Abstract
Background
Central nervous system (CNS) infections remain a major public health problem in Sub-Saharan Africa, causing 15%–25% of AIDS-related deaths. With widespread availability of antiretroviral therapy (ART) and the introduction of improved diagnostics, the epidemiology of infectious meningitis is evolving.
Methods
We prospectively enrolled adults presenting with HIV-associated meningitis in Kampala and Mbarara, Uganda, from March 2015 to September 2017. Participants had a structured, stepwise diagnostic algorithm performed of blood cryptococcal antigen (CrAg), CSF CrAg, Xpert MTB/RIF for tuberculous (TB) meningitis (TBM), Biofire multiplex polymerase chain reaction, and traditional microscopy and cultures.
Results
We screened 842 consecutive adults with HIV presenting with suspected meningitis: 57% men, median age 35 years, median CD4 26 cells/mcL, and 55% presented on ART. Overall, 60.5% (509/842) were diagnosed with first-episode cryptococcal meningitis and 7.4% (62/842) with second episode. Definite/probable TB meningitis was the primary diagnosis in 6.9% (58/842); 5.3% (n = 45) had microbiologically confirmed (definite) TB meningitis. An additional 7.8% (66/842) did not meet the diagnostic threshold for definite/probable TBM but received empiric TBM therapy. Bacterial and viral meningitis were diagnosed in 1.3% (11/842) and 0.7% (6/842), respectively. The adoption of a cost-effective stepwise diagnostic algorithm allowed 79% (661/842) to have a confirmed microbiological diagnosis at an average cost of $44 per person.
Conclusions
Despite widespread ART availability, Cryptococcus remains the leading cause of HIV-associated meningitis. The second most common etiology was TB meningitis, treated in 14.7% overall. The increased proportion of microbiologically confirmed TBM cases reflects the impact of new improved molecular diagnostics.
Keywords: bacterial meningitis, cryptococcal meningitis, HIV/AIDS, tuberculous meningitis, viral meningitis
Meningitis remains a major cause of mortality in Africa and is the medical condition associated with the highest risk of inpatient death [1]. Historically in Africa, Neisseria meningitidis and Streptococcus pneumoniae were the most common infectious pathogens, resulting in approximately 100 000 deaths between 1991 and 2010 in the meningitis belt [2]. The HIV epidemic, however, resulted in significant changes to etiologies of adult meningitis in Africa. In 2014, an estimated 250 000 incident cases of cryptococcal meningitis occurred, accounting for 15% of AIDS-related deaths [3]. Tuberculous meningitis (TBM) is the second commonest cause of HIV-associated meningitis [4]. The introduction of pneumococcal, meningococcal, and Haemophilus influenzae type b immunizations has dramatically decreased the incidence of pediatric bacterial meningitis, but the respective decline in adult bacterial meningitis incidence has been variable [4, 5]. Precise estimates on the incidence of viral meningitis in Sub-Saharan Africa are lacking.
With the widespread rollout of antiretroviral therapy (ART), coupled with introduction of an HIV “test and treat” policy, the landscape of meningitis epidemiology is again changing. Furthermore, the introduction of new molecular diagnostics, including the Xpert MTB/RIF Assay and commercial multiplex polymerase chain reaction (PCR) platforms for testing cerebrospinal fluid (CSF) for bacterial, viral, and fungal pathogens, is improving meningitis diagnostic capacity. This is most significant with respect to TBM. Although TBM accounts for between 8% and 17% of all meningitis cases in high–HIV prevalence settings [4], a lack of sensitive and timely diagnostics has historically made TBM diagnoses extremely difficult and disease burden estimates challenging. In 2013, the World Health Organization endorsed Xpert MTB/RIF (a cartridge-based, fully automated PCR molecular assay) as the preferred firstline TBM diagnostic. Performance data suggest that the sensitivity of Xpert MTB/RIF for TBM is ~50% [4]. This is significantly better than CSF microscopy for acid-fast bacilli (AFB), which has a sensitivity of ≤15%, and comparable to culture (sensitivity ~50%) [6, 7]. The re-engineered Xpert MTB/RIF Ultra assay (Cepheid Inc., Sunnyvale, CA) introduced in 2017 has further improved the analytical sensitivity for detecting TB [7].
In a 2010–2012 Ugandan cohort of 416 ART-naïve adults with HIV presenting with suspected meningitis, cryptococcal meningitis was diagnosed in 60%, acute bacterial meningitis in 1.6%, and tuberculous meningitis in 2.5% [8]. Since that time, improved Food and Drug Administration (FDA)–approved diagnostics have been introduced to our clinical research setting: (1) CrAg lateral flow assay has replaced the former CrAg latex agglutination platform; (2) GeneXpert MTB/RIF on large-volume centrifuged CSF; (3) BioFire FilmArray multiplex PCR Meningitis/Encephalitis panel. In the context of these new diagnostics and increased ART coverage, we aimed to describe the etiologies and frequencies of fungal, mycobacterial, bacterial, and viral meningitis among adults in Uganda.
