Abstract
Objectives:
HIV-positive pregnant women who are initiated on lifelong antiretroviral therapy (ART) and isoniazid preventive therapy (IPT) have lower adherence rates after delivery. We quantified maternal motivation to take preventive therapy before and after delivery among pregnant women newly diagnosed with HIV.
Methods:
We enrolled pregnant women (≥18 years) with a recent HIV diagnosis (<6 months) at 14 public primary health clinics in Matlosana, South Africa and followed them in the postpartum period. Participants received eight choice tasks comparing two mutually exclusive sub-sets of seven possible benefits related to preventive therapy identified through literature reviews and key informant interviews. Data was analyzed using conditional logit regression in the antepartum vs. postpartum periods. Coefficients are reported with 95% confidence intervals (CI).
Results:
Sixty-five women completed surveys both at enrollment and in the postpartum period. All women were already on ART, while 21 (32%) were receiving IPT at enrollment. The mean CD4 count was 436 (±246) cells/mm3. In the antepartum period, preventing HIV transmission to partners was the most important benefit (coefficients (ß)=0.87, 95% CI: 0.64, 1.11), followed by keeping healthy for family (ß =0.75, 95% CI: 0.52, 0.97). Such prioritization significantly decreased in the postpartum period (p<0.001). Compared to other motivators, keeping a high CD4 count was least prioritized in the antepartum period (ß =0.19, 95% CI: −0.04, 0.43) but was most prioritized in the postpartum period (ß =0.39, 95% CI 0.21, 0.57).
Conclusions and Implications:
These results highlight that messages on family might be particularly salient in the antepartum period, and keeping CD4 count high in the postpartum period. Understanding maternal motivation may help to design targeted health promotion messages to HIV-positive women around the time of delivery.
Keywords: Discrete choice experiment, Isoniazid preventive therapy, Antiretroviral therapy, Pregnant women, South Africa
INTRODUCTION
The World Health Organization set the ambitious target goals of eliminating HIV transmission from mother to child and reducing maternal deaths related to HIV by 2015 (1). There has been dramatic improvement globally, but 100,000 infants were still infected with HIV, and 20% of pregnant women living with HIV did not receive antiretroviral therapy (ART) in 2015 (2). It is during antenatal care (ANC) visits that many women first learn that they are infected with HIV and/or tuberculosis (TB). About 37% of women who attended ANC in South Africa were HIV-positive and of these, 23% reported one or more symptom of TB in 2011 (3). The prevalence of TB in HIV-positive pregnant women is similar to that of the general population, but they might be at aare at an estimated 10-fold increased risk of active TB compared to HIV-uninfected pregnant women (4) or men of similar age in sub-Saharan Africa (5).
The South African national guidelines for preventing mother-to-child transmission (PMTCT) recommend that all pregnant and breastfeeding HIV-positive women are initiated on lifelong ART, regardless of CD4 cell counts (6). If latently infected with TB, up to 36 months of isoniazid preventive therapy (IPT) up to 36 months is recommended. ART not only prevents vertical HIV transmission but also achieves better long-term maternal health outcomes, and IPT can reduce the risk of a patient developing TB by up to 60% among people living with HIV (PLWH) (6-8).
Despite the clear benefit of preventive therapy and freely available drugs at clinics, implementation of these guidelines remains challenging. Many pregnant women are often at an earlier stage of HIV disease without clinical symptoms so they are less likely to be motivated to take ART once they have protected their babies from infection (9). Recent studies in South Africa showed that up to half of women who initiated ART during pregnancy were lost to follow-up six months after delivery (10). Adherence rates to ART and IPT also significantly drop in the postpartum (11,12). In a meta-analysis among sub-Sharan African studies, the proportion of women with adequate adherence for ART (>80%) reduced from 76% in the antepartum to 53% during the postpartum period (13). Another study in Lesotho showed that 78.5% of HIV-positive women initiated IPT but only 65% of these women completed the 6- month IPT (14). Counseling and support systems could be important drivers to retain these women in care after delivery (15-17), but it remains unclear what benefits related to preventive therapy motivate HIV-positive women to take therapies before and after delivery.
Conjoint analysis has been increasingly used as a tool to elicit patients’ preferences, allowing them to make choices from sets of hypothetical alternatives, where each alternative is described by several characteristics (i.e. attributes) (18,19). We used conjoint analysis to quantitatively measure the relative importance of potential motivators to take preventive therapy among pregnant women with HIV in order to better understand the low uptake of preventive therapy in the postpartum period, and ensure better adherence and retention in care in the long-term.
