Abstract
BACKGROUND
Patients with a germline BRCA1 or BRCA2 mutation make up a small subgroup of those with metastatic pancreatic cancer. The poly(adenosine diphosphate–ribose) polymerase (PARP) inhibitor olaparib has had antitumor activity in this population.
METHODS
We conducted a randomized, double-blind, placebo-controlled, phase 3 trial to evaluate the efficacy of olaparib as maintenance therapy in patients who had a germline BRCA1 or BRCA2 mutation and metastatic pancreatic cancer and disease that had not progressed during first-line platinum-based chemotherapy. Patients were randomly assigned, in a 3:2 ratio, to receive maintenance olaparib tablets (300 mg twice daily) or placebo. The primary end point was progression-free survival, which was assessed by blinded independent central review.
RESULTS
Of the 3315 patients who underwent screening, 154 underwent randomization and were assigned to a trial intervention (92 to receive olaparib and 62 to receive placebo). The median progression-free survival was significantly longer in the olaparib group than in the placebo group (7.4 months vs. 3.8 months; hazard ratio for disease progression or death, 0.53; 95% confidence interval [CI], 0.35 to 0.82; P = 0.004). An interim analysis of overall survival, at a data maturity of 46%, showed no difference between the olaparib and placebo groups (median, 18.9 months vs. 18.1 months; hazard ratio for death, 0.91; 95% CI, 0.56 to 1.46; P = 0.68). There was no significant between-group difference in health-related quality of life, as indicated by the overall change from baseline in the global quality-of-life score (on a 100-point scale, with higher scores indicating better quality of life) based on the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire (between-group difference, −2.47 points; 95% CI, −7.27 to 2.33). The incidence of grade 3 or higher adverse events was 40% in the olaparib group and 23% in the placebo group (between-group difference, 16 percentage points; 95% CI, −0.02 to 31); 5% and 2% of the patients, respectively, discontinued the trial intervention because of an adverse event.
CONCLUSIONS
Among patients with a germline BRCA mutation and metastatic pancreatic cancer, progression-free survival was longer with maintenance olaparib than with placebo. (Funded by AstraZeneca and others; POLO ClinicalTrials.gov number, .)
METASTATIC PANCREATIC CANCER IS particularly refractory to treatment.1 Current standard-of-care first-line treatments are associated with a median progression-free survival of approximately 6 months, and fewer than 10% of patients are alive 5 years after the initial diagnosis.1–3
Loss-of-function mutations in BRCA1, BRCA2, or both (BRCA) genes are linked to an increased risk of ovarian and breast cancers4; such mutations are also associated with an increased risk of pancreatic cancer, and 4 to 7% of patients with pancreatic cancer have a germline BRCA mutation.5–8 BRCA genes code for proteins that are involved in homologous recombination repair of DNA double-strand breaks.9 Cells with a deficiency in homologous recombination repair, such as those with a BRCA mutation, are sensitive to poly(adenosine diphosphate–ribose) polymerase (PARP) inhibition through multiple mechanisms, including trapping of PARP on DNA at sites of single-strand breaks. These processes prevent repair of single-strand breaks and lead to generation of double-strand breaks in replicating cells, which cannot be repaired accurately in tumors with defects in homologous recombination repair. Thus, PARP inhibitors cause an accumulation of DNA damage and tumor-cell death.10 The PARP inhibitor olaparib has been shown to have clinical efficacy in patients with a germline BRCA mutation and ovarian or breast cancer,11,12 and in a phase 2 trial, olaparib had antitumor activity in heavily pretreated patients with a germline BRCA mutation and metastatic pancreatic cancer.13
No targeted therapies have been approved specifically for patients with a germline BRCA mutation and pancreatic cancer, although clinical evidence suggests that these patients may have improved outcomes when treated with platinum-based chemotherapies.14,15 Maintenance treatments that aim to extend progression-free and overall survival without compromising health-related quality of life are used in the management of many cancers and provide an opportunity to prolong responses.16 In ovarian cancer, maintenance olaparib has provided a clinically meaningful benefit with regard to progression-free survival among patients with a BRCA mutation.12,17 Maintenance treatment is a new concept in pancreatic cancer, although early studies of maintenance sunitinib and fluorouracil have shown promising results.18,19
We conducted the POLO (Pancreas Cancer Olaparib Ongoing) trial to evaluate the efficacy of maintenance therapy with olaparib in patients with a germline BRCA mutation and metastatic pancreatic adenocarcinoma that had not progressed during first-line platinum-based chemotherapy.
METHODS
PATIENTS
Patients were eligible if they were 18 years of age or older and had histologically or cytologically confirmed pancreatic adenocarcinoma and a documented deleterious or suspected deleterious germline mutation in BRCA1 or BRCA2. Eligibility was based on detection of a germline BRCA mutation by central testing with the use of the BRAC-Analysis CDx test (Myriad Genetic Laboratories) or by local testing with subsequent confirmation with the use of the BRACAnalysis CDx test after randomization.