METHODS
Setting and Participants
We conducted a prospective cohort study as part of screening for a cryptococcal meningitis trial from March 2015 to September 2017 (Adjunctive Sertraline for the Treatment of Cryptococcal Meningitis; ClinicalTrials.gov: NCT01802385) [9]. Consecutive adults presenting with suspected meningitis to Mulago National Referral Hospital, Kampala, or Mbarara Regional Referral Hospital were screened for infectious etiologies using a stepwise diagnostic algorithm [4]. All patients were initially screened at the bedside using a lateral flow assay (LFA) for cryptococcal antigen (CrAg LFA; IMMY, Norman, OK) in blood before lumbar puncture; CSF was then tested. CSF CrAg-negative patients underwent a targeted comprehensive evaluation for TBM, acute bacterial meningitis, and viral meningitis (Figure 1). Ninety-two patients with an unknown HIV status at the time of screening were offered a point-of-care rapid HIV test. Consented participants were followed until hospital discharge. Inclusion criteria were adults (≥18 years) presenting with suspected meningitis.
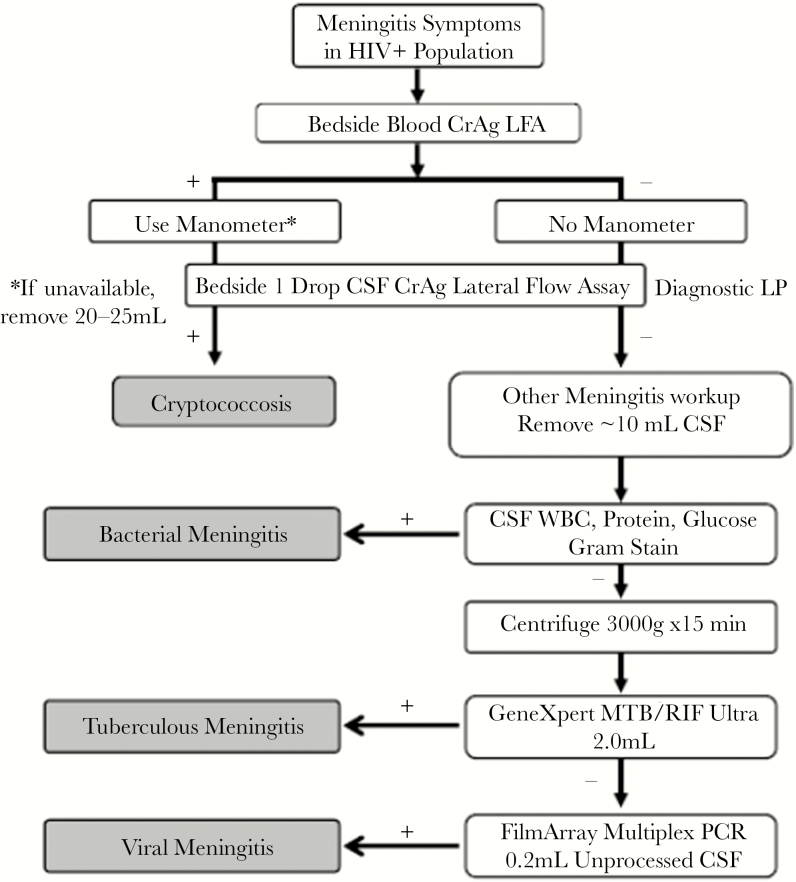
Figure 1.
Stepwise diagnostic algorithm used in investigation of suspected HIV-associated meningitis. Figure 1 demonstrates the diagnostic workup for adults with HIV, presenting with meningitis symptoms. This is a stepwise diagnostic algorithm, such that diagnostic tests are sequentially run until a positive diagnosis is made (as indicated in the gray boxes), and then no further investigations are employed. Participants with a positive finger-stick CrAg but negative CSF CrAg had additional “other meningitis” workup performed. Abbreviations: CSF, cerebrospinal fluid; LFA, lateral flow assay; LP, lumbar puncture; PCR, polymerase chain reaction; WBC, white blood cell count.
Stepwise Meningitis Diagnostic Algorithm
We used a diagnostic algorithm to maximize diagnostic yield in a cost-efficient manner (Figure 1). Participants with suspected meningitis were first screened with a finger-stick CrAg LFA performed at the bedside to identify patients with systemic cryptococcosis requiring control of their intracranial pressure [10]. We targeted collection of 10 mL of CSF, aware that centrifuged large-volume CSF testing increases the diagnostic yield for TB testing [11] with more CSF collected depending on CSF opening pressure. Cryptococcal meningitis was diagnosed by positive CSF CrAg LFA at the bedside and later confirmed by culture. CSF testing of white cell count, protein, and quantitative cryptococcal cultures was performed as described previously [12]. Cryptococcal paradoxical immune reconstitution inflammatory syndrome (IRIS) was as per the case definition [13]. Acute bacterial meningitis was diagnosed via gram stain, culture, and/or by multiplex PCR using the FilmArray Meningitis–Encephalitis panel (BioFire, Salt Lake City, UT). All CSF CrAg-negative patients were systematically evaluated for TBM, including those with symptomatic cryptococcal antigenemia [14]. In patients with confirmed cryptococcal meningitis, TBM co-infection was investigated at physician discretion. TBM evaluation included clinical examination, radiological assessment where possible (chest radiograph and neuroradiology in patients with focal neurological deficits), and CSF evaluation. CSF testing for TBM included CSF AFB smear using Ziehl-Nielsen stain (Mulago Hospital only) and GeneXpert TB/RIF. After routine microbiology and chemistry analysis, the remaining volume of CSF was centrifuged at 3000g for 20 minutes. We removed and cryopreserved all supernatant except for 2 mL, which was resuspended via vortexing for 15–20 seconds. We then used 0.5 mL for Xpert MTB/RIF testing and 0.5 mL for CSF Mycobacteria Growth Inhibitor Tube culture (MGIT; Becton Dickinson, Franklin Lakes, NJ). Between January 2016 and May 2017, a subset of consecutive diagnostic CSF samples underwent analysis using the BioFire FilmArray multiplex PCR Meningitis/Encephalitis panel, which includes detection of Cryptococcus, Streptococcus pneumoniae, Haemophilus influenzae, Escherichia coli, cytomegalovirus (CMV), herpes simplex virus 1 and 2 (HSV-1 and HSV-2), varicella zoster virus (VZV), human herpes viruses 6 and 7 (HHV6 and HHV7), enterovirus, and parechovirus.
