were dissected 3 days after eclosion.
Supplementary Material
Acknowledgments
We are grateful to B. A. Hassan, T. Chihara, J. Pielage, H. Aberle, and T. Préat for providing critical reagents for this study. We would like to thank the BDSC for Drosophila transgenic lines and DSHB for antibodies. We thank H. Yamada for providing Anopheles arabiensis. We thank B. Bergkirchner and A. Kasture for their help with filament tracing. We further thank L. Luo, Y. Chou, and M. Pende for stimulating discussions and members of the Hummel laboratory for critical comments on the manuscript. Funding: DFG (HU 992/21), Schram Foundation (T287/22478/2012), and ROL (254) Research Platform Vienna University supported this work. Author contributions: R.K.: conceptualization and investigation, acquisition of data, analysis and interpretation of data, and writing the article; M.S., S.H., N.G., and A.G.: acquisition and analysis of data; L.T.: identification of different fly species; L.T. and W.K.: antennal backfilling; T.H.: conceptualization, design, supervision, analysis and interpretation of data, writing (original article), and funding acquisition. Competing interests: The authors declare that they have no competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from the authors.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/5/10/eaaw5537/DC1
Fig. S1. Unilateral versus bilateral olfactory circuit organization within Diptera.
Fig. S2. Comprehensive analysis of unilateral and bilateral projecting antennal ORN axon in Neuroglian mutant (see also table in Fig. 1).
Fig. S3. Neuroglian expression during development.
Fig. S4. Neuroglian expression in midline glia cells is dispensable for bilateral ORN connectivity.
Fig. S5. Cell-specific loss of Neuroglian in cPINs affects bilateral olfactory map formation.
Fig. S6. Ablation of cPINs affects bilateral connectivity of ORNs.
Fig. S7. cPINs segregate from PNs in early AL development.
Fig. S8. Cell autonomous and non-autonomous function of Neuroglian in cPINs and ORNs.
Fig. S9. Neuroglian affects olfactory commissure development of CSD neurons.
Table S1. Genotype of experiments.
Reference (49)
REFERENCES AND NOTES
- 1.Suárez R., Gobius I., Richards L. J., Evolution and development of interhemispheric connections in the vertebrate forebrain. Front. Hum. Neurosci. 8, 497 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aboitiz F., Montiel J., One hundred million years of interhemispheric communication: The history of the corpus callosum. Brazilian J. Med. Biol. Res. 36, 409–420 (2003). [DOI] [PubMed] [Google Scholar]
- 3.Fenlon L. R., Richards L. J., Contralateral targeting of the corpus callosum in normal and pathological brain function. Trends Neurosci. 38, 264–272 (2015). [DOI] [PubMed] [Google Scholar]
- 4.Fame R. M., MacDonald J. L., Macklis J. D., Development, specification, and diversity of callosal projection neurons. Trends Neurosci. 34, 41–50 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dickson B. J., Molecular mechanisms of axon guidance. Science 298, 1959–1964 (2002). [DOI] [PubMed] [Google Scholar]
- 6.Evans T. A., Bashaw G. J., Axon guidance at the midline: Of mice and flies. Curr. Opin. Neurobiol. 20, 79–85 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.T. H. Huxley, Man’s Place in Nature (London, Williams and Norgate, 1863).
- 8.Richards L. J., Plachez C., Ren T., Mechanisms regulating the development of the corpus callosum and its agenesis in mouse and human. Clin. Genet. 66, 276–289 (2004). [DOI] [PubMed] [Google Scholar]
- 9.Brochtrup A., Hummel T., Olfactory map formation in the Drosophila brain: Genetic specificity and neuronal variability. Curr. Opin. Neurobiol. 21, 85–92 (2011). [DOI] [PubMed] [Google Scholar]
- 10.Imai T., Sakano H., Vosshall L. B., Topographic mapping—The olfactory system. Cold Spring Harb. Perspect. Biol. 2, a001776 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hildebrand J. G., Shepherd G. M., Mechanisms of olfactory discrimination: Converging evidence for common principles across phyla. Annu. Rev. Neurosci. 20, 595–631 (1997). [DOI] [PubMed] [Google Scholar]
- 12.Stocker R. F., The organization of the chemosensory system in Drosophila melanogaster: A review. Cell Tissue Res. 275, 3–26 (1994). [DOI] [PubMed] [Google Scholar]
- 13.Riabinina O., Task D., Marr E., Lin C. C., Alford R., O’Brochta D. A., Potter C. J., Organization of olfactory centres in the malaria mosquito Anopheles gambiae. Nat. Commun. 7, 13010 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duistermars B. J., Chow D. M., Frye M. A., Flies require bilateral sensory input to track odor gradients in flight. Curr. Biol. 19, 1301–1307 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Budick S. A., Dickinson M. H., Free-flight responses of Drosophila melanogaster to attractive odors. J. Exp. Biol. 209, 3001–3017 (2006). [DOI] [PubMed] [Google Scholar]
