Abstract
Introduction:
Western blotting is one of the most commonly used techniques in molecular biology and proteomics. Since western blotting is a multistep protocol, variations and errors can occur at any step reducing the reliability and reproducibility of this technique. Recent reports suggest that a few key steps, such as the sample preparation method, the amount and source of primary antibody used, as well as the normalization method utilized, are critical for reproducible western blot results.
Areas covered:
In this review, improvements in different areas of western blotting, including protein transfer and antibody validation, are summarized. The review discusses the most advanced western blotting techniques available and highlights the relationship between next generation western blotting techniques and its clinical relevance.
Expert commentary:
Over the last decade significant improvements have been made in creating more sensitive, automated, and advanced techniques by optimizing various aspects of the western blot protocol. New methods such as single cell-resolution western blot, capillary electrophoresis, DigiWest, automated microfluid western blotting and microchip electrophoresis have all been developed to reduce potential problems associated with the western blotting technique. Innovative developments in instrumentation and increased sensitivity for western blots offer novel possibilities for increasing the clinical implications of western blot.
Keywords: immunoblot, next generation Western blotting, protein purification, Western blotting, Western blotting techniques, protein sample preparation
1. Introduction
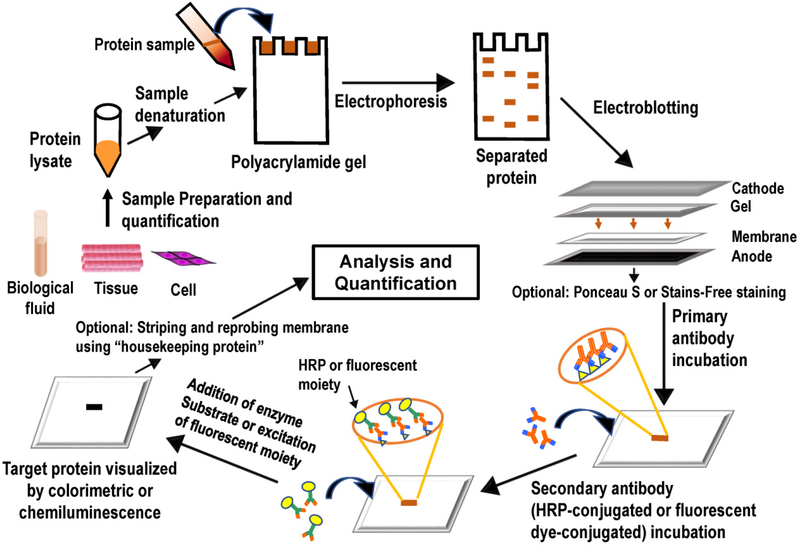
The western blot is a common method used to detect proteins as well as post-translational modifications on proteins, and can provide semi-quantitative or quantitative data about the target protein in simple or complex biological samples [1]. Western blotting is an extensively used technique for protein analysis [2–4]. It is a multistep procedure (Figure 1) that typically involves a) sample preparation (protein extraction and measurement of protein concentration) from cells or tissue lysates, b) separation of proteins by size on sodium dodecyl sulphate (SDS) polyacrylamide gel by electrophoresis, c) immobilization of separated proteins in a nitrocellulose or polyvinylidene fluoride (PVDF) membrane, d) blocking of non-specific proteins on membrane, e) probing of target proteins with specific primary antibodies, e) incubation with secondary antibody conjugated with labelled chemiluminescent or fluorescent molecule, f) detection of signal that reflects antigen/ antibody binding, g) densitometry analysis of protein bands of interest using a software [3,5]. Variations at any step of the process can influence the result. Thus, care must be taken to standardize each step of the western blot process for reproducibility and high sensitivity.
Figure 1.
Schematic representation of the Western Blotting Procedure
2. Sample Preparation
An often-overlooked aspect of reproducible western blotting is efficient sample preparation. An efficient protein extraction and purification step has a substantial impact on the results and interpretation of western blotting experiments [3]. To effectively extract proteins, a suitable homogenization method that can efficiently release the intracellular contents of the cell through the rupture of cell membranes should be selected [5]. In addition, an optimal lysis buffer should be chosen to facilitate the proper solubilization of proteins and prevent proteolytic degradation in order to obtain high amounts of target proteins [5,6]. Once the protein samples (cell lysates, tissue homogenates, and body fluids) are prepared, they are then subjected to electrophoretic separation based on their size on SDS polyacrylamide gel electrophoresis (PAGE), and immobilized on a membrane by electroblotting.