Costing Analysis
We assessed 3 different diagnostic strategies to identify the most cost-efficient use of the diagnostic tests available. Using the diagnostic algorithm outlined in Figure 1 and the sensitivity, specificity, and cost of each test, we calculated the costs of (a) using a stepwise algorithm without multiplex PCR testing, (b) using a stepwise algorithm with the addition of multiplex PCR testing, and (c) comprehensive “shotgun” testing of all running all tests on all patients. Real-world costs of testing were used, including shipping and labor costs. Disease prevalence was taken from our results. Test characteristics and costs are available in Supplementary Table 2.
Ethics
All participants (or a surrogate in cases of mental incapacity) provided written informed consent for lumbar puncture, CSF testing, CSF storage, and data collection. Approval for the study was obtained from the Uganda National Council for Science and Technology and institutional review boards in Uganda and at the University of Minnesota.
Statistical Analysis
Baseline demographics, clinical characteristics, and final diagnoses were summarized as percentages or medians (with interquartile ranges). The distribution of diagnoses was calculated for each ART group—ART-naïve, ART <3 months, ART >3 months—and the proportion of cryptococcal diagnoses among those with suspected meningitis was compared across ART groups using a chi-square test. The percentage of pathogens detected by multiplex PCR was calculated among those tested, and the prevalence was imputed among the population without a known diagnosis (negative for cryptococcosis or TBM) and among the entire cohort. All analyses were conducted using SAS, version 9.4 (SAS Institute, Cary, NC).
RESULTS
From March 2015 until September 2017, 846 Ugandan adults with suspected meningitis were screened for infectious etiologies: 553 (65%) in Kampala and 293 (35%) in Mbarara. Overall, 99.5% (842/846) were people with HIV who had advanced HIV/AIDS with a median CD4 count (interquartile range) of 26 (7–76) cells/μL. Analysis focused on 842 people with HIV. Participants presented with features typical of meningitis (Table 1). Overall, 55% (460/842) were on ART at presentation. The median duration for those receiving ART was 9 months, with 34% (156/460) of those on ART for <3 months and 61% (279/460) on ART for >3 months.
Table 1.
Demographics and Baseline Characteristics
| Characteristic | Initial CM | Prior CM | TBM (Definite or Probable) | Other | Overall | P Value |
|---|---|---|---|---|---|---|
| No. | 509 | 78 | 52 | 203 | 842 | |
| Male | 303 (60) | 40 (51) | 34 (65) | 100 (49) | 477 (57) | .03 |
| Age, y | 35 [29–40] | 34 [29–42] | 32 [30–40] | 37 [30–44] | 35 [30–42] | .16 |
| Receiving ART | 244 (48) | 73 (94) | 32 (62) | 111 (55) | 460 (55) | <.001 |
| Months on ART | 6 [1–35] | 8 [2–37] | 7 [3–22] | 15 [3–57] | 9 [1–36] | .01 |
| Clinical presentation | ||||||
| Fever | 260 (51) | 18 (23) | 45 (87) | 133 (66) | 456 (54) | <.001 |
| Headache | 493 (97) | 76 (97) | 50 (96) | 175 (86) | 794 (94) | <.001 |
| Duration of headache | 14 [7–28] | 14 [7–30] | 9 [7–14] | 10 [6–14] | 14 [7–21] | <.001 |
| Photophobia | 131 (26) | 20 (26) | 9 (17) | 24 (12) | 184 (22) | <.001 |
| Visual change | 149 (29) | 30 (38) | 15 (29) | 41 (20) | 235 (28) | .01 |
| Glasgow Coma Score <15 | 255 (50) | 22 (28) | 47 (90) | 145 (71) | 469 (56) | <.001 |
| Seizure | 87 (17) | 9 (12) | 6 (12) | 26 (13) | 128 (15) | .30 |
| Laboratory parameters | ||||||
| CD4 count per μL | 16 [6–43] | 27 [6–77] | 85 [47–131] | 73 [22–242] | 26 [7–76] | <.001 |
| CSF opening pressure, cm H2Oa | 26 [18–38] | 29 [18–43] | 21 [9–36] | 14 [10–20] | 25 [17–38] | <.001 |
| CSF OP <20 cm H20 | 139 (32) | 17 (28) | 6 (43) | 38 (76) | 200 (36) | <.001 |
| CSF white cells/ μL | <5 [<5–40] | <5 [<5–65] | 60 [12–140] | <5 [<5–5] | <5 [<5–40] | <.001 |
| CSF white cells <5 μL | 306 (63) | 37 (51) | 9 (20) | 125 (74) | 477 (62) | <.001 |
| CSF protein, mg/dL | 43 [23–100] | 52 [24–100] | 135 [47–280] | 45 [22–93] | 47 [23–107] | <.001 |
| CSF protein <45 mg/dL | 213 (51) | 31 (50) | 10 (23) | 70 (50) | 324 (49) | <.01 |
| Normal WBC + proteinb | 182 (44) | 23 (37) | 4 (10) | 63 (45) | 272 (41) | <.001 |
Table 1 presents the demographics and baseline clinical and laboratory characteristics of 842 Ugandan adults with HIV presenting with suspected meningitis. Patients with cryptococcal meningitis were overall more likely to be ART experienced and had lower median baseline CD4 counts. Data are presented as follows: No. (%) for categorical variables, median [IQR] for continuous variables. P values are from chi-square tests for categorical variables and Kruskal-Wallis tests for continuous variables. Among those with empiric TB diagnoses, 32 participants had both CSF protein (n = 32) and white cell count (n = 47). Among patients with cryptococcal meningitis, 3 were diagnosed with concurrent definite TB meningitis. One symptomatic cryptococcal antigenemia person was diagnosed with probable TB meningitis. One person with definite TB meningitis by Mycobacteria Growth Inhibitor Tube culture and 1 person with probable TB meningitis both had a history of cryptococcal meningitis, being CSF CrAg positive and fungal culture negative.