METHODS
Study Participants
This study was conducted in 14 primary care public health clinics in the Dr. Kenneth Kaunda health district in the North West province, South Africa from November 2014 to December 2016. These 14 clinics were selected to utilize the existing study structure of an ongoing cluster randomized trial and enroll a similar population. This trial compares the proportion with known TB infection status and IPT initiation among newly diagnosed HIV patients in clinics using two different diagnostic tests for latent TB infection (20,21). Clinics were chosen to cover a range of patient volumes, urban vs rural settings, geographical regions and clinic hours. Patients were eligible for enrollment if they were ≥18 years old, newly diagnosed with HIV in the preceding six months, currently pregnant and able to demonstrate reading in either English, Xhosa, Setswana or Zulu. We sought to enroll all eligible pregnant women coming to antenatal care services during the study period. All participants gave written informed consent. The study was approved by the institutional review boards at the Johns Hopkins School of Medicine and the University of Witwatersrand.
Study Design
We conducted a longitudinal survey using a conjoint analysis to elicit pregnant mothers’ motivation for IPT and ART. Conjoint analysis has been applied to measuring preferences for a wide range of health applications, including HIV prevention (22,23) and delivery services among women in rural settings (24). The advantage of conjoint analysis is that it allows us to quantify the degree of preferences (i.e. preference weights) associated with different attributes. One-onto- one in-depth interviews were conducted with 28 HIV-positive patients, 1 female and lmale from each of the 14 participating clinics to elicit patients’ perspectives about IPT and ART as part of the parent study (21)(25). Based on the qualitative interviews with patients and providers, literature review and expert consultations, we determined seven possible benefits of preventive therapy that could matter the most to patients.
We focused on examining positive benefits to inform health promotion messages that could be directly incorporated directly into counseling, clinical consultation and interventions such as SMS text messages (21,25). Table 1 shows the seven possible benefits of preventive therapy with example quotes from the qualitative interviews: (1) Keeps me healthy for my family (2) Keeps me from giving HIV to my partner (3) Keeps my HIV disease under control (4) Keeps my CD4 high (5) Prevents me from getting sick from infections (6) Keeps me healthy and working (7) Prevents me from getting TB. These benefits were not selected to be mutually exclusive and may represent similar constructs (i.e. keeping healthy), but we included a specific key term or concept related to perceived benefits of preventive therapy which seemed to be well accepted by patients. For example, previous studies showed that providing CD4 counts results at the time of HIV diagnosis increased the likelihood of ART initiation (26,27), while counseling messages focusing on a healthy and productive life with ART helped to reduce HIV-related stigma (28). Also, the concept of preventing TB or other infections may resonate better with patients, compared to keeping them generally healthy.
Table 1.
List of seven potential motivations for taking preventive therapy
| Motivator | Example quotes* |
|---|---|
| Keeps me from giving HIV to my partner | “[The doctor] tell me... Start your treatment for HIV because HIVpositive is dangerous If you sleep with someone without condom, you’re HIV-positive infected that one.” |
| Keeps me healthy and working | “[W]e’ve got something to help us to keep going.” |
| Keeps me healthy for my family | “This is my life, this one. I want to live for the children. I want to grew up this children.” |
| “I have to do this for myself and my children, my kids. I’m still raising them” | |
| Keeps my CD4 high | “I see that it is for my life, I must take it because I see that maybe when I started that time, maybe my CD4 count will be that, the way it was. But now it goes down, because I’m not taking the treatment.” |
| “So if I take [ARVs] again for the CD4 count, maybe it will be good then, it will | |
| Keeps my HIV disease under control | “It’s good because I want to stay in my life. I want to stay in my life because this life.”. |
| “My [immune] system is so low so that’s why I’m taking the ARV.” | |
| Prevents me from getting sick from infections | “Yes, it makes me feel good. That infection must not come near to me. They must before.” “I think to prevent it before you are infected is better that way.” |
| “I think to prevent it before you are infected is better that way.” | |
| Prevents me from getting TB | “I will take it, to prevent the TB, I will take it. Use it. “ |
| “I will take it, to prevent the TB, I will take it. Use it. “ “So I want to prevent TB it’s much better. It’s better than to get sitting at home and |
All quotes were extracted from the qualitative interviews with 28 patients newly diagnosed with HIV in the same 14 clinics.
All conjoint tasks were forced choices (i.e. respondent could not opt out or choose neither option). An example of conjoint tasks is shown in Figure 1. We used a fractional factorial experiment with an orthogonal main effect design to limit the number of choice tasks required to be answered by respondents out of all 27 possible combinations of the seven benefits.
Figure 1.