- 16.Chen W., Hing H., The L1-CAM, neuroglian, functions in glial cells for Drosophila antennal lobe development. Dev. Neurobiol. 68, 1029–1045 (2008). [DOI] [PubMed] [Google Scholar]
- 17.Das A., Chiang A., Davla S., Priya R., Reichert H., VijayRaghavan K., Rodrigues V., Identification and analysis of a glutamatergic local interneuron lineage in the adult Drosophila olfactory system. Neural Syst. Circuits 1, 4 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chou Y. H., Spletter M. L., Yaksi E., Leong J. C. S., Wilson R. I., Luo L., Diversity and wiring variability of olfactory local interneurons in the Drosophila antennal lobe. Nat. Neurosci. 13, 439–449 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jenett A., Rubin G. M., Ngo T. T. B., Shepherd D., Murphy C., Dionne H., Pfeiffer B. D., Cavallaro A., Hall D., Jeter J., Iyer N., Fetter D., Hausenfluck J. H., Peng H., Trautman E. T., Svirskas R. R., Myers E. W., Iwinski Z. R., Aso Y., DePasquale G. M., Enos A., Hulamm P., Lam S. C. B., Li H. H., Laverty T. R., Long F., Qu L., Murphy S. D., Rokicki K., Safford T., Shaw K., Simpson J. H., Sowell A., Tae S., Yu Y., Zugates C. T., A GAL4-driver line resource for drosophila neurobiology. Cell Rep. 2, 991–1001 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dacks A. M., Christensen T. A., Hildebrand J. G., Phylogeny of a serotonin-immunoreactive neuron in the primary olfactory center of the insect brain. J. Comp. Neurol. 498, 727–746 (2006). [DOI] [PubMed] [Google Scholar]
- 21.Liu W. W., Wilson R. I., Glutamate is an inhibitory neurotransmitter in the Drosophila olfactory system. Proc. Natl. Acad. Sci. U.S.A. 110, 10294–10299 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Siegenthaler D., Enneking E. M., Moreno E., Pielage J., L1CAM/Neuroglian controls the axon-axon interactions establishing layered and lobular mushroom body architecture. J. Cell Biol. 208, 1003–1018 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hong W., Mosca T. J., Luo L., Teneurins instruct synaptic partner matching in an olfactory map. Nature 484, 201–207 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stocker R. F., Singh R. N., Schorderet M., Siddiqi O., Projection patterns of different types of antennal sensilla in the antennal glomeruli of Drosophila melanogaster. Cell Tissue Res. 232, 237–248 (1983). [DOI] [PubMed] [Google Scholar]
- 25.Wong E. V., Kenwrick S., Willems P., Lemmon V., Mutations in the cell adhesion molecule L1 cause mental retardation. Trends Neurosci. 18, 168–172 (1995). [DOI] [PubMed] [Google Scholar]
- 26.Lovick J. K., Ngo K. T., Omoto J. J., Wong D. C., Nguyen J. D., Hartenstein V., Postembryonic lineages of the Drosophila brain: I. Development of the lineage-associated fiber tracts. Dev. Biol. 384, 228–257 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hartenstein V., Younossi-Hartenstein A., Lovick J. K., Kong A., Omoto J. J., Ngo K. T., Viktorin G., Lineage-associated tracts defining the anatomy of the Drosophila first instar larval brain. Dev. Biol. 406, 14–39 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sweeney L. B., Couto A., Chou Y. H., Berdnik D., Dickson B. J., Luo L., Komiyama T., Temporal target restriction of olfactory receptor neurons by Semaphorin-1a/PlexinA-mediated axon-axon interactions. Neuron 53, 185–200 (2007). [DOI] [PubMed] [Google Scholar]
- 29.Okumura M., Kato T., Miura M., Chihara T., Hierarchical axon targeting of Drosophila olfactory receptor neurons specified by the proneural transcription factors Atonal and Amos. Genes Cells 21, 53–64 (2016). [DOI] [PubMed] [Google Scholar]
- 30.Sakano H., Neural map formation in the mouse olfactory system. Neuron 67, 530–542 (2010). [DOI] [PubMed] [Google Scholar]
- 31.Imai T., Sakano H., Axon-axon interactions in neuronal circuit assembly: Lessons from olfactory map formation. Eur. J. Neurosci. 34, 1647–1654 (2011). [DOI] [PubMed] [Google Scholar]
- 32.Kalil K., Dent E. W., Branch management: Mechanisms of axon branching in the developing vertebrate CNS. Nat. Rev. Neurosci. 15, 7–18 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sen S., Cao D., Choudhary R., Biagini S., Wang J. W., Reichert H., VijayRaghavan K., Genetic transformation of structural and functional circuitry rewires the Drosophila brain. eLife 3, 1–27 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thum A. S., Leisibach B., Gendre N., Selcho M., Stocker R. F., Diversity, variability, and suboesophageal connectivity of antennal lobe neurons in D. melanogaster larvae. J. Comp. Neurol. 519, 3415–3432 (2011). [DOI] [PubMed] [Google Scholar]