Abbreviations: ART, antiretroviral therapy; CM, cryptococcal meningitis; CSF, cerebrospinal fluid; IQR, interquartile range; OP, Opening Pressure; TB, tuberculosis; TBM, tuberculous meningitis; WBC, white blood cell.
aCSF opening pressure was not measured routinely in noncryptococcal patients (n = 19 with TB meningitis, n = 1 with empiric TB meningitis therapy).
bCSF WBC <5 µL and protein <45 mg/dL.
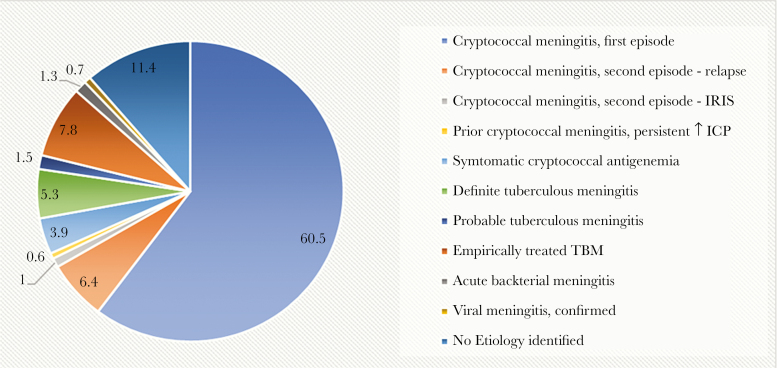
Overall, 60.5% (509/842) of participants were diagnosed with first-episode cryptococcal meningitis by CSF CrAg LFA and/or cryptococcal quantitative culture (Figure 2). In addition, 9.2% (78/842) reported a previous episode of cryptococcal meningitis, of whom 54 had culture-positive relapse, 8 probable paradoxical cryptococcal IRIS, 1 symptomatic antigenemia, 3 TBM (1 definite, 1 probable, 1 empiric), 1 bacterial pneumococcal meningitis, 5 persistent high intracranial pressures, and 6 unremarkable CSF/unknown diagnosis. Three participants with culture-positive cryptococcal meningitis had concurrent microbiologically confirmed TBM by Xpert. Additionally, 4% (33/842) of participants presenting with clinical meningitis had a positive blood CrAg LFA result with a negative CSF CrAg LFA and cryptococcal culture, that is, “symptomatic cryptococcal antigenemia” (Table 2) [14].
Figure 2.
Etiology of HIV-associated meningitis in Ugandan adults, 2015–2017. Figure 2 demonstrates the proportional frequencies (%) of fungal, mycobacterial, bacterial, and fungal meningitis in 842 Ugandan adults with HIV presenting with suspected meningitis. Abbreviations: ICP, Intra cranial pressure; IRIS, immune reconstitution inflammatory syndrome; TBM, tuberculous meningitis.
Table 2.
Primary Infective Etiologies
| Meningitis Etiology | No. (%) |
|---|---|
| Cryptococcal meningitis, first episodea | 509 (60.5) |
| Cryptococcal meningitis, second episode—relapse | 54 (6.4) |
| Cryptococcal meningitis, second episode—IRIS | 8 (1.0) |
| Prior cryptococcal meningitis, persistent ↑ICP | 5 (0.6) |
| Symptomatic cryptococcal antigenemiab | 33 (3.9) |
| Definite tuberculous meningitisa | 45 (5.3) |
| Probable tuberculous meningitisb | 13 (1.5) |
| Empirically treated TBM | 66 (7.8) |
| Acute bacterial meningitisc | 11 (1.3) |
| Viral meningitis, confirmed | 6 (0.7) |
| No etiology with normal CSFd | 42 (5.0) |
| No etiology identified | 54 (6.4) |
| Total cohort | 842 (100) |
Eight hundred forty-two Ugandan adults with HIV presenting with meningitis underwent a structured, stepwise diagnostic algorithm to diagnose infective etiologies. Cryptococcal meningitis was the most common cause of HIV-associated meningitis followed by tuberculous meningitis. Total etiologies n = 846 due to 4 participants with TB+ cryptococcal co-infection. Supplementary Table 4 lists the Marais et al. uniform criteria for TB meningitis.