Patients had received at least 16 weeks of continuous first-line platinum-based chemotherapy for metastatic pancreatic cancer; the duration was unlimited as long as no evidence of disease progression was noted by the investigator at randomization. The platinum component of chemotherapy could have been discontinued because of toxic effects at any time after 16 weeks. Toxic effects from previous treatment for cancer must have resolved to grade 1 (according to the National Cancer Institute Common Terminology Criteria for Adverse Events [NCI CTCAE], version 4.0) before randomization, except for alopecia, grade 3 peripheral neuropathy, and grade 2 anemia (if the hemoglobin level was ≥9 g per deciliter, with no blood transfusions in the preceding 28 days).
Eligible patients had normal organ and acceptable bone marrow function. Full eligibility criteria are provided in the trial protocol, available with the full text of this article at NEJM.org. All the patients provided written informed consent.
TRIAL DESIGN AND INTERVENTIONS
The randomized, double-blind, placebo-controlled, phase 3 POLO trial was conducted at 119 sites in 12 countries. With the use of a central interactive voice or Web response system that incorporated a block randomization scheme, patients were randomly assigned, in a 3:2 ratio, to receive maintenance olaparib tablets (300 mg twice daily) or matching placebo. No stratification factors were used. The maintenance trial intervention was initiated 4 to 8 weeks after the last dose of first-line chemotherapy had been administered. The intervention was continued until the occurrence of objective radiologic disease progression (determined by investigator assessment according to the modified Response Evaluation Criteria in Solid Tumors [RECIST], version 1.1) (see the Methods section in the Supplementary Appendix, available at NEJM.org) or unacceptable toxic effects.20 Crossover to olaparib was not permitted during this trial. After discontinuation of the trial intervention, subsequent therapies were administered at the investigators’ discretion.
END POINTS AND ASSESSMENTS
The primary end point was progression-free survival, which was defined as the time from randomization until objective radiologic disease progression, as assessed by blinded independent central review according to modified RECIST, version 1.1, or death from any cause. Prespecified sensitivity analyses of progression-free survival were performed (see the Methods section in the Supplementary Appendix).
Secondary end points included the following: overall survival; second progression-free survival, defined as the time from randomization to second progression (investigator-assessed objective radiologic or symptomatic progression) or death; the objective response rate, assessed by blinded independent central review according to modified RECIST, version 1.1, in patients with data that could be evaluated; and the change in scores for global health-related quality of life (measured as the adjusted mean change from baseline).
Computed tomography or magnetic resonance imaging of the chest, abdomen, and pelvis were performed at baseline, every 8 weeks for 40 weeks, and then every 12 weeks until disease progression. Survival, second progression, and subsequent treatments for cancer were assessed every 8 weeks after progression. Health-related quality of life was assessed with the use of the 30-item European Organization for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ-C30), which was completed by the patients at baseline, every 4 weeks until disease progression, at discontinuation of the trial intervention, and 30 days after the last dose of the trial agent. Global scores on the EORTC QLQ-C30 range from 0 to 100, with higher scores indicating better quality of life; an increase or decrease of at least 10 points was considered to be a clinically meaningful change.21 Adverse events were graded according to the NCI CTCAE, version 4.0.
TRIAL OVERSIGHT
This trial was performed in accordance with the principles of the Declaration of Helsinki, Good Clinical Practice guidelines, and the AstraZeneca policy of bioethics.22 The trial was designed by the first and last authors in collaboration with AstraZeneca. AstraZeneca was responsible for overseeing the collection, analysis, and interpretation of the data. All the authors had full access to the data. Olaparib is being codeveloped by AstraZeneca and Merck Sharp & Dohme (MSD, a subsidiary of Merck), and MSD provided input regarding the interpretation of the data. The manuscript was written with medical writing assistance funded by AstraZeneca and MSD, with critical review and input by the authors. The authors attest to the accuracy and completeness of the data and the fidelity of the trial to the protocol.
STATISTICAL ANALYSIS
We determined that with a sample size of 145 patients, 87 cases of disease progression or death would provide the trial with 80% power, at a one-sided significance level of 2.5% (one-sided because we did not think that the superiority of placebo was a possible outcome), to show a significant difference in progression-free survival between the olaparib group and the placebo group, assuming a hazard ratio for disease progression or death of 0.54. An interim futility analysis of progression-free survival was conducted after 44 events had occurred. A prespecified interim analysis of overall survival was performed at the time of the primary analysis of progression-free survival.
Data on efficacy were analyzed in the intention-to-treat population (all patients who underwent randomization). Data on safety were summarized in the safety population (patients who received ≥1 dose of a trial agent), and data on health-related quality of life were analyzed in all patients with a form on baseline health-related quality of life that could be evaluated (see the Methods section in the Supplementary Appendix).