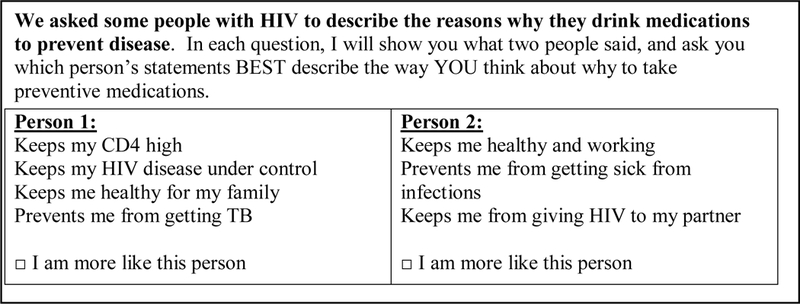
Example of a conjoint analysis task with seven motivators to take preventive therapy among HIV-positive pregnant women
Orthogonal main effect design is a simple, statistically efficient and valid design commonly used for discrete choice experiments, and allows for the to estimation ofe coefficients for main effects, assuming that two or higher-level interactions among factors are negligible (29). The experiment was also balanced such that each benefit and a pair of benefits appeared an equal number of times across eight choice tasks per respondent (30). Each respondent was asked the same eight choice tasks-both at enrollment and the 14 weeks postpartum visit. We pilot-tested the instrument with healthcare providers to refine potential wording and presentation of the instrument.
There is no standardized way to calculate sample size required for discrete choice experiments although there are is some published guidances (31,32). According to de Bekker- Grob et al. (2015), the a minimum sample size of 57 is required to estimate a parameter of 0.3 or above for our study design at a power of 80% and a 5% significance level when analyzed using multinomial logit.
Statistical analysis
The data was were analyzed using the a conditional logit model to estimate relative utility (i.e. preference weight) of each potential benefit to take preventive therapy in the antepartum vs. postpartum (33-35). The primary outcome was the choice between two sets of motivators in each given choice task, while the independent variables were a set of seven potential benefits presented in each task. The conditional logit model assumes that all respondents have the same underlying preferences and thate random errors associated with choices are independent and identically distributed (IID) and independent of irrelevant alternatives (IIA). We fitted two separate conditional logit models for each of the antepartum and postpartum periods. An additional model was fitted where both antepartum and postpartum visits and the interaction terms between each benefit and timing of visits were included in one a single model‥
We tested two hypotheses: first, whether each of the seven identified benefits are considered as an important motivator for taking preventive therapy among HIV-positive pregnant mothers (i.e. utility associated with the benefit is statistically significant and greater than zero) and second, whether this importance differs in the antepartum vs. postpartum periods.. Coefficients (referred to as preference weights) are presented with 95% confidence intervals and clustered standard errors to adjust for correlation within individuals. Coefficients can be interpreted to represent patients’ utility associated with each benefit (i.e. a higher coefficient means higher utility or value associated with that benefit). The p-values for the interaction terms between each benefit and the timing of visits were calculated from the Wald tests to test the statistical difference of the coefficients in the ante- vs. postpartum periods. P-values <0.05 were considered significant. All analysis was performed in STATA 13.0.
RESULTS
Participant Characteristics
A total of 72 people pregnant women newly diagnosed with HIV were enrolled and completed the questionnaire. Sixty-five women completed surveys at both baseline and follow- up visit (Table 2). At baseline, the median age was 27 years (Interquartile range [IQR]:22-31 years), with average time since initial HIV diagnosis of 51 (±75) days. The mean gestational week at enrollment was 18 (±7) weeks. All women were already on ART, and the median CD4 count was 404 (IQR: 228-609) cells/mm3 and about 30% (n=19) were on IPT. All participants reported currently having a partner or spouse, but 60% (n=38) were not living with partners, and 85% (n=55) were not employed.
Table 2.
Baseline characteristics among 65 HIV-positive pregnant women who completed the survey, South Africa*
| N=65 | |
|---|---|
| Age (years), Median (Q1, Q3) | 27 (22, 31) |
| Time since HIV diagnosis (days), Mean (±SD) | 55 ± 51 |
| Gestational weeks, Mean (±SD) | 18 ± 7 |
| CD4 cell count (cells/mm3), Median (Q1, Q3)‡ | 404 (228, 609) |
| n(%) | |
| Marital status | |
| Married | 6 (9.4) |
| Living with partner | 20 (31.3) |
| Not living with partner | 38 (59.4) |
| Employment status | |
| I work full time | 5 (7.7) |
| I work part time or piece jobs | 5 (7.7) |
| I am not employed | 55 (84.6) |
| Number of adults living in the household | |
| 0-1 | 24 (36.9) |
| 2+ | 41 (63.1) |
| Number of children living in the household | |
| 0-1 | 55 (84.6) |
| 2+ | 10 (15.4) |
| Transportation | |
| On foot | 36 (55.4) |
| Public taxi or bus | 29 (44.6) |
| TB treatment history | |
| Yes | 4 (6.2) |
| No | 61 (93.8) |
| Currently on isoniazid preventive therapy | |
| Yes | 19 (29.2) |
| No | 46 (70.8) |
| Currently on cotrimoxazole preventive therapy | |
| Yes | 4 (6.4) |
| No | 61 (93.6) |
Pearson χ2 test (discrete variables), t-test (mean comparison for continuous variables) and Mann-Whitney test (median comparison for continuous variables) were used.
Data was available for 48 participants.
