Abstract
O6-methylguanine-DNA methyltransferase (MGMT) is an enzyme that removes alkyl groups at the O6-position of guanine in DNA. MGMT expression is reduced or absent in many tumor types derived from a diverse range of tissues, most notably in glioma. Low MGMT expression confers significant sensitivity to DNA alkylating agents such as temozolomide (TMZ), providing a natural therapeutic index over normal tissue. In this study, we sought to identify novel approaches which could maximally exploit the therapeutic index between tumor cells and normal tissues based on MGMT expression, as a means to enhance selective tumor cell killing. TMZ, unlike other alkylators, activated the Ataxia Telangiectasia and Rad3-related (ATR)-Checkpoint Kinase 1 (Chk1) axis in a manner that was highly dependent on MGMT status. TMZ induced growth delay, DNA double-strand breaks, and G2/M cell cycle arrest, which led to ATR-dependent phosphorylation of Chk1; this effect was dependent on reduced MGMT expression. Treatment of MGMT-deficient cells with TMZ increased sensitivity to ATR inhibitors both in vitro and in vivo across numerous tumor cell types. Taken together, this study reveals a novel approach for selectively targeting MGMT-deficient cells with ATR inhibitors and TMZ. As ATR inhibitors are currently being tested in clinical trials, and TMZ is a commonly used chemotherapeutic, this approach is clinically actionable. Furthermore, this interaction potently exploits a DNA-repair defect found in many cancers.
Introduction
O6-methylguanine-DNA methyltransferase (MGMT) is a major protein involved in the repair of DNA alkylation damage, specifically at the O6-position of guanine (1). MGMT removes O6-alkylguanine adducts by transferring the lesions from guanine to the active site Cysteine-145 residue in the protein. The enzyme is then degraded via the ubiquitin proteolytic pathway (2). Importantly, MGMT expression levels can accurately predict the sensitivity of cells to alkylating agents that create lesions on O6-guanine, such as temozolomide (TMZ) (1).
It is now well-established that MGMT expression is either silenced or down-regulated in subsets of glioblastoma, where TMZ is used as a standard treatment component. An analysis of data from the landmark Stupp trial (3) demonstrated that MGMT promoter methylation was associated with almost a doubling of both progression-free survival and overall survival, in comparison to unmethylated glioblastoma. Emerging data now reveal that subsets of many different tumor types beyond glioma also harbor reduced MGMT expression, including lung squamous cell carcinoma, small cell lung cancer, colon adenocarcinoma, acute myeloblastic leukemia, and bladder cancer (4,5). Despite the fact that this natural therapeutic index exists in numerous tumor types, the clinical use of TMZ is confined largely to glioblastoma (4). Therefore, TMZ may be efficacious in these tumors, although effective treatment likely will require combinations with other agents, such as DNA repair inhibitors.
TMZ is a methylating agent that creates O6-methylguanine (O6MeG) lesions. In the absence of MGMT expression, TMZ-induced O6MeG lesions are left unrepaired and accumulate in the genome. O6MeG lesions mispair with thymine during DNA replication, and these mispairs activate the mismatch repair (MMR) pathway (6). MSH2 and MSH6 heterodimers (comprising the MutSα complex) specifically recognize O6MeG:T mismatches and recruit MLH1 and PMS2 heterodimers (referred to as the MutLα complex) (7). In vitro, selected studies have shown that MutSα and MutLα localize to O6MeG:T mismatches and recruit Ataxia Telangiectasia and Rad3-related protein (ATR) and its binding partner, ATR-interacting protein (ATRIP), activating ATR (7).
ATR activates the G2/M cell cycle checkpoint and prevents premature entry into mitosis in response to various forms of DNA damage that cause uncoupling of DNA polymerases and helicases during DNA replication, a state otherwise known as replication stress (8). As tumor cells harbor elevated levels of replication stress, ATR inhibitors have been developed as antitumor agents. However, beyond homologous recombination (HR) defects (9), there is a dearth of molecular biomarkers that have been described to identify tumors that will be most responsive to ATR inhibitors, especially in combination with cytotoxic agents.