The primary analysis of progression-free survival was performed with a log-rank test, with calculation of a hazard ratio, an accompanying 95% confidence interval, and a P value. Data on patients who had not had disease progression and who had not died at the time of the primary analysis, or who had progression or had died after two or more missed visits, were censored at the date of the last tumor assessment for which data could be evaluated. Subgroup and multivariate analyses of progression-free survival were conducted with the use of a Cox proportional-hazards model. All time-to-event curves were generated with the use of the Kaplan–Meier method and were used to calculate medians for each trial group.23 Overall survival and second progression-free survival (defined as the time from randomization to second progression occurring while the patient was receiving salvage therapy) were analyzed with the same method as that used for progression-free survival.
The adjusted mean change from baseline in the EORTC QLQ-C30 global health-related quality-of-life score was analyzed with a mixed model for repeated measures (see the Methods section in the Supplementary Appendix). A change of 10 points in either direction was considered to be a clinically significant difference.
A multiple-testing procedure was used to control for the type 1 error rate, with a test for progression-free survival to be performed first, followed by a planned interim test for overall survival if there was a significant difference in progression-free survival between the trial groups. The final overall survival analysis is planned after 106 deaths. All reported P values are two-sided and coincide with the reported two-sided confidence intervals. The statistical analysis plan is available with the protocol at NEJM.org.
RESULTS
PATIENTS
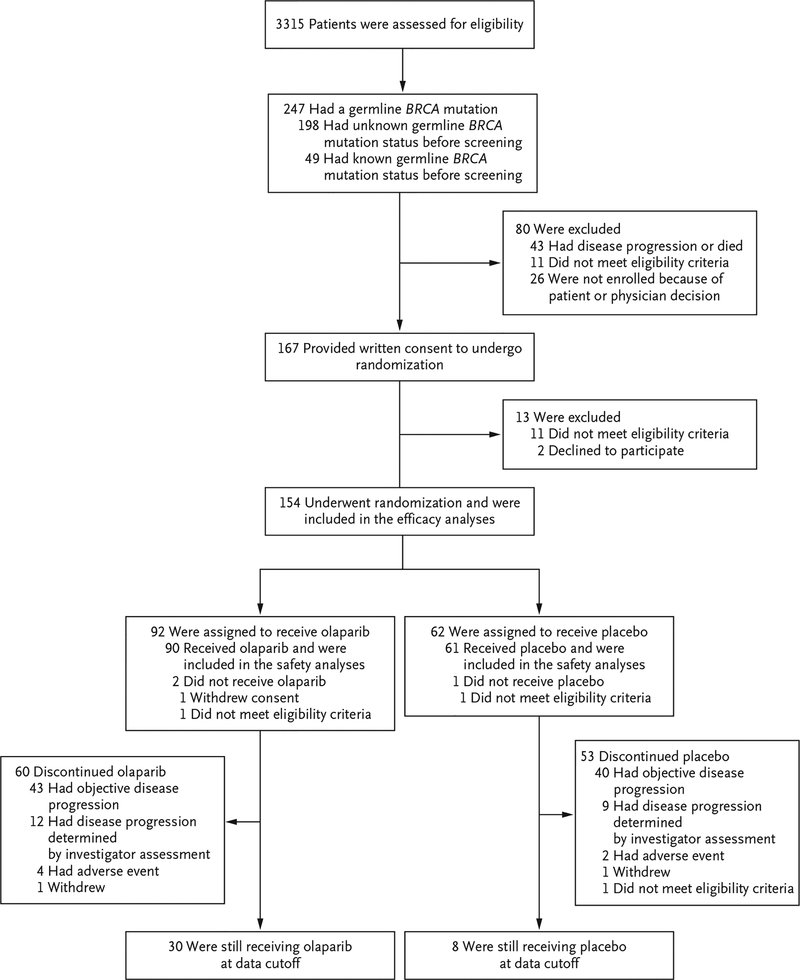
A total of 3315 patients were screened for trial entry; 247 (7.5%) of these patients had a germ-line BRCA mutation. Between January 2015 and January 2019, a total of 154 patients underwent randomization (Table S1 in the Supplementary Appendix), and 90 of 92 patients who were assigned to olaparib and 61 of 62 patients who were assigned to placebo received the trial intervention (Fig. 1). At the time of data cutoff for this analysis (January 15, 2019), 30 patients were still receiving olaparib and 8 were still receiving placebo. The median duration of follow-up for disease progression in patients with censored data was 9.1 months (range, 0 to 39.6) in the olaparib group and 3.8 months (range, 0 to 29.8) in the placebo group. The baseline characteristics of the patients are listed in Table 1, and in Table S2 in the Supplementary Appendix.
Figure 1. Screening, Enrollment, Randomization, and Intervention.
One patient in the placebo group incorrectly received olaparib and was included in the olaparib group for the safety analyses. After initiation of the trial intervention, another patient in the placebo group was found not to have met the eligibility criteria, and the intervention was discontinued on day 3 at the decision of the trial sponsor. In addition to this patient, one patient in each trial group was found, after randomization, not to have met the eligibility criteria. Both were included in the intention-to-treat efficacy analyses. Neither patient received a trial intervention, and so neither was included in the safety analyses.
