Abstract
OBJECTIVES:
To investigate whether low-dose aspirin (<300 mg/d) can influence the onset of cognitive impairment or dementia in observational studies and improve cognitive test scores in randomized controlled trials (RCTs) in participants without dementia.
DESIGN:
Systematic review and meta-analysis.
SETTING:
Observational and interventional studies.
PARTICIPANTS:
Individuals with no dementia or cognitive impairment initially.
MEASUREMENTS:
Odds ratios (ORs) and 95% confidence intervals (CIs), adjusted for the maximum number of covariates from each study, were used to summarize data on the incidence of dementia and cognitive impairment in observational studies. Standardized mean differences (SMDs) were used for cognitive test scores in RCTs.
RESULTS:
Of 2,341 potentially eligible articles, eight studies were included and provided data for 36,196 participants without dementia or cognitive impairment at baseline (mean age 66, 63% female). After adjusting for a median of three potential confounders over a median follow-up period of 6 years, chronic use of low-dose aspirin was not associated with onset of dementia or cognitive impairment (5 studies, N = 26,159; OR = 0.82, 95% CI = 0.55–1.22, P = .33, I2 = 67%). In three RCTs (N = 10,037; median follow-up 5 years), the use of low-dose aspirin was not associated with significantly better global cognition (SMD=0.005, 95% CI=−0.04–0.05, P = .84, I2 = 0%) in individuals without dementia. Adherence was lower in participants taking aspirin than in controls, and the incidence of adverse events was higher.
CONCLUSION:
This review found no evidence that low-dose aspirin buffers against cognitive decline or dementia or improves cognitive test scores in RCTs.
Keywords: aspirin, dementia, cognitive impairment, meta-analysis
Low-dose aspirin is often used for the prevention of cardiovascular disease (CVD) because of its antithrombotic properties.1,2 Aspirin has antiinflammatory properties, and inflammation plays an important role in several diseases.3 Preclinical models suggest that aspirin may decrease neuroinflammation and oxidative stress in the central nervous system.4,5 These pleiotropic mechanisms of action of aspirin could aid in the prevention of cognitive decline or dementia.
Cardiovascular and cerebrovascular conditions are established risk factors for poor cognitive status and dementia.6–8 The atherosclerotic process, which leads to plaque development and reduced oxygen availability to the brain, can explain the relationship between vascular disease and cognitive decline and dementia.9 Dementia and cardio- and cerebrovascular diseases share several risk factors, particularly with diabetes mellitus.10,11
There has been growing interest in the use of drugs of a similar nature to aspirin, such as nonsteroidal anti-inflammatory drugs (NSAIDs) for the prevention of dementia.13 A growing body of research is suggesting a potential role for aspirin in the prevention of cognitive decline. Because dementia is irreversible, understanding whether low-dose aspirin is useful for the prevention of cognitive decline and improvement of cognitive function of people without dementia is important. A Cochrane systematic review including one randomized controlled trial (RCT) and 70 participants published in 2000 demonstrated no benefit of aspirin in preventing vascular dementia.12 Since that time, further evidence has become available, and an updated comprehensive meta-analysis is required.
Given the aforementioned limitations in the literature, this study had the following aims: investigate whether low-dose aspirin use is associated with onset of dementia and decline in cognition in observational studies and whether low-dose aspirin use results in better cognitive test scores in RCTs of people without cognitive impairment or dementia. It was hypothesized that low-dose aspirin could have a favorable effect on cognition.
METHODS
This systematic review adhered to the Preferred Reporting Items for Systematic Reviews and Meta Analyses14 and Meta-analysis Of Observational Studies in Epidemiology15 statements and followed an a priori defined but unpublished protocol.