Here, we report an exquisite, MGMT-dependent synergistic interaction between ATR inhibitors and TMZ. Mechanistically, we find that TMZ induces double-strand breaks (DSBs) and activates the ATR-Chk1 axis, specifically in tumor cells with low MGMT expression. These findings argue that MGMT is an important molecular biomarker for ATR inhibitor and TMZ combinations. Notably, they form the basis for a clinically actionable DNA repair inhibitor and DNA damaging agent combination with a favorable therapeutic index.
Materials and Methods
Cell Culture
The LN229 MGMT- and MGMT+ cell lines were obtained from Bernd Kaina and confirmed negative of mycoplasma using MycoAlert (Lonza). NCI-H446, SW480, and SW620, HeLa, and U2OS were purchased from ATCC. Hap1 cells (parental and MGMT-) were purchased from Horizon. GBM22 (patient-derived xenograft (PDX) cells) were obtained from Jann Sarkaria. NCI-H446, SW480, and SW620 cells were cultured in RPMI-1640 with 10% FBS (Gibco). LN229, HeLa, GBM22, and U2OS cells were cultured in DMEM/10% FBS (Gibco). Hap1 cells were cultured in IMDM with 10% FBS (Gibco). Cell lines were not authenticated.
Antibodies and reagents
Drugs were purchased from Selleckchem or Sigma Aldrich. Compounds were resuspended in DMSO and stored at room temperature (O6BG) or −20˚C (all others). Please see supplemental methods for a table of antibodies used in the study.
Growth Delay and Drug Synergy Assays
High-throughput growth delay assays and synergy assays were performed and analyzed as described previously (10). Drug treatment was conducted for six days continuously with at least two replicates per treatment condition.
Comet Assay
Comet assays were performed and analyzed as described previously using neutral conditions (10).
Flow Cytometry
Cells were fixed in 3.7% paraformaldehyde (PFA) at room temperature for 20 minutes, washed with PBS with 1% BSA, and permeabilized in 90% methanol overnight at −20˚C. After permeabilization, cells were incubated in primary and secondary antibody solutions for 1 hour and 30 minutes, respectively, at room temperature. Cells were then stained in RNase/PI buffer (BD Biosciences), and data was acquired on a Cytek flow cytometer in accordance with the manufacturer’s instructions. FlowJo software was used for analysis. The Dead Cell Apoptosis Kit with Annexin V Alexa Fluor 488 & Propidium Iodide (Thermo Fisher Scientific) was used for apoptosis experiments.
Clonogenic Survival Assay
Cells were pre-treated in culture for 48–72 hours. Cells were then transferred in media without drug to six-well plates in triplicate at 3-fold dilutions ranging from 9000 to 37 cells per well. After 12–14 days, plates were washed with PBS and stained with crystal violet. Colonies were counted by hand. Counts were normalized to plating efficiency of corresponding treatment condition, unless otherwise noted.
Immunofluorescence analysis
Immunofluorescence analysis was carried out as described previously (11).
In vivo TMZ and VX-970 efficacy studies
Female athymic nu/nu mice (Hsd:Athymic Nude-Foxn1nu, Envigo) were used for in vivo xenograft studies. LN229 MGMT- or GBM22 cells were injected subcutaneously into the flank at a concentration of 500,000 cells per 100 μL of PBS. Prior to treatment, mice were sorted into groups of 7–10 animals such that average starting tumor volume of each group was approximately equal. Mice were treated for 3 weeks on a 4-day-on, 3-day-off regimen. TMZ was administered on days 1 and 3 of each cycle, and VX-970 was administered on days 2 and 4. TMZ was delivered via oral gavage at 3 or 5 mg/kg in 50% PEG-400/50% sterile PBS, while VX-970 was delivered at 60 mg/kg via oral gavage in the same vehicle. Tumors were measured using digital calipers. IACUC at Yale School of Medicine approved the studies.
Statistical analysis
Data are presented as mean ± SD or SEM. Comparisons were made using Student’s t test (comet, immunofluorescence) or Mann-Whitney test (xenograft study). All tests were two-sided. Statistical analyses were carried out using GraphPad Prism. A p-value of less than 0.05 was considered statistically significant.