AtIn the postpartum visits, >95% all (n=65) reported that they were still taking ART. Of 65 infants, 1 (1.5%) was confirmed as HIV-positive, 47 (72.3%) were HIV-negative, 4 (6.2%) had indeterminate status, and 13 (20.0%) did not receive PCR tests. Over 90% (n=61) of participants had ever breastfed their infants, and 67% (n=41) were still breastfeeding. While 51.5% (n=33) reported that they received some or a lot of support from friends and family to remind them to take medications, 95.2% (n=60) reported receiving such support from healthcare providers (p<0.001). Almost everyone (62/65) on ART reported that they were informed about the side effects and reasons to take ART, while 85.7% (12/14) of patients on IPT did so (p<0.001).
Preference weights in antepartum vs. postpartum period
Preference weights for each of the seven attributes are shown in Table 3. In the antepartum period, pPreventing HIV transmission had the highest coefficient (β=0.87, 95% CI: 0.64, 1.11), followed by keeping healthy for family (β=0.75, 95% CI: 0.52, 0.97), in the antepartum period where positive coefficients represent positive preferences for the motivators. Keeping CD4 high had the lowest coefficient (β=0.19, 95% CI: −0.04, 0.43) (Figure 2). When the coefficients of the motivators were compared, preventing HIV transmission to a partner had approximately 4.5 times higher utility than keeping CD4 high, and two times higher utility than benefits focused on individual health. Keeping healthy and working and, preventing oneself from getting sick from infections or TB were moderately ranked.
Table 3.
Coefficients from conditional logistic regression among 65 HIV-positive women in the antepartum vs. postpartum periods
| Benefits related to preventive therapy |
Antepartum | Postpartum | p- value† |
||
|---|---|---|---|---|---|
| Coefficient (SE) |
95% CI | Coefficient (SE) |
95% CI | ||
| Preventing HIV transmission | 0.87 (0.14)** | (0.59, 1.15) | 0.20 (0.09)* | (0.03, 0.38) | <0.01 |
| Keeping healthy and working | 0.41 (0.11)** | (0.19, 0.63) | 0.10 (0.09) | (−0.07, 0.27) | 0.02 |
| Keeping healthy for family | 0.75 (0.15)** | (0.45, 1.04) | 0.05 (0.08) | (−0.09, 0.20) | <0.01 |
| Keeping CD4 high | 0.19 (0.10) | (−0.01, 0.40) | 0.41 (0.10)** | (0.22, 0.59) | 0.13 |
| Keeping disease under control | 0.67 (0.11)** | (0.46, 0.87) | 0.24 (0.10)* | (0.04, 0.44) | 0.01 |
| Preventing infections | 0.40 (0.12)** | (0.16, 0.64) | 0.03 (0.10) | (−0.16, 0.22) | 0.02 |
| Preventing TB | 0.44 (0.11)** | (0.23, 0.66) | 0.21 (0.08)** | (0.05, 0.38) | 0.10 |
P-values from Wald tests are reported: p-value <0.05;
p-value <0.01
P-values were estimated from the model where both antepartum and postpartum visits and the interaction terms between each benefit and timing of visits (postpartum vs. antepartum) were fitted in one model.
Figure 2.
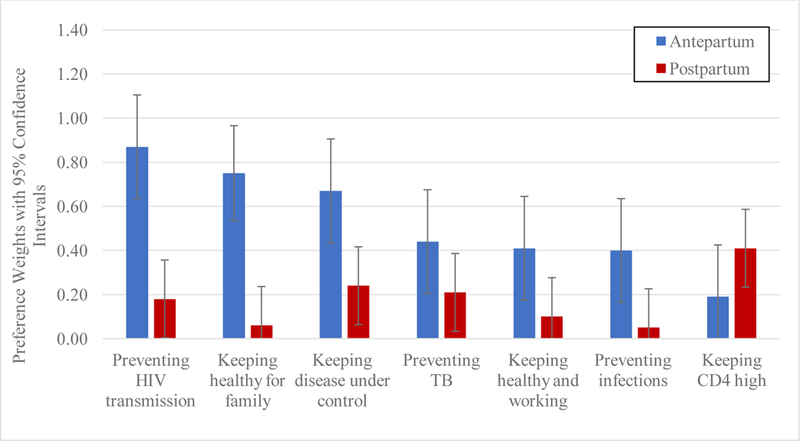
Preference weights estimated from conditional logit estimates for seven motivators to take preventive therapy among 65 HIV-positive pregnant women in South Africa in the antepartum vs. postpartum period. The bar range represents 95% confidence interval for the preference weights.