Data Sources and Literature Search Strategy
Two investigators (NV, MS) independently searched PubMed, EMBASE, SCOPUS, Cochrane Central Register of Controlled Trials, and Clinicaltrials.gov without language restrictions from database inception to September 1, 2016. Observational studies and RCTs investigating the effect of oral low-dose aspirin on the incidence of dementia or test scores assessing cognitive function in people without dementia or cognitive impairment at baseline were included. In PubMed, the following search strategy was used: (aspirin OR acetylsalicylic acid) AND (cognit*). Conference abstracts and reference lists of included articles were hand searched to identify any potential additional relevant articles. Any inconsistencies were resolved by consensus.
Study selection
Inclusion criteria for this meta-analysis were use of an RCT or longitudinal study design, use of low-dose aspirin (<300 mg/d16), reporting data on dementia diagnosed through validated criteria, and reporting data of cognitive tests of global (e.g., Mini-Mental State Examination (MMSE)17) or specific cognitive functions in adults without dementia or cognitive impairment.
Studies were excluded if they were not conducted in humans, used a nonplacebo control group (active controls in RCTs), or used dosages of aspirin with antiinflammatory aims (≥300 mg/d or for brief periods, <1 year) or if a standardized mean difference (SMD) or odds ratio (OR) could not be computed from the available data. If studies were encountered that did not provide sufficient data for the meta-analysis, the authors were contacted twice over a period of a month to request additional data.
Data extraction
Two investigators (NV, BS) extracted data from the articles in a standardized file, and a third independent investigator (MS) validated data extraction. Data were extracted about authors, year of publication, country, setting, demographic characteristics (sample size, mean age, percentage of women), follow-up duration, diagnostic criteria or tests used for diagnosis of dementia and cognitive impairment, and daily dose of aspirin. In longitudinal studies, information was extracted on number and type of covariates used in multivariate analyses. Data were extracted according to treatment group (low-dose aspirin and controls). If information was missing, the first or corresponding author of the original article was contacted at least two times in a month.
Outcomes
For longitudinal studies, the primary outcome was incidence of dementia or cognitive impairment during follow-up in the longitudinal studies. Dementia was ascertained through validated criteria, and cognitive impairment was ascertained according to predefined cut-offs of standardized test results for assessing cognitive status (e.g., MMSE score ≥24).17
For RCTs, changes between follow-up and baseline on tests assessing cognition (e.g., global cognition, verbal memory or fluency) were determined for participants without dementia or cognitive impairment at baseline in the group taking aspirin and in the control group (placebo, no intervention).
Assessment of Study Quality
Two authors (NV, BS) assessed the quality of studies using the Newcastle-Ottawa Scale (NOS) to evaluate longitudinal studies18 and the Jadad scale19 to assess RCTs.
Data Synthesis and Statistical Analysis
All analyses were performed using Comprehensive Meta-analysis 3 (Biostat, Englewood, NJ).
In the primary analysis, data regarding incidence of dementia and cognitive impairment in people using low-dose aspirin were compared with data of those receiving no treatment using the ORs with their 95% confidence intervals (CIs) adjusted for the maximum number of covariates available for each study. In the co-primary analysis, cognitive tests values of participants treated with low-dose aspirin were compared with those of controls (placebo or no intervention). Differences between the means of the treatment and control groups were calculated using change between follow-up and baseline data for each group, using SMDs with 95% CIs. In all analyses, a random-effects model was applied.20
Heterogeneity across studies was assessed using the I2 metric and Cochran Q chi-square statistics with a value of 50% or greater for the first and P < .05 indicating the presence of significant heterogeneity.21
Publication bias was assessed by visually inspecting funnel plots and using the Begg-Mazumdar Kendall tau22 and the Egger bias test.23 The trim-and-fill method was used to account for publication bias, to adjust for any potential unpublished (imputed) studies.24
For all analyses, P < .05 was considered statistically significant.
RESULTS
Search Results
The search yielded 2,341 nonduplicated articles. After excluding 2,325 articles based on title and abstract review, 16 articles were retrieved for full-text review, and eight studies (5 longitudinal,25–29 3 RCTs30–32) were included (Figure S1). The other eight studies were excluded because they included active controls (people taking another drug, n = 3); investigated acute effects of aspirin on cognition (dose >300 mg/d used for analgesic aims, n = 2); were reviews (n = 1) or protocols (n = 1); or reported data as linear regression estimates and thus were not metaanalyzable (n = 1).