Abbreviations: CSF, cerebrospinal fluid; ICP, Intra cranial pressure; IRIS, immune reconstitution inflammatory syndrome; TB, tuberculosis; TBM, tuberculous meningitis.
aThree patients were co-infected with cryptococcal and TB meningitis.
bOne patient had symptomatic cryptococcal antigenemia and probable TB meningitis.
cOne patient had acute bacterial meningitis and a history of cryptococcosis, being CSF CrAg positive.
dCSF WBC <5 µL and protein <45 mg/dL, of whom n = 6 had prior cryptococcal meningitis, of whom n = 4 were CSF CrAg negative.
Definite or probable TBM was the primary diagnosis in 6.9% (58/842), with 45 (5.3%) having microbiologically confirmed (definite) TBM based on Xpert MTB/RIF (n = 39) and/or MGIT culture (n = 22) and 13/842 (1.5%) having probable TBM per the uniform clinical case definition (Table 2) [15]. An additional 7.8% (66/842) did not meet the diagnostic threshold for definite or probable TBM but received empiric TBM therapy due to a high index of clinical suspicion.
Overall, 1.3% (11/842) of participants were diagnosed with acute bacterial meningitis, based on gram stain (n = 3) and/or bacterial culture (n = 0) and/or PCR (n = 2) or CSF neutrophilic pleocytosis with clinical diagnosis (n = 6) (Table 2).
Between January 2016 and May 2017, 45 diagnostic CSF samples in patients without a confirmed diagnosis of cryptococcal meningitis, TB meningitis, or symptomatic cryptococcemia underwent testing for common viral and bacterial meningitis pathogens using the BioFire FilmArray. Two cases of acute bacterial meningitis were diagnosed: Streptococcus pneumoniae (1) and Haemophilus influenzae (1); both cases were CSF culture negative. Six patients (0.7%, 6/842) were diagnosed with a viral meningitis; these cases had a single viral pathogen detected by PCR (Supplementary Table 1). Diagnoses were confirmed by clinical case note review and adjudication by 2 independent clinicians.
We assessed 3 strategies for using diagnostic tests efficiently. Supplementary Table 3 demonstrates that total costs of the 3 algorithms of interest. The least expensive algorithm used the stepwise approach without Biofire PCR. In this stepwise strategy, where tests were sequentially run until a positive test result occurred (and then further diagnostics stopped), the total cost was $1359 for 100 persons, with a cost per person of $13.59 and 75 people with a microbiologic diagnosis. Adding the multiplex PCR to the stepwise approach, whereby the multiplex PCR would be run as the last test, cost a total of $4434 ($44.34 per person) and resulted in 78 people with a confirmed diagnosis. For these additional 3 diagnoses, the cost per diagnosis was $917. Performing all diagnostic tests on all patients cost $16,495 ($164.95 per person) with no additional diagnoses (78 people diagnosed in total).
Table 3.
Infectious Etiologies in Adults Presenting With HIV-Associated Meningitis Stratified by ART Status
| ART-Naïve | ART <3 mo |
ART >3 mo |
P Value | |
|---|---|---|---|---|
| No. | 379 | 156 | 279 | |
| Cryptococcal meningitisa | 268 (71) | 116 (74) | 172 (62) | <.01 |
| Second-episode cryptococcal meningitis | 5 (2) | 9 (8) | 39 (23) | |
| Other etiologies | ||||
| Symptomatic crag antigenemia | 16 (14) | 5 (13) | 11 (10) | |
| Definite tuberculous meningitisb | 17 (15) | 5 (13) | 12 (11) | |
| Probable tuberculous meningitis | 3 (3) | 3 (8) | 6 (6) | |
| Empirically treated TBM | 27 (24) | 6 (15) | 33 (31) | |
| Acute bacterial meningitis | 6 (5) | 1 (3) | 4 (4) | |
| Viral meningitis, confirmed | 3 (3) | 1 (3) | 2 (2) | |
| No etiology with normal CSFc | 17 (15) | 7 (18) | 13 (12) | |
| No etiology identified | 22 (20) | 12 (30) | 26 (24) | |
| Demographics | ||||
| Men | 212 (56) | 98 (63) | 153 (55) | 0.24 |
| Age, y | 35 [29–40] | 35 [30–43] | 35 [30–42] | 0.36 |
| Clinical presentation | ||||
| Fever | 203 (54) | 72 (46) | 161 (58) | .07 |
| Headache | 354 (93) | 151 (97) | 267 (96) | .20 |
| Duration of headache (d) | 14 [7–21] | 14 [7–30] | 14 [7–21] | .31 |
| Photophobia | 78 (21) | 37 (24) | 62 (22) | .71 |
| Visual change | 114 (30) | 45 (29) | 69 (25) | .31 |
| Glasgow Coma Score <15 | 207 (55) | 91 (58) | 153 (55) | .71 |
| Seizure | 65 (17) | 24 (15) | 36 (13) | .33 |
| Laboratory parameters | ||||
| CD4 count per μL | 18 [6–60] | 30 [11–70] | 33 [7–95] | .008 |
| CSF opening pressure, cm H2O | 24 [17–40] | 25 [17–38] | 26 [17–36] | .98 |
| CSF opening pressure <20 cm H2O | 94 (36) | 44 (36) | 55 (34) | .83 |
| CSF white cells per μL | 4 [4–40] | 4 [4–60] | 4 [4–30] | .04 |
| CSF WBC <5 μL | 226 (64) | 76 (52) | 159 (63) | .04 |
| CSF protein, mg/dL | 46 [22–105] | 50 [25–109] | 47 [24–107] | .66 |
| CSF protein <45 mg/dL | 151 (48) | 60 (49) | 101 (49) | .95 |
| Normal WBC and protein | 131 (42) | 47 (39) | 83 (41) | .82 |
P value from chi-square test comparing the proportion of cryptococcal meningitis diagnoses vs other diagnoses across ART groups. The distribution of diagnoses was calculated for each ART group—ART-naïve, ART <3 months, ART >3 months—and the proportion of cryptococcal diagnoses compared with other diagnoses. Cryptococcal and TB meningitis remained the most common infectious etiologies in both art-naïve and art-experienced participants. Those on ART for less than 3 months had the highest proportion of cryptococcal diagnoses. Persons with altered mental status and unknown ART status (n = 3) or duration (n = 25) were excluded.