Results
TMZ selectively induces enhanced tumor cell growth delay, DNA damage, and cell cycle arrest in MGMT-deficient cells
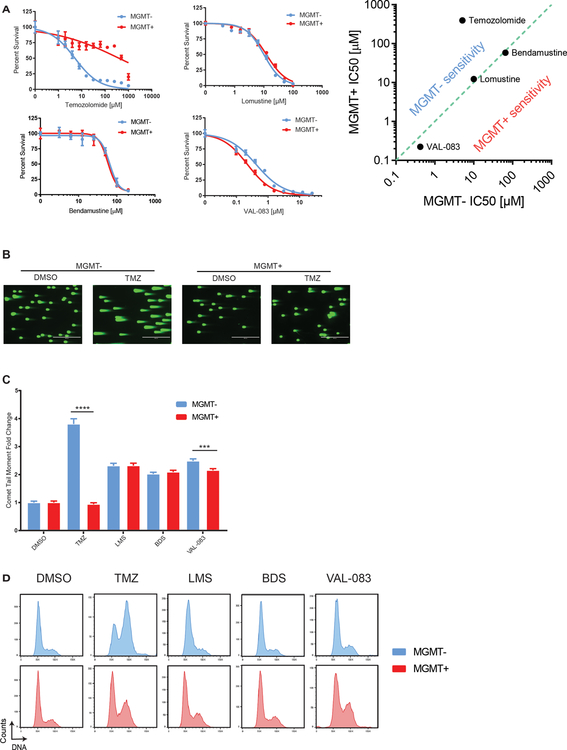
First, we sought to characterize the effects of several structurally unique and functionally dissimilar alkylators (TMZ, lomustine (LMS), bendamustine (BDS), and VAL-083) on cells proficient and deficient in MGMT. Unlike TMZ, LMS, BDS, and VAL-083 create DNA interstrand crosslinks (12). Specifically, we tested the MGMT promoter-methylated glioblastoma cell line LN229 and a clone stably complemented with an MGMT open reading frame (ORF; referred to as MGMT- and MGMT+). Short-term viability assays revealed that MGMT status was important for the activity of TMZ but not crosslinking alkylators (compare TMZ versus LMS, BDS, and VAL-083 in Fig. 1A).
Figure 1.
TMZ selectively induces enhanced tumor cell growth delay, DNA damage, and cell cycle arrest in MGMT-deficient cells. A, Six-day growth delay curves of alkylators TMZ, LMS, BDS, and VAL-083 on LN229 cells. Log-log plot displays relative IC50 values for the different drugs in MGMT- and MGMT+ cells. B, LN229 cells were treated for 24h hours with TMZ [20 μM], LMS [20 μM], BDS [50 μM], or VAL-083 [5 μM] and released for 48h before neutral comet assay. Representative images for TMZ are shown. C, Comet tail moment fold change normalized to the DMSO treatment condition for the corresponding cell line. D, LN229 cells were treated with TMZ [20 μM], LMS [20 μM], BDS [50 μM], or VAL-083 [10 μM] for 48h. DNA content histograms are shown.
We hypothesized that the MGMT-dependent alkylator sensitivity observed with TMZ but not with crosslinking alkylators would correlate with differences in the type of DNA damage induced by these agents. Neutral comet assays revealed that TMZ induces a dramatic increase in DSBs, specifically in LN229 MGMT- cells, but not in the complemented line (LN229 MGMT+). This was evidenced by 4-fold higher mean comet tail moment levels (p < 0.0001) levels in LN229 MGMT- cells compared to MGMT+ cells (Figs. 1B, 1C, S1A).
We also hypothesized that TMZ-mediated growth delay could result from cell cycle arrest. We found that MGMT- cells treated with TMZ accumulated in the G2/M phase of the cell cycle, whereas MGMT+ cells were not affected (Fig. 1D). This MGMT-specific effect was not observed upon treatment with the crosslinking alkylating agents, which induced similar cell cycle effects on LN229 cells regardless of MGMT status.