Study and Participant Characteristics
Full descriptive details of the included studies are reported in Tables S1 and S2.
This meta-analysis included 36,196 participants, of whom 8,484 (23.4%) received low-dose aspirin. Mean age was 66, and 63% of participants were female. All participants were community-dwelling and lived in Europe (6 studies) or the United States (2 studies).
Longitudinal Studies
The five longitudinal studies25–29 included 26,159 community-dwelling participants with a mean age of 65.1, mainly women, for a median follow-up of 6 years. Three studies25,27,28 investigated dementia as an outcome, and the other two26,29 considered cognitive impairment (Table S1). The quality of the studies was sufficient (NOS mean 6, range: 6–7).18
Of the 26,159 participants, 3,035 (11.6%) used low-dose aspirin. Participants using low-dose aspirin at baseline were significantly older (78.1 ± 5.3 vs 75.9 ± 6.4, P < .001) but no different emerged in terms of percentage of women (64% vs 69%, P = .15).
RCTs
The three RCTs30–32 (two vs placebo30,32 and one31 as add-on therapy) included 10,037 participants with an average age of 66.8; 74% were female. Median follow-up was 5 years (range 3–9.6 years). One RCT31 used MMSE score to determine cognitive status, and the other two30,32 used several tests (Table S2). The quality of the studies was good.19
Meta-analysis of Longitudinal Studies
After adjusting for a median of three potential confounders (range 0–7), the use of low-dose aspirin was not significantly associated onset of dementia or cognitive impairment (5 studies: OR = 0.82, 95% CI = 0.55–1.22, P = .33, I2=67%; Figure 1). Considering each study separately, only one study,27 with the largest cohort available (n = 23,915 participants at baseline), reported a significantly lower risk of dementia at follow-up, taking into account four potential confounders (Table S2). Even though the three studies using dementia as the outcome25,27,28 reported a lower risk (OR = 0.59 (95% CI = 0.33–1.05, P = .84, I2 = 33%) than the other two which examined cognitive impairment26,29 (OR = 0.96, 95% CI = 0.62–1.51, P = .66, I2=59%), the interaction between aspirin use and outcome was not significant (P = .18). After removing one study28 (a conference abstract), the results did not significantly change (OR = 0.72, 95% CI = 0.47–1.10, P = .14, I2=70%), with the studies assessing dementia reporting an OR equal to 0.59, (95% CI = 0.33–1.05, P = .07, I2 = 33%).
Figure 1.
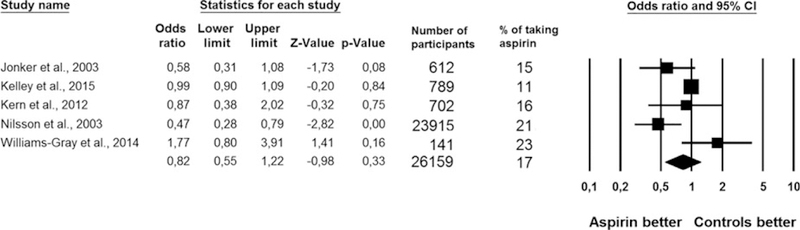
Effect of low-dose aspirin on the onset of dementia or cognitive impairment in longitudinal studies.
Meta-analysis of Randomized Controlled Trials
The use of low-dose aspirin was not associated with better global cognitive test scores in the three RCTs including 10,037 participants30–32 (SMD = 0.00, 95% CI = −0.04 to 0.05, P = .84, I2 = 0%) (Figure 2). Publication bias was unlikely.
Figure 2.
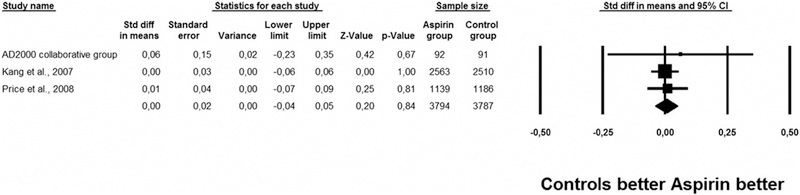
Effect of low-dose aspirin on global cognitive tests in randomized controlled trials.