Table 1.
Baseline Characteristics of the Patients.*
| Olaparib | Placebo | |
|---|---|---|
| Characteristic | (N = 92) | (N = 62) |
| Age at randomization — yr | ||
| Median | 57 | 57 |
| Range | 37–84 | 36–75 |
| Age ≥ 65 yr at randomization — no. (%) | 28 (30) | 13 (21) |
| Male sex — no. (%) | 53 (58) | 31 (50) |
| ECOG performance status — no. (%) | ||
| 0, normal activity | 65 (71) | 38 (61) |
| 1, restricted activity | 25 (27) | 23 (37) |
| Missing data | 2 (2) | 1 (2) |
| Germline BRCA mutation — no. (%)† | ||
| BRCA1 | 29 (32) | 16 (26) |
| BRCA2 | 62 (67) | 46 (74) |
| Both BRCA1 and BRCA2 | 1 (1) | 0 |
| Time from diagnosis to randomization — mo | ||
| Median | 6.9 | 7.0 |
| Range | 3.6–38.4 | 4.1–30.2 |
| First-line platinum-based chemotherapy — no. (%)‡ | ||
| FOLFIRINOX variants | 79 (86) | 50 (81) |
| Gemcitabine-cisplatin | 2 (2) | 3 (5) |
| Other platinum-based treatments | 10 (11) | 8 (13) |
| Missing data | 1 (1) | 1 (2) |
| Duration of first-line chemotherapy before randomization | ||
| Median — mo | 5.0 | 5.1 |
| Range — mo | 2.5–35.2 | 3.4–20.4 |
| 16 wk-6 mo — no. (%) | 61 (66) | 40 (65) |
| >6 mo — no. (%) | 30 (33) | 21 (34) |
| Missing data — no. (%) | 1 (1) | 1 (2) |
| Best response with first-line chemotherapy — no. (%) | ||
| Complete or partial response | 46 (50) | 30 (48) |
| Stable disease | 45 (49) | 31 (50) |
| Missing data | 1 (1) | 1 (2) |
Percentages may not total 100 because of rounding. ECOG denotes Eastern Cooperative Oncology Group, and FOLFIRINOX fluorouracil, leucovorin, irinotecan, and oxaliplatin.
Four patients who underwent randomization on the basis of results of a local germline BRCA mutation test did not undergo confirmatory BRACAnalysis CDx testing as part of the trial (see the Results section in the Supplementary Appendix). BRACAnalysis CDx results from the remaining 150 patients are provided in Table S3 in the Supplementary Appendix.
Further details regarding first-line platinum-based chemotherapy regimens received before randomization are provided in Table S4 in the Supplementary Appendix.
EFFICACY
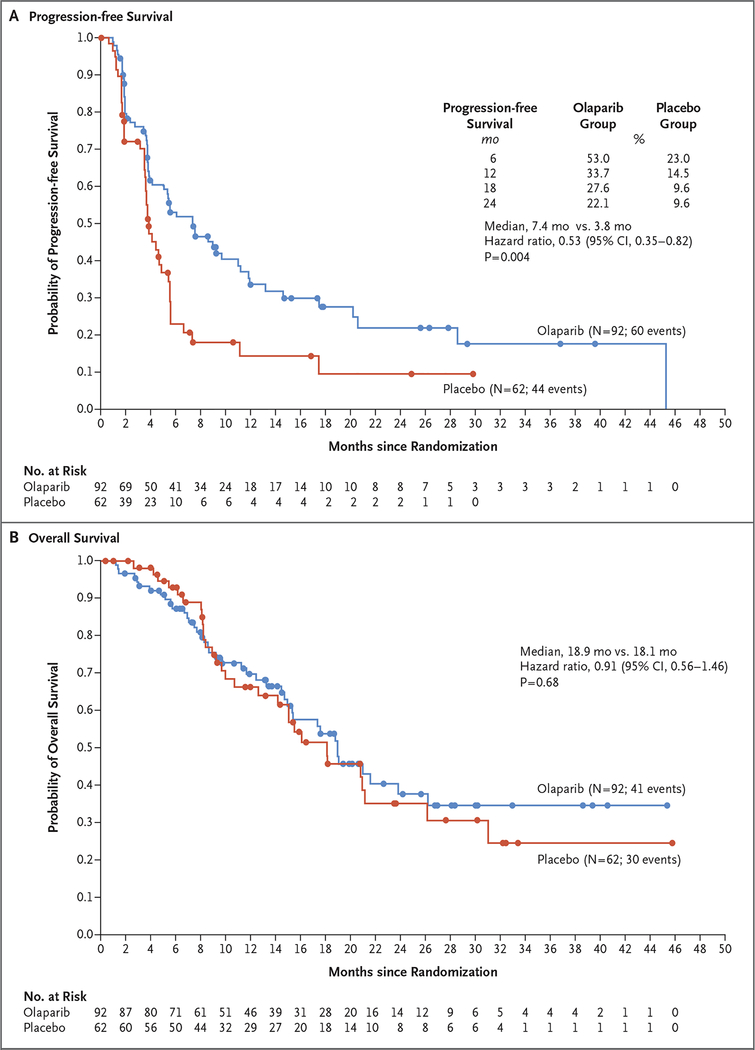
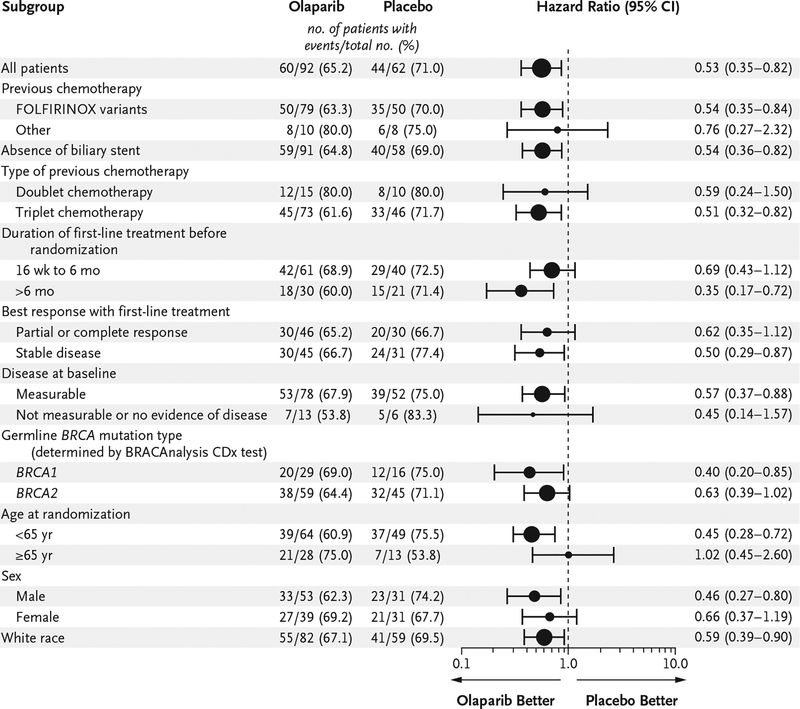
The analysis of the primary end point was performed after 104 of 154 patients had had disease progression (assessed by blinded independent central review) or had died (data maturity, 68%). The median progression-free survival was significantly longer in the olaparib group than in the placebo group (7.4 months vs. 3.8 months; hazard ratio for disease progression or death, 0.53; 95% confidence interval [CI], 0.35 to 0.82; P = 0.004), and from 6 months onward, the percentage of patients who were alive and free