In the postpartum period, the preference weight for keeping CD4 high was the highest (β=0.41, 95% CI: 0.23, 0.59), and associated with more than twice the utility compared to preventing HIV transmission and keeping healthy for family. The preference weights for preventing HIV transmission (β=0.18, 95% CI: 0.00, 0.35) and keeping healthy for family (β=0.06, 95% CI: −0.12, 0.24) were reduced and no longer significant. Keeping disease under control (β=0.24, 95% CI: 0.06, 0.42) and preventing oneself from getting TB (β=0.24, 95% CI: 0.03, 0.39) were moderately ranked and statistically significant. Compared to the antepartum period, tThe preference weights significantly reduced for preventing HIV transmission (p<0.01) and keeping healthy for family (p<0.01) in the postpartum as well as keeping disease under control (p=0.01) in the postpartum period.
DISCUSSION
Pregnant women newly diagnosed with HIV face the “triple burden” of accepting that they are pregnant, newly diagnosed with HIV, and the need to make the decision to initiate lifelong treatment (36). We demonstrate that pregnant women initiated on lifelong ART prioritize benefits related to preventive therapy differently in the antepartum vs. postpartum periods. We found that HIV-positive pregnant women were most attentive to medication benefits related to social responsibility such as preventing HIV transmission to partners or supporting family during pregnancy. The extent of such prioritization was subdued in the postpartum and keeping high CD4 counts and medication benefits related to their own health were more prioritized. These results provide important insights to help develop and strengthen health promotion messages to pregnant women with HIV around the time of delivery.
This is one of the first studies to quantify relative utility of ART and IPT as perceived by women initiated on lifelong therapy during pregnancy. We observed that the relative utility related to preventing HIV transmission or supporting family was much higher in the antepartum period. Every participant in this study was tested for HIV and received ART during pregnancy. Several quantitative studies have reported that women initiating ART during pregnancy have a strong motivation to take medications to prevent HIV transmission to their infants and then they have fallen out of care as they perceive their own health as relatively less unimportant compared to delivering healthy infants (9,36). Providing care for children and family was also considered as an important motivator for women to remain healthy during pregnancy (37)(37). Our results also underscore that it is crucial to understand and support the social roles of HIV-positive pregnant women.
There was no clear ranking among different benefits in the postpartum period, and the relative utility gain for most benefits significantly decreased compared to in the antepartum.
There are a few possible explanations. First, mothers seem to perceive the benefits of preventive therapy for their own health more highly compared to other benefits after delivery. The South African government recommends HIV-positive women initiated on lifelong ART during pregnancy have CD4 counts checked within between 3-6 days after delivery in postpartum and continue to receive HIV care and counseling at monthly visits aligned with infant’s visits (6). Almost everyone in the study reported that healthcare providers explained the side effects and benefits of ART and IPT to them during their last clinic visits. Maternal self-reported adherence to ART and IPT in the postpartum was 95% and 92%, respectively, and such rates continued even after termination of breastfeeding, indicating that mothers in our study were likely to continue taking therapy for their own health.
This could be that HIV counseling support was well accepted by mothers in this setting. A recent study among HIV-positive women in Ethiopia and Zambia reported that counseling support services were an important driver in maintaining regular clinic visits, while types of clinics or transportation costs were less prioritized in the postpartum period (38). Similarly, positive attitudes towards treatment and its perceived benefits were reported as the facilitators for adherence to ART and retention in care (39,40). Other predictors of disengagement and/or poor adherence in the postpartum included non-disclosure of HIV status, feeling well, and inadequate knowledge about PMTC (10,36). About 80% of our participants had disclosed their HIV status to their partner, potentially contributing to better adherence rates.
Second, Kkeeping high CD4 counts was associated with the largest utility gain in the postpartum period. Most of the participants had been on ART an average of 2 two months at enrollment, but potentially became more familiar with the clinical implications of CD4 counts through repeated HIV counseling and regular clinic visits in the ante- and early postpartum periods. A study among HIV-positive pregnant women in South Africa showed that providing CD4 count results might have helped mothers take their HIV diagnosis more seriously and resulted in improved retention in care and adherence to ART after delivery (36). Other messages related to individual health benefits, such as preventing infections or keeping them healthy, resulted in similar utility gain. Our study results highlight that counseling in the postpartum period needs to be geared towards communicating CD4 cell counts as well as promoting the benefits of ART and IPT for maternal health.
The South African National Department of Health started the MomConnect program in 2014 for pregnant women to receive weekly messages related to pregnancy and delivery up to 1 one year after delivery. It subsequently launched a pilot PMTCT MomConnect program in 2016, targeted at enrollingwith a targeting to enroll all pregnant women and mothers of infants diagnosed with HIV by 2018 to deliver specific messages related to PMTCT (41). The findings from this study can help to develop targeted messages important to mothers in the ante and postpartum periods in South Africa and similar settings. Further studies are warranted to specify how these health promotion and specific counseling messages could improve clinic attendance and adherence to ART and IPT.