Two studies30,32 reported information regarding verbal memory and executive function and fluency tests. Although no significant differences emerged in terms of verbal memory tests (SMD = −0.02, 95% CI = −0.06–0.03, P = .42, I2=0%), the use of low-dose aspirin was associated with slightly better executive function and fluency test results (SMD = 0.06, 95% CI = 0.02–0.11, P = .006, I2 = 0%). This difference was estimated to correspond to 2.6 years younger age and a 20% lower risk of cognitive decline than with placebo32 in a single study.
Adherence and Adverse Effects
In the three RCTs included, a lower percentage of participants using low-dose aspirin completed the RCTs (69.9%) than of controls (75.9%, chi-square test P = .005).
Only two studies (one longitudinal,25 one RCT31) reported information regarding side effects. The prevalence of gastrointestinal adverse events was 10 times as high in people taking aspirin (15.2%) as in controls (1.4%, P < .001). Although the RCT31 did not report the type of gastrointestinal side effects, the longitudinal study25 reported a similar incidence of gastric ulcers between those taking and not taking aspirin (P = .17).
DISCUSSION
This meta-analysis found that the use of low-dose aspirin did not appear to be associated with lower incidence of dementia and cognitive impairment in observational studies. Across RCTs, no evidence was found of improvement in cognitive test scores in people who were free of dementia or cognitive impairment, although people using low-dose aspirin had a higher frequency of side effects (particularly gastrointestinal); this was limited to data from two studies.
Several hypotheses might explain these findings. First, the average age of the participants was 65. Previous research has suggested that the pathological changes typical of dementia happen 20 years before the clinical symptoms present33, so the use of low-dose aspirin at this age may be “too little, too late.” Second, participants may have been taking low-dose aspirin for the secondary prevention of CVD or related conditions. Thus, preexisting comorbidities at baseline may have interfered with potential cognitive beneficial effects of low-dose aspirin and explain the null result. Finally, genetic factors could play a role in this lack of effect of low-dose aspirin. One study investigated whether the role of the apolipoprotein (APO)E gene altered the association between NSAID use and dementia risk and found lower risk of AD only in NSAID users with an APOE ε4 allele.34 Specific research is needed to clarify this potential hypothesis. Other factors may have accounted for the result across RCTs included in this meta-analysis, which also need to be considered. First, the number of people lost during follow-up could have influenced the results. Moreover, some of the cognitive tests, such as the MMSE, have important limitations, such as overestimation of cognitive impairments in persons aged 60 and older and in those with less education.35 The current meta-analysis was designed to overcome this by using all of the tests available that assessed cognition and subcomponents such as executive function and fluency tests that aspirin use may improve, although the very small effect size detected in the latter suggests that the clinical significance of these findings is probably limited.
These findings are in partial agreement with two recent metaanalyses considering the effect of other NSAIDs on cognitive outcomes. Although participants in the longitudinal studies who took NSAIDs were 28% less likely to develop dementia,13 the findings from the RCTs included in another meta-analysis did not support these observational findings.36 Taken these findings together, it appears there is no consistent evidence that the use of NSAIDs may delay or prevent the onset of dementia or cognitive impairment over time, although future, adequately powered real-world investigations are required to better understand whether aspirin and other NSAIDs can delay or prevent dementia. Based on the current study findings, future RCTs should include more men and younger people. In this context, the ASPirin in Reducing Events in the Elderly Study, an ongoing trial including 19,000 healthy participants aged 65 and older could help better understand the possible role of aspirin in the prevention of dementia and mild cognitive impairment,37 although particular attention should also be paid to adherence and any adverse events from such studies, and low-cost, lower-risk alternatives such as physical activity38,39 and nutrition interventions40 should also be considered.