from disease progression was more than twice as high in the olaparib group as in the placebo group (Fig. 2A). The results of a prespecified sensitivity analysis of investigator-assessed progression-free survival, performed to evaluate for ascertainment bias, were consistent with those of the primary analysis (hazard ratio for disease progression or death, 0.51; 95% CI, 0.34 to 0.78) (Table S5 in the Supplementary Appendix). Prespecified subgroup analyses of progression-free survival showed no evidence of differences with respect to the benefit of olaparib, including between the subgroup of patients who had had a response to previous platinum-based chemotherapy and those who had stable disease (Fig. 3). A multivariate Cox regression analysis showed that the treatment effect was not affected by imbalances in baseline factors between the trial groups (Table S6 in the Supplementary Appendix).
Figure 2. Kaplan–Meier Estimates of Progression-free Survival and Overall Survival.
Panel A shows Kaplan–Meier estimates of progression‑free survival (based on blinded independent central review), and Panel B shows Kaplan–Meier estimates of overall survival in the olaparib group and the placebo group.
Figure 3. Subgroup Analysis of Progression-free Survival.
Subgroups in which fewer than five progression‑free survival events occurred per group were not included in the analysis. The sizes of the circles are proportional to the number of events. The dashed line indicates the point of no effect (hazard ratio = 1). The prespecified gemcitabine–cisplatin subgroup included two patients in the olaparib group and three patients in the placebo group; this subgroup did not meet the threshold for inclusion in the subgroup analysis. Patients who received gemcitabine–cisplatin were not included in the “other” subcategory of the “previous chemotherapy” subgroup but are included in the “doublet chemotherapy” subgroup. Race was determined from patient records.
At the primary data cutoff date, 41 patients in the olaparib group and 30 patients in the placebo group had died. A planned interim analysis of overall survival that took place at a data maturity of 46% showed no evidence of a difference in overall survival between the groups (median, 18.9 months in the olaparib group and 18.1 months in the placebo group; hazard ratio for death, 0.91; 95% CI, 0.56 to 1.46; P = 0.68) (Fig. 2B). A primary analysis of overall survival was planned after 106 events (data maturity, 69%). One patient in the olaparib group and 9 patients in the placebo group received treatment with a PARP inhibitor after discontinuation of the trial intervention (Table S7 in the Supplementary Appendix).