Abbreviations: ART, antiretroviral therapy; CSF, cerebrospinal fluid; TB, tuberculosis; TBM, tuberculous meningitis; WBC, white blood cell count.
aThree patients were co-infected with cryptococcal and TB meningitis.
bOne patient was co-infected with TB and bacterial meningitis.
cCSF white cells <5 μL and protein <45 mg/dL.
Of all participants screened, 21% (181/842) had no confirmed microbiological diagnosis made. When diagnoses were stratified by ART experience, cryptococcal and TB meningitis remained the most common infectious etiologies in both ART-naïve and ART-experienced participants (Table 3). There was a statistically significant difference in the distribution of cryptococcal diagnoses across ART groups; patients on ART for <3 months had a higher proportion of cryptococcal diagnoses compared with those on ART for >3 months (69% vs 48%; P < .01).
DISCUSSION
Despite significant improvements in HIV management, CNS infections continue to be a major cause of morbidity and mortality in advanced HIV. Similar to previous studies from Sub-Saharan Africa [8, 16–18], cryptococcosis remains the leading cause of adult meningitis, accounting for 68% of cases, 7% of which are cases of second-episode symptomatic recurrences. In addition to CSF CrAg-positive cases of cryptococcal meningitis, the stepwise diagnostic algorithm adopted allowed for identification of a new disease entity: symptomatic cryptococcal antigenemia, which accounted for 4% of meningitis cases in our cohort [14]. This subgroup of patients with advanced HIV presented with classical meningitis symptoms with negative CSF studies (negative CSF CrAg LFA and cryptococcal culture) but positive blood CrAg LFA; this is postulated to represent early cryptococcal meningoencephalitis [14]. In the absence of CrAg testing blood, these diagnoses would have been missed, demonstrating the need for systematic CrAg screening of blood (as well as CSF analysis) in immunocompromised patients with CNS infections.
The second most common meningitis in our cohort was TBM; 6.9% of participants were diagnosed with definite or probable TBM. An additional 7.8% of participants were treated empirically for TBM based on clinician judgment. These participants did not meet the diagnostic threshold for definite or probable TBM, predominately due to a lack of microbiological and/or radiological data to comprehensively investigate for evidence of extra-CNS TB. This highlights the challenges of using diagnostic scores in resource-limited settings and, despite significant improvements in TB diagnostics, the ongoing importance of clinician judgment in diagnosing TBM.
The proportion of meningitis due to TBM (14.7%) was higher than that reported previously in Ugandan adults with HIV presenting with meningitis (2010–2012), when only 2.5% of cases were reported to be due to TBM, before the routine availability of Xpert [8]. Findings from our current study are, however, similar to other meningitis case series of predominately adults with HIV from Zimbabwe [17], South Africa [18], and Malawi [16], which found TBM to be the infective etiology in 12%, 13%, and 17% respectively. The increased proportion of TBM cases in our current Ugandan series almost certainly reflects historical underdiagnosis of TBM in Uganda and the impact of new improved molecular diagnostics. This is supported by a recently published large retrospective cohort study of TBM in Ugandan adults (2010–2017), which demonstrated that the proportion of microbiologically confirmed TBM cases has increased over time [19]. Given the diagnostic challenges of HIV-associated TBM, it is possible that the proportion classified as TBM in our current study still represents an underestimate of the true burden. In 2017, we introduced the next-generation Xpert MTB/RIF Ultra, which has a reported sensitivity of up to 95% against a microbiological composite outcome [7]. Used as part of a stepwise meningitis diagnostic algorithm, we anticipate that Xpert MTB/RIF Ultra will further increase confirmed TBM.
The prevalence of acute bacterial meningitis was low (1.3%). Importantly, only 27% (3/11) of bacterial meningitis diagnoses were made on microscopy and culture, and 18% (2/11) were made via the BioFire multiplex PCR. Although further research into the clinical utility of multiplex PCR platforms in resource-limited settings is warranted, as bacterial meningitis is less commonly seen by clinicians and laboratory personnel, these results may highlight the role of automated PCR platforms in the diagnosis of bacterial meningitis.