Although few studies provided evidence on adherence and adverse events, the limited available evidence suggests that a higher incidence of adverse events could in part explain poorer adherence. Gastrointestinal side effects appear to be particularly troublesome. Some experts have suggested that aspirin may lead to an increase in gut permeability (leaky gut), which may lead to the translocation of bacterial products (e.g., lipopolysaccharide), which may increase microglial activation and therefore neuroinflammation.5,41,42 Bearing in mind the possible limitations of the current study, these results suggest not only a lack of evidence that aspirin could protect against cognitive decline and dementia but that it may increase adverse gastrointestinal events.
The findings of this meta-analysis should be interpreted within its limitations. First, only a limited number of RCTs were identified. Although these studies were of high quality, with large sample sizes and long follow-up, other studies are needed to reach a definitive conclusion. Second, the observational studies did not use propensity score in their analyses, which is the best method for comparing a population taking a drug with another one that is not.43 Moreover, the possibility of selection bias in longitudinal studies and the fact that a consistent percentage of people not taking aspirin took other NSAIDs might have created another important bias in the results. In addition, one study included the overwhelming majority of participants and thus could have had a large influence on the overall result.26 Another limitation is the high heterogeneity observed in the longitudinal studies and that no metaregression analysis was possible because of the limited number of studies for each outcome.44 Fourth, no study assessed the effect of low-dose aspirin in reducing dementia risk according to APOE ε4 status, which could be a relevant factor in establishing a link between aspirin use and cognitive decline. It may be that only people with this mutation had a lower risk of dementia when taking aspirin, as shown by a large study analyzing the interaction between APOE ε4 status and NSAIDs.34 Fifth, conclusions on side effects of aspirin are based on only two studies. Finally, although potential confounding factors were accounted for when data were available by using adjusted effect estimates in the pooled analyses, it was not possible to consider other potentially important, unidentified confounders. For instance, people taking low-dose aspirin are more likely to be at risk of, or have a history of CVD than those not taking aspirin, and the indications for aspirin may vary across countries, and these may be influential factors. Thus, future research should attempt to consider, where possible, the effect of such factors on the relationship between aspirin and cognitive outcomes.
The strengths of this work include a comprehensive search of several databases and the inclusion of the largest possible number of participants; the long follow-up of each study; and that, to the best of our knowledge, this is the first systematic review and meta-analysis to consider this question.
In conclusion, this preliminary meta-analysis suggests that low-dose aspirin does not decrease the risk of cognitive decline (in terms of dementia or cognitive impairment) or improve cognitive test scores. Moreover, adherence to aspirin may be lower than to control conditions, and aspirin may cause adverse events. Future trials considering dementia onset as the outcome are needed to determine whether low-dose aspirin decreases cognitive decline and to test the possibility that low-dose aspirin has beneficial effects when taken over a longer period and at an earlier age than the observed population did.
Supplementary Material
Figure S1. PRISMA flow-chart.
Table S1. Descriptive characteristics of the longitudinal studies included.
Table S2. Descriptive characteristics of the randomized controlled trials included.
ACKNOWLEDGMENTS
We thank Dr. Caroline Williams-Gray, John Van Geest Centre for Brain Repair, University of Cambridge, United Kingdom, for providing data.