There are several limitations to our study. First, these surveys were done in primary health clinics in a mix of rural and urban areas where regular HIV testing and counseling programs were provided and the disclosure status was quite high. Thus the results reflect such programmatic settings, and can only be generalized to other similar contexts. Second, we did not directly ask about the benefits of preventing HIV transmission to infants, which could be the most important concern for mothers. Preventing HIV transmission to partners may pose similar social responsibility, and factors related to infants were tested in another discrete choice experiment and presented elsewhere (42)(43). Third, the an objective measure of self-reported adherence to ART, such as viral load suppression, was not available. SThe self-reported adherence rate could have been overstated, but we used the validated self-reported adherence questionnaire with 4 days of recall, and this short recall period was shown to be well associated with more objective measures (43-46). We also cannot discount that mothers were paying less attention to the surveys in the postpartum visits and therefore chose more randomly. Given that mothers had already taken the surveys once and were familiar with the choice task, we believe that such bias would have been minimal. In addition, we could not test any effect of potential geographic heterogeneity due to the small number of participants recruited from each clinic. Lastly, the sample size of our study is quite small, and we could not test interactions with other factors like gestational weeks at first ANC visits or number of pregnancies related to lower adherence in the postpartum period (47). Latent class model or random parameter logit model could not be fitted to account for heterogeneity in preferences across individuals or groups, given the small sample size. Future studies would be needed to examine potential heterogeneity in maternal preferences for preventive therapies.
CONCLUSION
We observed that prevention of HIV transmission and supporting family members were the most important motivators to for takinge preventive therapy in the antepartum period among HIV-positive pregnant women. Such prioritization diminished in the postpartum period and keeping a high CD4 count became a more important motivator. Understanding maternal motivation may help to design and deliver targeted health promotion messages to HIV-positive women around the time of delivery and to enhance adherence and retention in care after delivery in this setting.
Acknowledgement:
This work was supported by NIH supplement R01AI095014 02S1. We thank all study participants for devoting their time to take part in this study. We thank our study coordinators, Sandy Chon, Cokiswa Quomfo, Juanita Market, Mmabatho Malegotsia, Elvis Rangxa, Thembekile Mmmoledi as well as all study staff who helped in data collection.
Funding: This study was funded by NIH supplement R01AI095014 02S1.
Footnotes
Compliance with Ethical Standards:
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent: Informed consent was obtained from all individual participants included in the study.
Conflict of interest: H-YK declares that she has no conflict of interest. DWD declares that he has no conflict of interest. NAM declares that he has no conflict of interest. DK declares that she has no conflict of interest. CT declares that she has no conflict of interest. JG declares that he has no conflict of interest. JFPB declares that he has no conflict of interest. CFH declares that she has no conflict of interest.
References
- 1.UNAIDS. Global plan towards the elimination of new HIV infections among children by 2015 and keeping their mothers alive. Geneva; 2011. [Google Scholar]
- 2.Idele P, Hayashi C, Porth T, Mamahit A, Mahy M. Prevention of mother-to-child transmission of HIV and paediatric HIV care and treatment monitoring: from measuring process to impact and elimination of mother-to-child transmission of HIV. AIDS Behav. 2017;21(S1):1—11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gounder CR, Wada NI, Kensler C, Violari A, McIntyre J, Chaisson RE, et al. Active Tuberculosis Case-Finding Among Pregnant Women Presenting to Antenatal Clinics in Soweto, South Africa. J Acquir Immune Defic Syndr. 2011;57(4):e77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pillay T, Khan M, Moodley J. The increasing burden of tuberculosis in pregnant women, newborns and infants under 6 months of age in Durban, KwaZulu-Natal. South African Med J. 2001;91(11):983—7. [PubMed] [Google Scholar]
- 5.Karim QA, Sibeko S, Baxter C. Preventing HIV infection in women: a global health imperative. Clin Infect Dis. 2010;50(Suppl 3):S122–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Department of Health Republic of South Africa. National consolidated guidelines for the prevention of mother-to-child transmission of HIV (PMTCT) and the management of HIV in children, adolescents and adults. 2015. [Google Scholar]
- 7.World Health Organization. Global Tuberculosis Report 2017. Geneva; 2017. [Google Scholar]