The BioFire FilmArray multiplex PCR Meningitis/Encephalitis panel enabled us to diagnose viral meningitis in 0.7% of our cohort. HSV-1 was the most common viral pathogen isolated in the subset of cases we investigated using the Biofire PCR panel (n = 45); if we impute the positive Biofire results for all cases without a diagnosis of cryptococcosis or definite/probable TBM (n = 84), we predict we would have diagnosed 8 cases of HSV-1 meningoencephalitis (Supplementary Table 1). HSV-1 meningoencephalitis is a treatable clinical condition, and our data therefore support the expansion of viral meningitis diagnostics as part of a stepwise diagnostic algorithm in HIV-associated meningitis.
Our data demonstrate that the majority of patients with HIV-associated meningitis are now presenting while receiving ART (55%). This is a heterogeneous group, consisting of primarily 2 populations: first, patients presenting with clinical meningitis soon after ART initiation (typically <3 months), a clinical phenotype consistent with unmasking IRIS [13]; second, patients on ART for ≥6 months with virological and immunological failure. ART defaulters were less common (13% of those with ART history). Despite the rapid rollout of ART in much of Sub-Saharan Africa, the predominant infective etiologies of HIV-associated adult meningitis have not changed. Cryptococcal meningitis and TBM remain the most common causes of meningitis in both ART-experienced and ART-naïve patients. Sixty-nine percent of patients presenting <3 months after ART initiation were diagnosed with cryptococcal meningitis. Patients presenting with unmasking cryptococcosis after recently initiating ART have poorer clinical outcomes when compared with ART-naïve patients [20]; there is a risk that the recent upscaling of “HIV test and treat”—unless combined with systematic CrAg screen before initiation—will result in increases in the number of patients presenting with unmasking CM-IRIS [21].
Our cost analysis demonstrates that use of a stepwise diagnostic algorithm that sequentially utilizes microbiological tests as key differential diagnoses are excluded is a cost-efficient approach. The cheapest of the diagnostic algorithms explored was the stepwise approach without multiplex PCR, with a cost per person of $13.59. Although further research into the clinical utility of multiplex PCR is underway, given the Ugandan GDP per capita of $604, we would support this diagnostic approach in Uganda. None of the algorithms tested included TB culture in the cost analysis; in a study that evaluated the diagnostic performance of Xpert, Xpert Ultra, and TB culture in the diagnosis of TBM in 129 Ugandan adults with HIV, only 1 case of definite TBM meningitis was diagnosed on TB culture alone [7]. Furthermore, the median time to positive TB culture was 16 days [7], which severely limits the clinical utility of a positive result. At the current cost of $35 per TB culture in Uganda, the number needed to treat of 129 would equate to a cost of $4515 per additional case of TBM detected. Therefore, at present, we do not recommend the use of TB culture in the investigation of HIV-associated meningitis outside the research setting.
One strength of our study is that we studied a large cohort of 842 with HIV; this sample size is larger than that achieved in comparable studies in Malawi (n = 573) [19] and Zimbabwe (n = 406) [17], reflecting the considerable burden of opportunistic CNS infections in Uganda. At our 2 hospitals in Uganda, the number of meningitis cases has increased [22], despite the rollout of ART. We do, however, recognize some limitations to our study. This study was conducted at 2 referral hospitals well known in Uganda for conducting cryptococcal meningitis studies. To facilitate referrals, our team has periodically conducted outreach sensitization sessions on cryptococcal screening at medical facilities in the surrounding areas; this could have resulted in a referral bias, with a greater proportion of cryptococcal cases and under-representation of tuberculous, bacterial, and/or viral meningitis. Second, due to availability of the test, we performed BioFire multiplex PCR only on a subset of noncryptococcal, non-TBM cases. We have therefore estimated the predicted frequency and 95% confidence interval for the proportion of viral and bacterial meningitis cases (as diagnosed by PCR) for all cases without a confirmed microbiological diagnosis from the 45 Biofire tests performed.
In conclusion, we used a comprehensive diagnostic algorithm to investigate patients with HIV-associated meningitis in Uganda. Overall, 79% of patients had a definitive microbiologically confirmed diagnosis, dominated by cryptococcal and TB meningitis. Similar to Botswana, where with 90-90-90 targets are being reached [23], cryptococcosis persists in Uganda. Using cutting-edge diagnostics in a systematic, stepwise way can optimize diagnostic capacity while minimizing cost [4].
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
We thank all the participants and their families and gratefully acknowledge the support offered by the clinical trial staff.
Financial support. This work was supported by the National Institute of Neurologic Diseases and Stroke (R01NS086312), the Fogarty International Center (K01TW010268, R25TW009345), the National Institute of Allergy and Infectious Diseases (T32AI055433), United Kingdom Medical Research Council/DfID/Wellcome Trust Global Clinical Trials (M007413/1), and the Wellcome Trust (210772/Z/18/Z). We thank the University of Minnesota Foundation for provision of the Bactec MGIT culture system for the MSF Epicentre Laboratory in Mbarara, Uganda.
Conflicts of interest. The authors declare that they have no competing interests.