Footnotes
SUPPORTING INFORMATION
Additional Supporting Information may be found in the online version of this article:
Please note: Wiley-Blackwell is not responsible for the content, accuracy, errors, or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
REFERENCES
- 1.Bartolucci AA, Tendera M, Howard G. Meta-analysis of multiple primary prevention trials of cardiovascular events using aspirin. Am J Cardiol 2011;107:1796–1801. [DOI] [PubMed] [Google Scholar]
- 2.Berger JS, Lala A, Krantz MJ et al. Aspirin for the prevention of cardiovascular events in patients without clinical cardiovascular disease: A meta-analysis of randomized trials. Am Heart J 2011;162:115.e2–124.e2. [DOI] [PubMed] [Google Scholar]
- 3.Soysal P, Stubbs B, Lucato P et al. Inflammation and frailty in the elderly: A systematic review and meta-analysis. Ageing Res Rev 2016;31:1–8. [DOI] [PubMed] [Google Scholar]
- 4.Wu Y, Zhai H, Wang Y et al. Aspirin-triggered lipoxin A 4 attenuates lipopolysaccharide- induced intracellular ROS in BV2 microglia cells by inhibiting the function of NADPH oxidase. Neurochem Res 2012;37:1690–1696. [DOI] [PubMed] [Google Scholar]
- 5.Wang Y-P, Wu Y, Li L-Y et al. Aspirin-triggered lipoxin A4 attenuates LPS-induced pro-inflammatory responses by inhibiting activation of NF-KB and MAPKs in BV-2 microglial cells. J Neuroinflammation 2011;8:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tatemichi TK, Paik M, Bagiella E et al. Risk of dementia after stroke in a hospitalized cohort: Results of a longitudinal study. Neurology 1994;44:1885–1891. [DOI] [PubMed] [Google Scholar]
- 7.Newman AB, Fitzpatrick AL, Lopez O et al. Dementia and Alzheimer’s disease incidence in relationship to cardiovascular disease in the Cardiovascular Health Study cohort. J Am Geriatr Soc 2005;53:1101–1107. [DOI] [PubMed] [Google Scholar]
- 8.Harrison SL, Ding J, Tang EYH et al. Cardiovascular disease risk models and longitudinal changes in cognition: A systematic review. PLoS ONE 2014;9:e114431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trevisan C, Veronese N, Bolzetta F et al. Low hemoglobin levels and the onset of cognitive impairment in older people: The PRO.V.A. Study. Rejuvenation Res 2016;19:447–455. [DOI] [PubMed] [Google Scholar]
- 10.Li L, Hölscher C. Common pathological processes in Alzheimer disease and type 2 diabetes: A review. Brain Res Rev 2007;56:384–402. [DOI] [PubMed] [Google Scholar]
- 11.Katon W, Pedersen HS, Ribe AR et al. Effect of depression and diabetes mellitus on the risk for dementia: A national population-based cohort study. JAMA Psychiatry 2015;98195:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williams PS, Rands G, Orrel M et al. Aspirin for vascular dementia. Cochrane Database Syst Rev 2000;4:CD001296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang J, Tan L, Wang H-F et al. Anti-inflammatory drugs and risk of Alzheimer’s disease: An updated systematic review and meta-analysis. J Alzheimers Dis 2015;44:385–396. [DOI] [PubMed] [Google Scholar]
- 14.Liberati A, Altman DG, Tetzlaff J et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Med 2009;6:e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stroup DF, Berlin J, Morton SC et al. Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283: 2008–2012. [DOI] [PubMed] [Google Scholar]
- 16.Lloyd J, Bochner F. Aspirin: How low is low dose? Aust Prescr 1996;19:79–81. [Google Scholar]
- 17.Folstein M, Robins L, Helzer J. The Mini-Mental State Examination. Arch Gen Psychiatry 1983;40:812. [DOI] [PubMed] [Google Scholar]
- 18.Wells G, Shea B, O’Connell D et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality if nonrandomized studies in meta-analyses [online]. Available at http//www.ohri.ca/programs/clinical_epidemiology/oxford.asp Accessed on 1st October 2016.