At a data maturity of 46%, an analysis of second progression-free survival showed a median time from randomization to second disease progression or death of 13.2 months in the olaparib group and 9.2 months in the placebo group (hazard ratio, 0.76; 95% CI, 0.46 to 1.23) (Fig. S1 in the Supplementary Appendix).
Overall, 18 patients in the olaparib group (20%) and 6 patients in the placebo group (10%) had a response. Among patients with measurable disease at baseline, the response rate as assessed by blinded independent central review was 23% in the olaparib group (18 of 78 patients) and 12% in the placebo group (6 of 52 patients) (odds ratio, 2.30; 95% CI, 0.89 to 6.76). Two patients, both in the olaparib group, had a complete response; both complete responses were ongoing at the time of data cutoff. The median duration of response was 24.9 months (95% CI, 14.8 to could not be calculated) and 3.7 months (95% CI, 2.1 to could not be calculated) and the median time to response was 5.4 months and 3.6 months in the olaparib and placebo groups, respectively.
SAFETY
In the 151 patients who received a trial agent, the total median duration of treatment was 6.0 months (range, 0.8 to 45.3) in the olaparib group and 3.7 months (range, 0.1 to 30.1) in the placebo group (related data on dose intensity are provided in the Results section in the Supplementary Appendix). Table 2 shows the most common adverse events in the trial groups. Serious adverse events occurred in 24% of the patients who received olaparib and in 15% of the patients who received placebo.
Table 2.
Summary of Adverse Events.*
| Variable | Olaparib (N = 91) | Placebo (N = 60) | Between-Group Difference (95% CI) | |||
|---|---|---|---|---|---|---|
| Any Grade | Grade ≥ 3 | Any Grade | Grade ≥ 3 | Any Grade | Grade ≥ 3 | |
| number (percent) | percentage points | |||||
| Adverse event | ||||||
| Any | 87 (96) | 36 (40) | 56 (93) | 14 (23) | 2 (−5 to 12) | 16 (−0.02 to 31) |
| Fatigue or asthenia | 55 (60) | 5 (5) | 21 (35) | 1 (2) | 25 (7 to 41) | 4 (−4 to 11) |
| Nausea | 41 (45) | 0 | 14 (23) | 1 (2) | 22 (4 to 36) | −2 (−9 to 3) |
| Anemia† | 25 (27) | 10 (11) | 10 (17) | 2 (3) | 11 (−3 to 24) | 8 (−2 to 17) |
| Abdominal pain | 26 (29) | 2 (2) | 15 (25) | 1 (2) | 4 (−12 to 18) | 1 (−8 to 6) |
| Diarrhea | 26 (29) | 0 | 9 (15) | 0 | 14 (−1 to 26) | NC |
| Decreased appetite | 23 (25) | 3 (3) | 4 (7) | 0 | 19 (5 to 30) | 3 (−3 to 9) |
| Constipation | 21 (23) | 0 | 6 (10) | 0 | 13 (−0.02 to 25) | NC |
| Vomiting | 18 (20) | 1 (1) | 9 (15) | 1 (2) | 5 (−9 to 17) | −1 (−8 to 5) |
| Back pain | 17 (19) | 0 | 10 (17) | 1 (2) | 2 (−12 to 14) | −2 (−9 to 3) |
| Arthralgia | 14 (15) | 1 (1) | 6 (10) | 0 | 5 (−7 to 16) | 1 (−5 to 6) |
| Interruption of intervention owing to adverse event | 32 (35) | NA | 3 (5) | NA | 30 (17 to 42) | NA |
| Dose reduction owing to adverse event | 15 (16) | NA | 2 (3) | NA | 13 (2 to 23) | NA |
| Discontinuation of intervention owing to adverse event | 5 (5) | NA | 1 (2) | NA | 4 (−4 to 11) | NA |
The table includes adverse events of any grade that occurred in at least 15% of the patients in the safety population of either trial group during the trial intervention or up to 30 days after discontinuation of the trial intervention. Adverse events were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0. NA denotes not applicable, and NC not calculated.
The anemia category includes anemia, decreased hemoglobin level, decreased red-cell count, decreased hematocrit, erythropenia, macrocytic anemia, normochromic anemia, normochromic normocytic anemia, and normocytic anemia.
No adverse events that occurred during the trial intervention resulted in death. One stent-related duodenal perforation in a patient who received olaparib occurred during the 30-day follow-up period after discontinuation of therapy, and that patient subsequently died (see the Results section in the Supplementary Appendix).
Adverse events led to discontinuation of the trial agent in 5% of the patients who received olaparib and in 2% of the patients who received placebo (Table S8 in the Supplementary Appendix); adverse events were usually managed by dose interruption or reduction. No cases of myelodysplastic syndrome or acute myeloid leukemia were reported in either group. In two patients who received placebo, new primary cancers developed more than 30 days after the end of the trial intervention (one patient had rectal cancer, and the other had a possible ovarian cancer, which resulted in death). One diagnosis of grade 1 pneumonitis in a patient in the olaparib group was not considered by the investigators to be causally related to the trial intervention (see the Results section in the Supplementary Appendix).