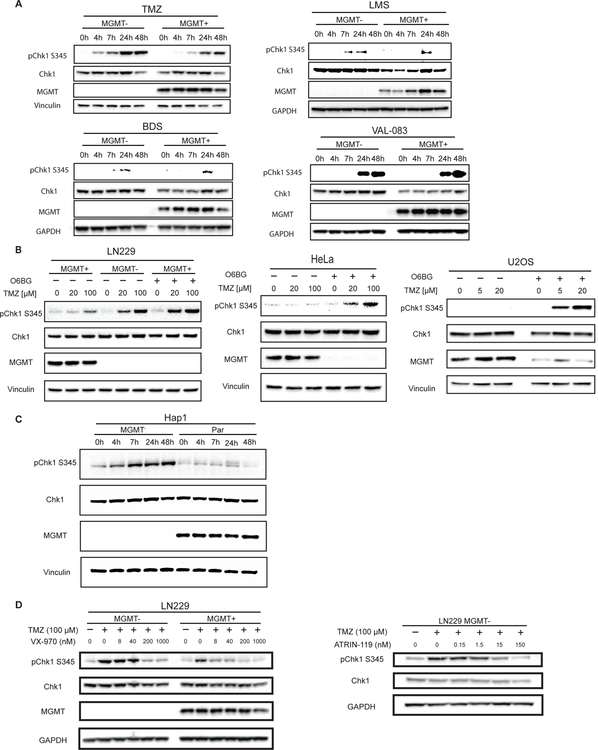
TMZ specifically activates the ATR-Chk1 axis in MGMT-deficient cells
As discussed previously, ATR regulates the intersection between DNA damage and cell cycle arrest. Given the MGMT-dependent DNA damage and cell cycle arrest that we observed upon TMZ treatment, we hypothesized that TMZ activates the ATR axis specifically in MGMT-deficient cells. To this end, we examined the levels of phosphorylated Chk1 as a readout of ATR activity. We found that treatment with TMZ in LN229 cells activated the ATR-Chk1 axis in an MGMT-dependent manner, with higher levels of Chk1 (S345) phosphorylation in LN229 MGMT- cells (Fig. 2A). TMZ treatment resulted in MGMT-dependent phosphorylation of RPA32 (Fig. S1B), another downstream target of ATR (8). Treatment with LMS, BDS, and VAL-083 induced no MGMT-dependent differential in pChk1 levels (Fig. 2A). As described above, the TMZ-mediated activation of ATR is dependent on a functional MMR system, and we have corroborated this in our work: knockdown of MLH1 with siRNA prevents phosphorylation of Chk1 after TMZ treatment (Fig. S1C).
Figure 2.
TMZ specifically activates the ATR-Chk1 axis in MGMT-deficient cells. A, LN229 cells were treated for various times with TMZ [12.5 μM], LMS [12.5 μM], BDS [25 μM], or VAL-083 [1 μM] before western blotting analysis. B, (Left) LN229 MGMT+ cells were treated with O6BG [500 nM] for 24h prior to treatment with TMZ [20 or 100 μM] for 6h. (Middle) HeLa cells were treated with DMSO or O6BG [500 nM] for 24h prior to treatment with TMZ [20 or 100 μM] for 6h. (Right) U2OS cells were treated with O6BG [20 μM] for 24h prior to treatment with TMZ [5 or 20 μM] for 5h then allowed to recover in medium containing 20 μM O6BG for 48h prior to pelleting. C, Hap1 cells were treated with TMZ [3 μM] for the time shown prior to immunoblotting analysis. D, (Left) LN229 cells were co-treated with VX-970 at various concentrations and TMZ [100 μM] for 24h. (Right) LN229 MGMT- cells were co-treated with TMZ and varying concentrations of ATR inhibitor ATRIN-119 for 24h.
We next hypothesized that TMZ-mediated activation of the ATR-Chk1 axis would apply to other MGMT-deficient tumor models. O6-benzylguanine (O6BG) is a competitive inhibitor of MGMT that mimics the structure of O6MeG and thereby depletes MGMT levels (1). Upon pre-treatment of LN229 MGMT+ cells with O6BG, we created a state of MGMT deficiency that potentiated the induction of pChk1 after TMZ treatment to similar levels found in LN229 MGMT- cells (Fig. 2B). We repeated this result in HeLa and U2OS cell lines, which express MGMT endogenously (Fig. 2B). Using isogenic Hap1 (chronic myelogenous leukemia) cell lines differing only on expression of MGMT, we determined that TMZ activates ATR in an MGMT-dependent manner (Fig. 2C). TMZ also induced ATR activation in MGMT-deficient cell lines SW620 (colon cancer) and GBM22 (glioblastoma PDX) (Fig. S1D).