- 19.Jadad AR, Moore RA, Carroll D et al. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control Clin Trials 1996;17:1–12. [DOI] [PubMed] [Google Scholar]
- 20.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–188. [DOI] [PubMed] [Google Scholar]
- 21.Higgins JPT, Thompson SG, Deeks JJ et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088–1101. [PubMed] [Google Scholar]
- 23.Egger M, Davey Smith G, Schneider M et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duval S, Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000;56:455–463. [DOI] [PubMed] [Google Scholar]
- 25.Kern S, Skoog I, Östling S et al. Does low-dose acetylsalicylic acid prevent cognitive decline in women with high cardiovascular risk? A 5-year follow-up of a non-demented population-based cohort of Swedish elderly women BMJ Open 2012;3;2(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kelley BJ, McClure LA, Unverzagt FW, et al. Regular aspirin use does not reduce risk of cognitive decline. J Am Geriatr Soc. 2015;63:390–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nilsson SE, Johansson ÆB, Takkinen ÆS. Does aspirin protect against Alzheimer’s dementia? A study in a Swedish population-based sample aged > or = 80 years. Eur J Clin Pharmacol 2003;59:313–319. [DOI] [PubMed] [Google Scholar]
- 28.Williams-Gray C, Mason S, Foltinye T et al. Williams Gray.pdf 2014;24: S553. [Google Scholar]
- 29.Jonker C, Comijs HC, Smit JH. Does aspirin or other NSAIDs reduce the risk of cognitive decline in elderly persons? Results from a population-based study. Neurobiol Aging 2003;24:583–588. [DOI] [PubMed] [Google Scholar]
- 30.Price JF, Stewart MC, Deary IJ et al. Low dose aspirin and cognitive function in middle aged to elderly adults: Randomised controlled trial. BMJ 2008;337:a1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.AD2000 Collaborative Group. Aspirin in Alzheimer’s disease (AD2000): A randomised open-label trial. Lancet Neurol 2008;7:41–49. [DOI] [PubMed] [Google Scholar]
- 32.Kang JH, Cook N, Manson J et al. Low dose aspirin and cognitive function in the women’s health study cognitive cohort. BMJ 2007; 334:987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reiman EM, Quiroz YT, Fleisher AS et al. Brain imaging and fluid biomarker analysis in young adults at genetic risk for autosomal dominant Alzheimer’s disease in the presenilin 1 E280A kindred: A case-control study. Lancet Neurol 2012;11:1048–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Szekely CA, Breitner JCS, Fitzpatrick AL et al. NSAID use and dementia risk in the Cardiovascular Health Study: Role of APOE and NSAID type. Neurology 2008;70:17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Naugle RI, Kawczak K. Limitations of the Mini-Mental State Examination. Cleve Clin J Med 1989;56:277–281. [DOI] [PubMed] [Google Scholar]
- 36.Miguel-Alvarez M, Santos-Lozano A, Sanchis-Gomar F et al. Non-steroidal anti-inflammatory drugs as a treatment for Alzheimer’s disease: a systematic review and meta-analysis of treatment effect. Drugs Aging 2015;32:139–147. [DOI] [PubMed] [Google Scholar]
- 37.ASPREE Investigator Group. Study design of ASPirin in Reducing Events in the Elderly (ASPREE): A randomized, controlled trial. Contemp Clin Trials 2013;36:555–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ahlskog JE, Geda YE, Graff-Radford NR et al. Physical exercise as a preventive or disease-modifying treatment of dementia and brain aging. Mayo Clin Proc 2011;86:876–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lautenschlager NT, Cox KL, Flicker L et al. Effect of physical activity on cognitive function in older adults at risk for Alzheimer disease: A randomized trial. JAMA 2008;300:1027–1037. [DOI] [PubMed] [Google Scholar]
- 40.Ngandu T, Lehtisalo J, Solomon A et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): A randomised controlled trial. Lancet 2015;385:2255–2636. [DOI] [PubMed] [Google Scholar]
- 41.Carabotti M, Scirocco A, Maselli MA et al. The gut-brain axis: Interactions between enteric microbiota, central and enteric nervous systems. Ann Gastroenterol 2015;28:203–209. [PMC free article] [PubMed] [Google Scholar]
- 42.Hollander D. Intestinal permeability, leaky gut, and intestinal disorders. Curr Gastroenterol Rep 1999;1:410–416. [DOI] [PubMed] [Google Scholar]
- 43.Haukoos JS, Lewis RJ. The propensity score. JAMA 2015;314:1637–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. PRISMA flow-chart.
Table S1. Descriptive characteristics of the longitudinal studies included.
Table S2. Descriptive characteristics of the randomized controlled trials included.