PATIENT-REPORTED OUTCOMES
No clinically meaningful change from baseline was noted in the EORTC QLQ-C30 global health-related quality-of-life score in either group, and there was no significant difference in the overall change from baseline between the groups. The adjusted mean (±SE) change from baseline across all time points was −1.20±1.42 (95% CI, −4.01 to 1.62) in 84 patients in the olaparib group and 1.27±1.95 (95% CI, −2.58 to 5.12) in 54 patients in the placebo group. The corresponding estimated between-group difference of −2.47 points (95% CI, −7.27 to 2.33) on a 100-point scale was not clinically meaningful.
DISCUSSION
The primary analysis of the trial showed that patients who had a germline BRCA mutation and metastatic pancreatic cancer that had not progressed during first-line platinum-based chemotherapy had significantly longer progression-free survival with maintenance olaparib than with placebo. Separation between the Kaplan–Meier curves for the two trial groups can be seen from approximately 5 months; this result provides support for the observed 47% reduction in the risk of disease progression or death; at 2 years, 22.1% of the patients in the olaparib group versus 9.6% of patients in the placebo group did not have disease progression. Patients received platinum-based chemotherapy immediately before maintenance olaparib; therefore, the combined first-line platinum-based chemotherapy and maintenance olaparib strategy resulted in a progression-free survival of more than 1 year among patients with a germline BRCA mutation who had disease that had not progressed while they were receiving previous platinum-based chemo-therapy. However, our data do not include patients with disease that progressed during the first 4 months of platinum-based treatment or who may have continued to receive chemotherapy for an extended period.2,3
The POLO trial was a global trial, and when the trial was designed, 6-month treatment with FOLFIRINOX (fluorouracil, leucovorin, irinotecan, and oxaliplatin) was a standard of care.2 Eligible patients could undergo randomization after at least 16 weeks of chemotherapy; no maximum length of first-line chemotherapy was mandated. In some clinical practices, patients may receive platinum-based chemotherapy for more than 6 months; however, peripheral neuropathy and other cumulative treatment-related side effects limit an extended duration, making placebo a suitable comparator to maintenance treatment after first-line chemotherapy.24
Overall survival data from an interim analysis at a data maturity of 46% (the final analysis was planned at a data maturity of 69%) show no significant difference in survival between the trial groups. Overall survival may be confounded by subsequent therapies, including that of nine patients in the placebo group (15%) who went on to receive a PARP inhibitor after disease progression during the trial intervention (Table S7 in the Supplementary Appendix).13 The POLO trial was designed as a maintenance trial, and imaging analyses were performed regularly; therefore, patients in the placebo group with disease that progressed during the trial intervention went on to receive additional DNA-damaging agents, which are known to have some activity in this patient population.15
An analysis of the time to second disease progression suggested a trend toward benefit from maintenance olaparib through the next treatment. Although there is no precedent in pancreatic cancer, this end point has been assessed in studies of other tumor types and has been interpreted as a possible indicator of treatment benefit beyond disease progression.12
The adverse-effect profile of maintenance olaparib was similar to that observed in other tumor types.11,12,17 Health-related quality of life was preserved with olaparib, and there was no significant difference between the groups.
Of 3315 patients screened for entry in the POLO trial, 7.5% had a germline BRCA mutation. A substantial percentage of these potentially eligible patients had disease progression during platinum-based chemotherapy before trial entry, consistent with observations from previous FOLFIRINOX trials.2 In this trial, dual selection criteria were used to identify patients who would be likely to benefit from PARP inhibition. Patients had to have a germline BRCA mutation and no disease progression during at least 16 weeks of platinum-based chemotherapy. In an unselected population with metastatic pancreatic cancer, it is expected that approximately 30% of patients will have disease progression during their first 4 months of treatment with FOLFIRINOX.2 A total of 43 of the 198 patients (21.7%) with a germline BRCA mutation who were screened for trial entry before first-line treatment had disease progression during platinum-based chemotherapy before randomization.
In conclusion, the POLO trial showed that maintenance olaparib provided a significant progression-free survival benefit to patients with a germline BRCA mutation and metastatic pancreatic cancer that had not progressed during platinum-based chemotherapy.
Supplementary Material
POSTING PRESENTATIONS FROM MEDICAL MEETINGS ONLINE.
Online posting of an audio or video recording of an oral presentation at a medical meeting, with selected slides from the presentation, is not considered prior publication. Authors should feel free to call or send email to the Journal’s Editorial Offices if there are any questions about this policy.
Acknowledgments
Supported by AstraZeneca (as part of an alliance between AstraZeneca and Merck Sharp & Dohme, a subsidiary of Merck) and by a grant (P30–17 CA008748) from the National Institutes of Health National Cancer Institute Cancer Center.