As TMZ specifically activates the ATR-Chk1 axis in MGMT-deficient tumor cells, we hypothesized that ATR inhibitors would abrogate the normal response to TMZ. We observed a dose-dependent attenuation of pChk1 signal in LN229 MGMT- cells after co-treatment with the ATR inhibitor VX-970 and TMZ (Fig. 2D). Similar results were obtained using the ATR inhibitor ATRIN-119 in LN229 MGMT- cells (Fig. 2D). In combination with TMZ, ATR inhibitors decrease pChk1 levels and increase γH2AX levels in a dose-dependent manner in PDX cell line GBM22 (Fig. S1D).
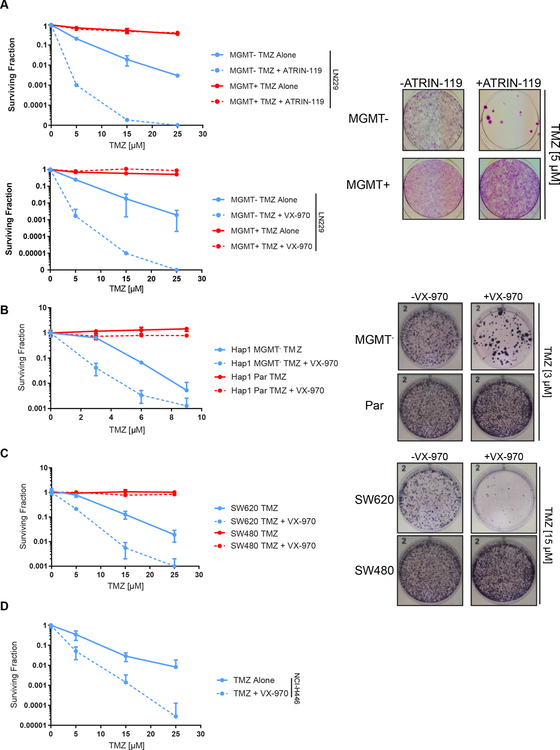
TMZ sensitizes MGMT-deficient tumors to ATR inhibitors
Next, we tested the cytotoxicity of ATR inhibitors and TMZ. First, we observed that combinations of TMZ with ATR inhibitors ATRIN-119, AZ20, or BAY-1895344 demonstrated strong synergy in LN229 MGMT- cells, but only mild synergy in LN229 MGMT+ cells (Fig. S2A). Unlike with ATR inhibitors, Wee1 inhibitor AZD1775 and Chk1/Chk2 inhibitor AZD7762 did not display marked specificity for synergy in MGMT-deficient cells with TMZ (Fig. S2B, S2C). TMZ and ATR inhibitors also demonstrated marked MGMT-dependent synergy in other cell lines, including GBM22, NCI-H446 (small cell lung cancer), SW620, and Hap1 MGMT- cells (Fig. S3A, S3B, S3C). This synergy also recapitulated in cell lines that express endogenous levels of MGMT such as SW480 (colon cancer), Hap1, and U2OS, albeit under conditions that deplete the levels of MGMT such as O6BG pre-treatment or with very high concentrations of TMZ (Fig. S3B, S3C, S3D).
TMZ synergized with ATR inhibitors in clonogenic survival assays, where we observed 100–1000 fold increased sensitivity in LN229 MGMT- cells treated with TMZ plus ATR inhibitors relative to TMZ treatment alone (Fig. 3A). As expected, no such sensitization was observed in LN229 MGMT+ cells. In other tumor types, the addition of ATR inhibitor decreased the surviving fraction of MGMT-deficient Hap1, SW620, and NCI-H446 cells 10–1000 fold relative to TMZ alone (Fig. 3B-D).
Figure 3.
TMZ sensitizes MGMT-deficient tumors to ATR inhibitors in vitro. A, LN229 cells were treated with TMZ with or without ATR inhibitors ATRIN-119 [15 nM] or VX-970 [50 nM] and allowed to form colonies for 14 days. B, Hap1 cells were treated with TMZ with or without VX-970 [50 nM] for 72h and allowed to form colonies for 12 days. C, SW480 and SW620 cells were treated with TMZ with or without VX-970 [50 nM] for 72h and allowed to form colonies for 12 days. D, NCI-H446 cells were treated with TMZ with or without VX-970 [50 nM] for 72h and allowed to form colonies for 16 days.