- 8.Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365(6):493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clouse K, Schwartz S, Van Rie A, Bassett J, Yende N, Pettifor A. “What they wanted was to give birth; nothing else.” J Acquir Immune Defic Syndr. 2014;67(1):e12–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clouse K, Pettifor A, Shearer K, Maskew M, Bassett J, Larson B, et al. Loss to follow-up before and after delivery among women testing HIV positive during pregnancy in Johannesburg, South Africa. Trop Med Int Heal. 2013;18(4):451–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rowe KA, Makhubele B, Hargreaves JR, Porter JD, Hausler HP, Pronyk PM. Adherence to TB preventive therapy for HIV-positive patients in rural South Africa: implications for antiretroviral delivery in resource-poor settings? Int J Tuberc Lung Dis. 2005;9(3):263–9. [PubMed] [Google Scholar]
- 12.Golub J, Pronyk P, Mohapi L, Thsabangu N, Moshabela M, Struthers H, et al. Isoniazid preventive therapy, HAART and tuberculosis risk in HIV-infected adults in South Africa: a prospective cohort. AIDS. 2009;23(5):631–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nachega JB, Uthman OA, Anderson J, Peltzer K, Wampold S, Cotton MF, et al. Adherence to antiretroviral therapy during and after pregnancy in low-income, middle- income, and high-income countries: a systematic review and meta-analysis. AIDS. 2012;26(16):2039–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tiam A, Machekano R, Gounder CR, Maama-maime LBM, Ntene-sealiete K, Sahu M, et al. Preventing tuberculosis among HIV-infected pregnant women in Lesotho: the case for rolling out active case finding and isoniazid preventive therapy. J Acquir Immune Defic Syndr. 2014;67(1):e5–11. [DOI] [PubMed] [Google Scholar]
- 15.Reda AA, Biadgilign S. Determinants of adherence to antiretroviral therapy among HIV- infected patients in Africa. AIDS Res Treat. 2012;2012:574656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carrieri MP, Leport C, Protopopescu C, Cassuto JP, Bouvet E, Peyramond D, et al. Factors associated with nonadherence to highly active antiretroviral therapy: a 5-year follow-up analysis with correction for the bias induced by missing data in the treatment maintenance phase. J Acquir Immune Defic Syndr. 2006;41(4):477–85. [DOI] [PubMed] [Google Scholar]
- 17.Holtzman C, Brady K, Yehia B. Retention in care and medication adherence: current challenges to antiretroviral therapy success. Drugs. 2015;75(5):445–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bridges J, Hauber AB, Marshall D, Lloyd A, Prosser LA, Regier DA, et al. Conjoint analysis applications in health--a checklist: a report of the ISPOR Good Research Practices for Conjoint Analysis Task Force. Value Heal. 2011;14(4):403–13. [DOI] [PubMed] [Google Scholar]
- 19.Louviere J, Hensher D, Swait J. Stated Choice Methods: Analysis and Applications. Cambridge: Cambridges University Press; 2000. [Google Scholar]
- 20.Golub J, Lebina L, Qomfu C, Chon S, Cohn S, Masonoke K, et al. Implementation of QuantiFERON®- TB Gold In-Tube test for diagnosing latent tuberculosis among newly diagnosed HIV-infected patients in South Africa. In: In: 46th World Conference on Lung Health of the International Union Against Tuberculosis and Lung Disease (The Union) Cape Town, South Africa; 2015. p. [Abstract OA-399-05]. [Google Scholar]
- 21.Kerrigan D, Tudor C, Motlhaoleng K, Lebina L, Qomfu C, Variava E, et al. Relevance and acceptability of using the Quantiferon gold test (QGIT) to screen CD4 blood draws for latent TB infection among PLHIV in South Africa: formative qualitative research findings from the TEKO trial. BMC Health Serv Res. 2018;18(1):288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carballo-Diéguez A, Stein Z, Sáez H, Dolezal C, Nieves-Rosa L, Díaz F. Frequent use of lubricants for anal sex among men who have sex with men: the HIV prevention potential of a microbicidal gel. Am J Public Health. 2000;90(7):1117–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Newman PA, Cameron MP, Roungprakhon S, Tepjan S, Scarpa R. Acceptability and preferences for hypothetical rectal microbicides among a community sample of young men who have sex with men and transgender women in Thailand: a discrete choice experiment. AIDS Behav. 2016;20(11):2588–601. [DOI] [PubMed] [Google Scholar]
- 24.Kruk ME, Paczkowski M, Mbaruku G, de Pinho H, Galea S. Women’s preferences for place of delivery in rural Tanzania: a population-based discrete choice experiment. Am J Public Health. 2009;99(9):1666–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Finitsis DJ, Pellowski JA, Johnson BT. Text message intervention designs to promote adherence to antiretroviral therapy (ART): a meta-analysis of randomized controlled trials. PLoS One. 2014;9(2):e88166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Faal M, Naidoo N, Glencross DK, Venter WDF, Osih R. Providing immediate CD4 count results at HIV testing improves ART initiation. J Acquir Immune Defic Syndr. 2011;58(3):e54–9. [DOI] [PubMed] [Google Scholar]