- 35.Lin H. H., Chu L. A., Fu T. F., Dickson B. J., Chiang A. S., Parallel neural pathways mediate CO2 avoidance responses in Drosophila. Science 340, 1338–1341 (2013). [DOI] [PubMed] [Google Scholar]
- 36.Gaudry Q., Hong E. J., Kain J., de Bivort B. L., Wilson R. I., Asymmetric neurotransmitter release enables rapid odour lateralization in Drosophila. Nature 493, 424–428 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Varadarajan S. G., Kong J. H., Phan K. D., Kao T. J., Panaitof S. C., Cardin J., Eltzschig H., Kania A., Novitch B. G., Butler S. J., Netrin1 produced by neural progenitors, not floor plate cells, is required for axon guidance in the spinal cord. Neuron 94, 790–799.e3 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dominici C., Moreno-Bravo J. A., Puiggros S. R., Rappeneau Q., Rama N., Vieugue P., Bernet A., Mehlen P., Chédotal A., Floor-plate-derived netrin-1 is dispensable for commissural axon guidance. Nature 545, 350–354 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Akin O., Zipursky S. L., Frazzled promotes growth cone attachment at the source of a Netrin gradient in the Drosophila visual system. eLife 5, e20762 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jhaveri D., Saharan S., Sen A., Rodrigues V., Positioning sensory terminals in the olfactory lobe of Drosophila by Robo signaling. Development 131, 1903–1912 (2004). [DOI] [PubMed] [Google Scholar]
- 41.Shimosako N., Hadjieconomou D., Salecker I., Flybow to dissect circuit assembly in the Drosophila brain. Methods Mol. Biol. 1082, 57–69 (2014). [DOI] [PubMed] [Google Scholar]
- 42.Lee T., Luo L., Mosaic analysis with a repressible cell marker for studies of gene function in neuronal morphogenesis. Neuron 22, 451–461 (1999). [DOI] [PubMed] [Google Scholar]
- 43.Jhaveri D., Sen A., Rodrigues V., Mechanisms underlying olfactory neuronal connectivity in Drosophila-the atonal lineage organizes the periphery while sensory neurons and glia pattern the olfactory lobe. Dev. Biol. 226, 73–87 (2000). [DOI] [PubMed] [Google Scholar]
- 44.Couto A., Alenius M., Dickson B. J., Molecular, anatomical, and functional organization of the Drosophila olfactory system. Curr. Biol. 15, 1535–1547 (2005). [DOI] [PubMed] [Google Scholar]
- 45.Vosshall L. B., Wong A. M., Axel R., An olfactory sensory map in the fly brain. Cell 102, 147–159 (2000). [DOI] [PubMed] [Google Scholar]
- 46.Stocker R. F., Heimbeck G., Gendre N., de Belle J. S., Neuroblast ablation in Drosophila P[GAL4] lines reveals origins of olfactory interneurons. J. Neurobiol. 32, 443–456 (1997). [DOI] [PubMed] [Google Scholar]
- 47.Solari P., Corda V., Sollai G., Kreissl S., Galizia C. G., Crnjar R., Morphological characterization of the antennal lobes in the Mediterranean fruit fly Ceratitis capitata. J. Comp. Physiol. A Neuroethol. Sensory Neural Behav. Physiol. 202, 131–146 (2016). [DOI] [PubMed] [Google Scholar]
- 48.Pende M., Becker K., Wanis M., Saghafi S., Kaur R., Hahn C., Pende N., Foroughipour M., Hummel T., Dodt H. U., High-resolution ultramicroscopy of the developing and adult nervous system in optically cleared Drosophila melanogaster. Nat. Commun. 9, 4731 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wiegmann B. M., Trautwein M. D., Winkler I. S., Barr N. B., Kim J.-W., Lambkin C., Bertone M. A., Cassel B. K., Bayless K. M., Heimberg A. M., Wheeler B. M., Peterson K. J., Pape T., Sinclair B. J., Skevington J. H., Blagoderov V., Caravas J., Kutty S. N., Schmidt-Ott U., Kampmeier G. E., Christian Thompson F., Grimaldi D. A., Beckenbach A. T., Courtney G. W., Friedrich M., Meier R., Yeates D. K., Episodic radiations in the fly tree of life. Proc. Natl. Acad. Sci. U.S.A. 108, 5690–5695 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/5/10/eaaw5537/DC1
Fig. S1. Unilateral versus bilateral olfactory circuit organization within Diptera.
Fig. S2. Comprehensive analysis of unilateral and bilateral projecting antennal ORN axon in Neuroglian mutant (see also table in Fig. 1).
Fig. S3. Neuroglian expression during development.
Fig. S4. Neuroglian expression in midline glia cells is dispensable for bilateral ORN connectivity.
Fig. S5. Cell-specific loss of Neuroglian in cPINs affects bilateral olfactory map formation.
Fig. S6. Ablation of cPINs affects bilateral connectivity of ORNs.
Fig. S7. cPINs segregate from PNs in early AL development.
Fig. S8. Cell autonomous and non-autonomous function of Neuroglian in cPINs and ORNs.
Fig. S9. Neuroglian affects olfactory commissure development of CSD neurons.
Table S1. Genotype of experiments.
Reference (49)