Because ATR inhibitors are known radiosensitizing agents (13), we tested the efficacy of ionizing radiation (IR), TMZ, and ATR inhibitor in combination in LN229 MGMT- cells. The addition of all three agents profoundly decreased the surviving fraction of MGMT- cells (Fig. S4), suggesting that this combination could be efficacious in tumor types amenable to IR such as glioblastoma.
Mechanistically, the inhibition of ATR in MGMT- cells decreased the G2/M arrest induced by TMZ and increased the level of γH2AX relative to cells treated with TMZ alone (Fig. S5, S6). The degrees of reduction in percentage of G2/M cells and induction in percentage of γH2AX-positive cells were dependent on the dose of ATR inhibitor and specific for MGMT- cells (Fig. S7). Combination treatment with TMZ and ATR inhibitor also increased the percentage of apoptotic cells compared to treatment with TMZ or ATR inhibitor alone (Fig. S8).
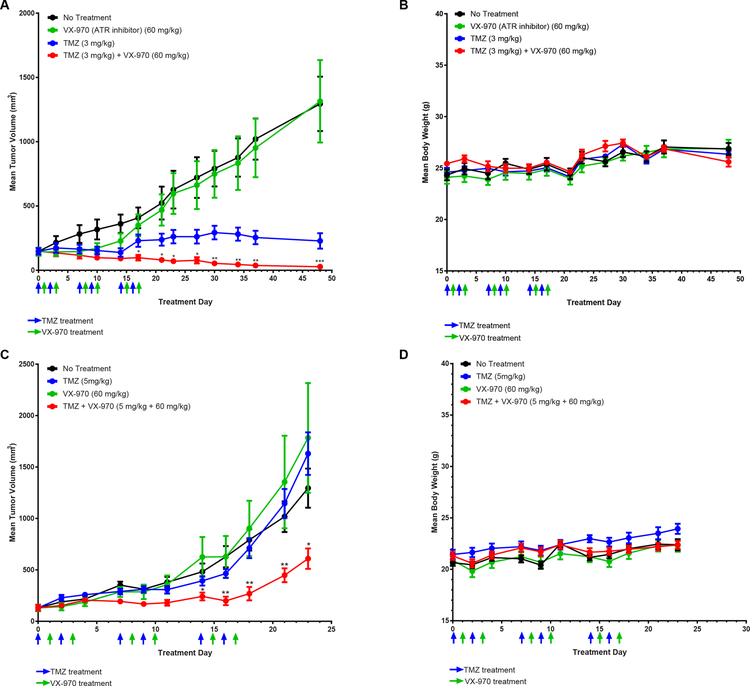
We next tested the combination of TMZ and VX-970 in 2 flank tumor models using LN229 MGMT- cells and GBM22 cells in vivo. We empirically derived a dose and treatment course of VX-970 (Fig. S9), which suggested that alternating VX-970 and TMZ treatments, every 24 hours for 4 days, would be the most optimal regimen. This combination treatment induced a significant tumor growth delay relative to TMZ alone or VX-970 alone in LN229 MGMT- flank tumors but did not cause a significant drop in body weight (Fig. 4A, 4B). In mice bearing PDX model GBM22 tumors, the combination treatment also significantly delayed tumor growth relative to TMZ or ATR inhibitor alone but did not result in significant drops in body weight (Fig. 4C, 4D).
Figure 4.
TMZ sensitizes MGMT-deficient tumors to ATR inhibitors in vivo. A, Mean tumor volume with SEM of LN229 MGMT- flank tumors treated with no treatment (n=8), TMZ alone (3 mg/kg) (n=7), VX-970 (60 mg/kg) (n = 7), or TMZ (3mg/kg) and VX-970 (60mg/kg) offset by 24h (n = 8). Mice were treated for 3 1-week cycles. Statistical significance between combination and TMZ alone is marked with an astrix. B, Mean body weight with SEM of mice during LN229 flank tumor experiment. C, Mean tumor volume with SEM of GBM22 flank tumors treated with no treatment (n=10), TMZ alone (5 mg/kg) (n=10), VX-970 (60 mg/kg) (n = 9), or TMZ (5mg/kg) and VX-970 (60mg/kg) offset by 24h (n = 10). Statistical significance between combination and TMZ alone is marked with an astrix. D, Mean body weight with SEM of mice during GBM22 flank tumor experiment.