- 27.Sutton R, Lahuerta M, Abacassamo F, Ahoua L, Tomo M, Lamb MR, et al. Feasibility and acceptability of health communication interventions within a combination intervention strategy for improving linkage and retention in HIV care in Mozambique. J Acquir Immune Defic Syndr. 2017;74(Suppl 1):S29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zuch M, Lurie M. “A virus and nothing else”: the effect of ART on HIV-related stigma in rural South Africa. AIDS Behav. 2012;16(3):564–70. [DOI] [PubMed] [Google Scholar]
- 29.Johnson F, Lancsar E, Marshall D, Kilambi V, Mühlbacher A, Regier DA, et al. Constructing experimental designs for discrete-choice experiments: report of the ISPOR conjoint analysis experimental design good research practices task. Value Heal. 2013;16(1):3–13. [DOI] [PubMed] [Google Scholar]
- 30.Montgomery DC. Design and analysis of experiments. John Wiley & Sons; 2017. [Google Scholar]
- 31.Orme B Getting started with conjoint analysis: strategies for product design and pricing research. Madison, WI: Research Publishers LLC; 2006. [Google Scholar]
- 32.de Bekker-Grob EW, Donkers B, Jonker MF, Stolk EA. Sample size requirements for discrete-choice experiments in healthcare: a practical guide. Patient. 2015;8(5):373–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Bekker-Grob EW, Ryan M, Gerard K. Discrete choice experiments in health economics: a review of the literature. Health Econ. 2012;21(2):145–72. [DOI] [PubMed] [Google Scholar]
- 34.Ryan M, Major K, Skátun D. Using discrete choice experiments to go beyond clinical outcomes when evaluating clinical practice. J Eval Clin Pract. 2005;11(4):328–38. [DOI] [PubMed] [Google Scholar]
- 35.Hauber AB, González JM, Groothuis-Oudshoorn CGM, Prior T, Marshall DA, Cunningham C, et al. Statistical methods for the analysis of discrete choice experiments: a report of the ISPOR conjoint analysis good research practices task force. Value Heal. 2016;19(4):300–15. [DOI] [PubMed] [Google Scholar]
- 36.Black S, Zulliger R, Marcus R, Mark D, Myer L, Bekker L-G. Acceptability and challenges of rapid ART initiation among pregnant women in a pilot programme, Cape Town, South Africa. AIDS Care. 2014;26(6):736–41. [DOI] [PubMed] [Google Scholar]
- 37.Lake Snell Perry & Associates Inc. The healthcare experiences of women with HIV/AIDS: Insights from focus groups. Henry Kaiser Family Foundation; [Internet]. 2003. [Google Scholar]
- 38.Kruk ME, Riley PL, Palma AM, Adhikari S, Ahoua L, Arnaldo C, et al. How can the health system retain women in HIV treatment for a lifetime? a discrete choice experiment in Ethiopia and Mozambique. PLoS One. 2016;11(8):e0160764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Penn C, Watermeyer J, Evans M. Why don’t patients take their drugs? The role of communication, context and culture in patient adherence and the work of the pharmacist in HIV/AIDS. Patient Educ Couns. 2011;83(3):310–8. [DOI] [PubMed] [Google Scholar]
- 40.Loggerenberg F, Gray D, Gengiah S, Kunene P, Gengiah TN, Naidoo K, et al. A qualitative study of patient motivation to adhere to combination antiretroviral therapy in South Africa. AIDS Patient Care STDS. 2015;29(5):299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Department of Health Republic of South Africa. One Year of Operation: A Case Study MomConnect. 2015. [Google Scholar]
- 42.Kim H-Y, Dowdy DW, Martinson NA, E Golub J, Bridges JFP, Hanrahan CF. Maternal priorities for preventive therapy among HIV-positive pregnant women before and after delivery in South Africa: a best-worst scaling survey. J Int AIDS Soc. 2018;21(7):e25143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chesney MA, Ickovics JR, Chambers DB, Gifford AL, Neidig J, Zwickl B, et al. Self-reported adherence to antiretroviral medications among participants in HIV clinical trials: the AACTG adherence instruments. AIDS Care. 2000;12(3):255–66. [DOI] [PubMed] [Google Scholar]
- 44.Carrieri MP, Leport C, Protopopescu C, Cassuto JP, Bouvet E, Peyramond D. Factors associated with nonadherence to highly active antiretroviral therapy: a 5-year follow-up analysis with correction for the bias induced by missing data in the treatment maintenance phase. J Acquir Immune Defic Syndr. 2006;41(4):477–85. [DOI] [PubMed] [Google Scholar]
- 45.Dal Fabbro MMFJ, Cunha RV da, Paniago AMM, Lindenberg ADSC, Freitas GMB De, Nogueira SA Prospective study on the prevention of vertical transmission of HIV in Campo Grande, Mato Grosso do Sul, Brazil, from 1996 to 2001. Braz J Infect Dis. 2005;9(1):20–7. [DOI] [PubMed] [Google Scholar]
- 46.Franco M, Diego R, Claudio A, Giampietro G, Giampaolo Q, Gary Antezana C, et al. Simpler regimens may enhance adherence to antiretrovirals in HIV-infected patients. HIV Clin Trials. 2002;3(5):371–8. [DOI] [PubMed] [Google Scholar]
- 47.Mellins CA, Chu C, Malee K, Allison S, Smith R, Harris L, et al. Adherence to antiretroviral treatment among pregnant and postpartum HIV-infected women. AIDS Care.2008;20(8):958–68. [DOI] [PubMed] [Google Scholar]