We thank the patients who participated in this trial and their families, our co-investigators, and Elin Pyke, M.Chem., of Mud-skipper, who provided medical writing assistance with an earlier version of the manuscript.
Footnotes
References
- 1.Rawla P, Sunkara T, Gaduputi V. Epidemiology of pancreatic cancer: global trends, etiology and risk factors. World J Oncol 2019; 10: 10–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for meta-static pancreatic cancer. N Engl J Med 2011; 364: 1817–25. [DOI] [PubMed] [Google Scholar]
- 3.Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 2013; 369: 1691–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Welcsh PL, King MC. BRCA1 and BRCA2 and the genetics of breast and ovarian cancer. Hum Mol Genet 2001; 10: 705–13. [DOI] [PubMed] [Google Scholar]
- 5.Ghiorzo P Genetic predisposition to pancreatic cancer. World J Gastroenterol 2014; 20: 10778–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holter S, Borgida A, Dodd A, et al. Germline BRCA mutations in a large clinic-based cohort of patients with pancreatic adenocarcinoma. J Clin Oncol 2015; 33: 3124–9. [DOI] [PubMed] [Google Scholar]
- 7.Friedenson B BRCA1 and BRCA2 pathways and the risk of cancers other than breast or ovarian. MedGenMed 2005; 7: 60. [PMC free article] [PubMed] [Google Scholar]
- 8.Golan T, Kindler HL, Park JO, et al. Geographic and ethnic heterogeneity in the BRCA1/2 pre-screening population for the randomized phase III POLO study of olaparib maintenance in metastatic pancreatic cancer (mPC). J Clin Oncol 2018; 36: Suppl: 4115 abstract. [Google Scholar]
- 9.Walsh CS. Two decades beyond BRCA1/2: homologous recombination, hereditary cancer risk and a target for ovarian cancer therapy. Gynecol Oncol 2015; 137: 343–50. [DOI] [PubMed] [Google Scholar]
- 10.O’Connor MJ. Targeting the DNA damage response in cancer. Mol Cell 2015; 60: 547–60. [DOI] [PubMed] [Google Scholar]
- 11.Robson M, Im SA, Senkus E, et al. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N Engl J Med 2017; 377: 523–33. [DOI] [PubMed] [Google Scholar]
- 12.Moore K, Colombo N, Scambia G, et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med 2018; 379: 2495–505. [DOI] [PubMed] [Google Scholar]
- 13.Kaufman B, Shapira-Frommer R, Schmutzler RK, et al. Olaparib monotherapy in patients with advanced cancer and a germline BRCA1/2 mutation. J Clin Oncol 2015; 33: 244–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Waddell N, Pajic M, Patch AM, et al. Whole genomes redefine the mutational landscape of pancreatic cancer. Nature 2015; 518: 495–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Golan T, Kanji ZS, Epelbaum R, et al. Overall survival and clinical characteristics of pancreatic cancer in BRCA mutation carriers. Br J Cancer 2014; 111: 1132–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khalique S, Hook JM, Ledermann JA. Maintenance therapy in ovarian cancer. Curr Opin Oncol 2014; 26: 521–8. [DOI] [PubMed] [Google Scholar]
- 17.Pujade-Lauraine E, Ledermann JA, Selle F, et al. Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol 2017; 18: 1274–84. [DOI] [PubMed] [Google Scholar]
- 18.Reni M, Cereda S, Milella M, et al. Maintenance sunitinib or observation in metastatic pancreatic adenocarcinoma: a phase II randomised trial. Eur J Cancer 2013; 49: 3609–15. [DOI] [PubMed] [Google Scholar]
- 19.Dahan L, Phelip JM, Le Malicot K, et al. FOLFIRINOX until progression, FOLFIRINOX with maintenance treatment, or sequential treatment with gemcitabine and FOLFIRI.3 for first-line treatment of metastatic pancreatic cancer: a randomized phase II trial (PRODIGE 35-PANOPTIMOX). J Clin Oncol 2018; 36: Suppl: 4000 abstract. [Google Scholar]
- 20.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45: 228–47. [DOI] [PubMed] [Google Scholar]
- 21.Osoba D, Rodrigues G, Myles J, Zee B, Pater J. Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol 1998; 16: 139–44. [DOI] [PubMed] [Google Scholar]
- 22.AstraZeneca. AstraZeneca global policy: bioethics. 2016. (https://www.astrazeneca.com/content/dam/az/PDF/2016/Bioethics_policy.pdf). [Google Scholar]
- 23.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958; 53: 457–81. [Google Scholar]
- 24.Tahara J, Shimizu K, Otsuka N, Akao J, Takayama Y, Tokushige K. Gemcitabine plus nab-paclitaxel vs. FOLFIRINOX for patients with advanced pancreatic cancer. Cancer Chemother Pharmacol 2018; 82: 245–50. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.