Discussion
Here, we report a clinically actionable approach to maximally exploit the DNA repair defect induced by loss of MGMT expression. We found that TMZ activates the ATR-Chk1 axis in a manner that is highly dependent on MGMT status. Combined treatment with TMZ and ATR inhibitors was associated with exquisite synergistic tumor cell killing in both in vitro and in vivo models. Importantly, we demonstrated this synergy with multiple structurally unique ATR inhibitors, which suggests a class-effect related to the direct inhibition of ATR. Furthermore, this synergy recapitulated in multiple cell lines from a wide range of tumor types, suggesting that this interaction is applicable to the treatment of various cancers. In vivo, the combination treatment resulted in minimal drops in body weight, arguing that this treatment will be tolerable.
Recent work has elucidated that ATR plays a critical role in the induction of senescence after treatment of tumor cells with TMZ (14). This senescent phenotype is characterized by reduced MMR capacity and reduced DNA replication, 2 cellular processes necessary for the mechanism of cytotoxicity of TMZ (14). We have demonstrated that concomitant treatment of MGMT-deficient tumor cells with TMZ and ATR inhibitors reduces the percentage of cells arrested in G2/M and increases both DNA damage and the percentage of apoptotic cells relative to either drug alone. Taken together, these data and this recent work (14) suggest that abrogation of the ATR-mediated checkpoint allows TMZ-treated cells to continue DNA replication, amplifying DNA damage and eventually resulting in apoptosis. Experiments are ongoing in our laboratory to further characterize this fascinating interaction between a DNA damaging agent and a DNA repair inhibitor.
Emerging data suggests that a diverse range of both solid and liquid tumors harbor low levels of MGMT expression (4). In parallel, recent clinical studies have demonstrated the activity of TMZ in chemotherapy-refractory tumors such as metastatic small cell lung and colorectal cancers, which appears to correlate with MGMT status (15–17). These and other studies have supported the combination of TMZ with other DNA repair inhibitors, such as PARP inhibitors, with enrichment for patients with MGMT-deficient tumors (18,19). To our knowledge, this study represents the first report of the potential for combining ATR inhibitors with TMZ specifically in MGMT-deficient tumors. As there are now a number of ATR inhibitors being developed and tested in clinical trials (13), our work forms the basis of clinical trials testing their combination with TMZ, using MGMT as a key molecular biomarker for patient selection.
Supplementary Material
Statement of significance:
Monofunctional alkylating agents sensitize MGMT-deficient tumor cells to ATR inhibitors.
References
- 1.Verbeek B, Southgate TD, Gilham DE, Margison GP. O6-Methylguanine-DNA methyltransferase inactivation and chemotherapy. Br Med Bull 2008;85:17–33 doi 10.1093/bmb/ldm036. [DOI] [PubMed] [Google Scholar]
- 2.Srivenugopal KS, Yuan XH, Friedman HS, Ali-Osman F. Ubiquitination-dependent proteolysis of O6-methylguanine-DNA methyltransferase in human and murine tumor cells following inactivation with O6-benzylguanine or 1,3-bis(2-chloroethyl)-1-nitrosourea. Biochemistry 1996;35(4):1328–34 doi 10.1021/bi9518205. [DOI] [PubMed] [Google Scholar]
- 3.Hegi ME, Diserens A-C, Gorlia T, Hamou M-F, de Tribolet N, Weller M, et al. MGMT Gene Silencing and Benefit from Temozolomide in Glioblastoma. New England Journal of Medicine 2005;352(10):997–1003 doi 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]
- 4.Thomas A, Tanaka M, Trepel J, Reinhold WC, Rajapakse VN, Pommier Y. Temozolomide in the Era of Precision Medicine. Cancer Res 2017;77(4):823–6 doi 10.1158/0008-5472.CAN-16-2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Esteller M, Hamilton S, Burger P, Baylin S, Herman J. Inactivation of the DNA Repair Gene O6-Methylguanine-DNA Methyltransferase by Promoter Hypermethylation is a Commen Event in Primary Human Neoplasia. Cancer Research 1999;59:5. [PubMed] [Google Scholar]