References
- 1. SanJoaquin MA, Allain TJ, Molyneux ME, et al. . Surveillance Programme of IN-patients and Epidemiology (SPINE): implementation of an electronic data collection tool within a large hospital in Malawi. PLoS Med 2013; 10:e1001400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. World Health Organization. Number of Suspected Meningitis Cases and Deaths Reported. Geneva: World Health Organization; 2015. Available at: http://www.who.int/gho/epidemic_diseases/meningitis/suspected_cases_deaths_text/en/. Accessed 9 April 2018. [Google Scholar]
- 3. Rajasingham R, Smith RM, Park BJ, et al. . Global burden of disease of HIV-associated cryptococcal meningitis: an updated analysis. Lancet Infect Dis 2017; 17:873–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Durski KN, Kuntz KM, Yasukawa K, et al. . Cost-effective diagnostic checklists for meningitis in resource-limited settings. J Acquir Immune Defic Syndr 2013; 63:e101–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wall EC, Everett BD, Mukaka M, et al. . Bacterial meningitis in Malawian adults, adolescents, and children during the era of antiretroviral scale-up and haemophilus influenzae type B vaccination, 2000–2012. Clin Infect Dis 2014; 58:e137–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wilkinson RJ, Rohlwink U, Misra UK, et al. ; Tuberculous Meningitis International Research Consortium Tuberculous meningitis. Nat Rev Neurol 2017; 13:581–98. [DOI] [PubMed] [Google Scholar]
- 7. Bahr NC, Nuwagira E, Evans EE, et al. ; ASTRO-CM Trial Team Diagnostic accuracy of Xpert MTB/RIF Ultra for tuberculous meningitis in HIV-infected adults: a prospective cohort study. Lancet Infect Dis 2018; 18:68–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rajasingham R, Rhein J, Klammer K, et al. . Epidemiology of meningitis in an HIV-infected Ugandan cohort. Am J Trop Med Hyg 2015; 92:274–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rhein J, Morawski BM, Hullsiek KH, et al. ; ASTRO-CM Study Team Efficacy of adjunctive sertraline for the treatment of HIV-associated cryptococcal meningitis: an open-label dose-ranging study. Lancet Infect Dis 2016; 16:809–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Williams DA, Kiiza T, Kwizera R, et al. . Evaluation of fingerstick cryptococcal antigen lateral flow assay in HIV-infected persons: a diagnostic accuracy study. Clin Infect Dis 2015; 61:464–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bahr NC, Tugume L, Rajasingham R, et al. . Improved diagnostic sensitivity for tuberculous meningitis with Xpert(®) MTB/RIF of centrifuged CSF. Int J Tuberc Lung Dis 2015; 19:1209–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dyal J, Akampurira A, Rhein J, et al. . ASTRO-CM Trial Team Reproducibility of CSF quantitative culture methods for estimating rate of clearance in cryptococcal meningitis. Med Mycol 2016; 54:361–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Haddow LJ, Colebunders R, Meintjes G, et al. . International Network for the Study of HIV-associated IRIS (INSHI) Cryptococcal immune reconstitution inflammatory syndrome in HIV-1-infected individuals: proposed clinical case definitions. Lancet Infect Dis 2010; 10:791–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ssebambulidde K, Bangdiwala AS, Kwizera R, et al. . Adjunctive Sertraline for Treatment of HIV-associated Cryptococcal Meningitis Team Symptomatic cryptococcal antigenemia presenting as early cryptococcal meningitis with negative cerebral spinal fluid analysis. Clin Infect Dis 2019; 68:2094–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Marais S, Thwaites G, Schoeman JF, et al. . Tuberculous meningitis: a uniform case definition for use in clinical research. Lancet Infect Dis 2010; 10:803–12. [DOI] [PubMed] [Google Scholar]
- 16. Cohen DB, Zijlstra EE, Mukaka M, et al. . Diagnosis of cryptococcal and tuberculous meningitis in a resource-limited African setting. Trop Med Int Health 2010; 15:910–7. [DOI] [PubMed] [Google Scholar]
- 17. Hakim JG, Gangaidzo IT, Heyderman RS, et al. . Impact of HIV infection on meningitis in Harare, Zimbabwe: a prospective study of 406 predominantly adult patients. AIDS 2000; 14:1401–7. [DOI] [PubMed] [Google Scholar]
- 18. Jarvis JN, Meintjes G, Williams A, et al. . Adult meningitis in a setting of high HIV and TB prevalence: findings from 4961 suspected cases. BMC Infect Dis 2010; 10:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cresswell FV, Bangdiwala AS, Bahr NC, et al. . Can improved diagnostics reduce mortality from tuberculous meningitis? Findings from a 6.5-year cohort in Uganda. Wellcome Open Res 2018; 3:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rhein J, Hullsiek KH, Evans EE, et al. . ASTRO-CM study team Detrimental outcomes of unmasking cryptococcal meningitis with recent ART initiation. Open Forum Infect Dis 2018; 5(X):XXX–XX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Abassi M, Rhein J, Meya DB, Boulware DR. Cryptococcal disease in the era of “test and treat”: is there cause for concern? Open Forum Infect Dis 2018; 5(X):XXX–XX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Flynn AG, Meya DB, Hullsiek KH, et al. . Evolving failures in the delivery of human immunodeficiency virus care: lessons from a Ugandan meningitis cohort 2006–2016. Open Forum Infect Dis 2017; 4(X):XXX–XX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tenforde MW, Mokomane M, Leeme T, et al. . Advanced human immunodeficiency virus disease in botswana following successful antiretroviral therapy rollout: incidence of and temporal trends in cryptococcal meningitis. Clin Infect Dis 2017; 65:779–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.