- 6.Fu D, Calvo JA, Samson LD. Balancing repair and tolerance of DNA damage caused by alkylating agents. Nature Reviews Cancer 2012;12:104 doi 10.1038/nrc3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yoshioka K, Yoshioka Y, Hsieh P. ATR kinase activation mediated by MutSalpha and MutLalpha in response to cytotoxic O6-methylguanine adducts. Mol Cell 2006;22(4):501–10 doi 10.1016/j.molcel.2006.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saldivar JC, Cortez D, Cimprich KA. The essential kinase ATR: ensuring faithful duplication of a challenging genome. Nat Rev Mol Cell Biol 2017;18(10):622–36 doi 10.1038/nrm.2017.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krajewska M, Fehrmann RS, Schoonen PM, Labib S, de Vries EG, Franke L, et al. ATR inhibition preferentially targets homologous recombination-deficient tumor cells. Oncogene 2015;34(26):3474–81 doi 10.1038/onc.2014.276. [DOI] [PubMed] [Google Scholar]
- 10.Sulkowski Parker L, Corso Christopher D, Robinson Nathaniel D, Scanlon Susan E, Purshouse Karin R, Bai Hanwen, et al. 2-Hydroxyglutarate produced by neomorphic IDH mutations suppresses homologous recombination and induces PARP inhibitor sensitivity. Science Transl Med 2017;9:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Surovtseva YV, Jairam V, Salem AF, Sundaram RK, Bindra RS, Herzon SB. Characterization of Cardiac Glycoside Natural Products as Potent Inhibitors of DNA Double-Strand Break Repair by a Whole-Cell Double Immunofluorescence Assay. J Am Chem Soc 2016;138(11):3844–55 doi 10.1021/jacs.6b00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Avendano Carmen, Menendez JC. Medicinal Chemistry of Anticancer Drugs. 2015.
- 13.Karnitz LM, Zou L. Molecular Pathways: Targeting ATR in Cancer Therapy. Clin Cancer Res 2015;21(21):4780–5 doi 10.1158/1078-0432.CCR-15-0479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aasland D, Gotzinger L, Hauck L, Berte N, Meyer J, Effenberger M, et al. Temozolomide Induces Senescence and Repression of DNA Repair Pathways in Glioblastoma Cells via Activation of ATR-CHK1, p21, and NF-kappaB. Cancer Res 2019;79(1):99–113 doi 10.1158/0008-5472.CAN-18-1733. [DOI] [PubMed] [Google Scholar]
- 15.Zauderer MG, Drilon A, Kadota K, Huberman K, Sima CS, Bergagnini I, et al. Trial of a 5-day dosing regimen of temozolomide in patients with relapsed small cell lung cancers with assessment of methylguanine-DNA methyltransferase. Lung Cancer 2014;86(2):237–40 doi 10.1016/j.lungcan.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pietanza MC, Kadota K, Huberman K, Sima CS, Fiore JJ, Sumner DK, et al. Phase II trial of temozolomide in patients with relapsed sensitive or refractory small cell lung cancer, with assessment of methylguanine-DNA methyltransferase as a potential biomarker. Clin Cancer Res 2012;18(4):1138–45 doi 10.1158/1078-0432.CCR-11-2059. [DOI] [PubMed] [Google Scholar]
- 17.Pietrantonio F, de Braud F, Milione M, Maggi C, Iacovelli R, Dotti KF, et al. Dose-Dense Temozolomide in Patients with MGMT-Silenced Chemorefractory Colorectal Cancer. Target Oncol 2016;11(3):337–43 doi 10.1007/s11523-015-0397-2. [DOI] [PubMed] [Google Scholar]
- 18.Pietanza MC, Waqar SN, Krug LM, Dowlati A, Hann CL, Chiappori A, et al. Randomized, Double-Blind, Phase II Study of Temozolomide in Combination With Either Veliparib or Placebo in Patients With Relapsed-Sensitive or Refractory Small-Cell Lung Cancer. J Clin Oncol 2018;36(23):2386–94 doi 10.1200/JCO.2018.77.7672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gupta SK, Kizilbash SH, Carlson BL, Mladek AC, Boakye-Agyeman F, Bakken KK, et al. Delineation of MGMT Hypermethylation as a Biomarker for Veliparib-Mediated Temozolomide-Sensitizing Therapy of Glioblastoma. J Natl Cancer Inst 2016;108(5) doi 10.1093/jnci/djv369. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.