3. Improvement in protein purification over last decade
Protein samples used in western blot are very diverse, ranging from purified proteins to highly complex samples such as cell/tissue lysates that contains cellular debris, protein aggregates, fats and proteases. To isolate an intracellular protein the cell membranes must be ruptured to free the cellular contents using a suitable lysis buffer to obtain a high yield of solubilized target protein. The expression, conformation, and stability of proteins varies significantly with the buffer and experimental conditions utilized [7]. The principal factors that can influence sample processing include the time between tissue collection and its freezing, frequent freeze/thaw cycles of tissue samples, poor homogenization techniques, and use of inappropriate lysis buffer [8]. A report by Glatter et al., 2015 showed that the detected total proteome mass of a sample was highly dependent on the sample preparation protocol [9]. Some lysis buffers resulted in incomplete protein extraction of certain protein groups such as membrane proteins, proteasome subunits, and ribosomal proteins [9]. Tissues are more complex than cultured cells and require more mechanical intervention than cultured cells for effective sample preparation.
To improve western blot accuracy, samples should be quickly harvested in ice cold buffer (neutral pH) and should be immediately frozen in liquid nitrogen and stored at −80 °C until use to avoid proteolytic degradation by endogenous proteases (as sometimes indicated by gel streaks) [10]. Moreover, multiple freeze/thaw cycles of protein samples must be avoided to prevent protein degradation [5]. Homogenization techniques (mechanical, sonication, chemical) employed for extraction of target proteins vary with types of tissues (liver, kidney, muscle, heart) or cells (suspended or cultured primary cells or cell lines) [11,12].
Although an ideal disruption method would involve only one step, many laboratories utilize two or more methods to obtain efficient sample disruption. The method of sample preparation depends on desired use of the sample. If active proteins are needed for subsequent native gel electrophoresis [13] the homogenization procedure should avoid processes that generate heat or cause foaming. If subcellular fractions are needed, simple cutting of tissue with scissors, then use of a handheld homogenizer for course grinding and subsequent use of glass Dounce homogenizer for shearing could be utilized. Dounce homogenizers are inexpensive and effective at mildly lysing cells. The shearing force of Dounce homogenizers depends on the diameters of the pestle and container. Glass-teflon homogenizers, Dounce hand homogenizers, or polytron homogenizers are generally used to mince tissues to release target proteins in homogenization buffer [12]. In some cases, to achieve higher yield of target proteins that are expressed at lower levels, additional sonication of samples is done to disrupt cellular membranes [5]. Sonication utilizes high frequency sound waves created by a probe that rapidly enlarges and contracts at high frequencies. These sound waves can disrupt most cell samples in seconds. Although very effective at disrupting cells and partly homogenized tissue, sonication is not very effective for solid tissue. Because sonication generates a significant amount of heat, it is important that this procedure should be carried out on ice using multiple short bursts.
With the increasing need for high throughput homogenizers in applications such as in screening strategies, various approaches were developed to increase the throughput of homogenization. One strategy our lab employs is a mixer mill which utilizes the effectiveness of bead beating and can process numerous samples in different types of tubes including microwell plates. Several companies make high throughput homogenizers including Retsch® Mixer Mill and the HT Homogenizer®. These homogenizers can homogenize most samples within 2 minutes.
Cyrogenic grinding, also known as cryogrinding, is the act of reducing material such as plant or animal tissue into a small particle size [14]. Cyrogrinding is commonly used for the extraction of ribonucleic acid (RNA) to keep samples at very low temperatures [14], but is also an alternative for thermally labile proteins. One such cryogrinding device is the SPEX Certiprep freezer mill which utilizes small magnetic bars to pulverize samples inside a self-contained chamber liquid nitrogen container and insulated case to allow pulverization at ultra-low temperatures. Although cyrogrinding is not widely used in proteomic applications, its benefits for preventing proteolysis as well as changes to post-translational modifications suggest that this method should be more commonly used for preparing tissue samples for more efficient homogenization.
Lysis buffers vary with their ability to solubilize proteins and have effects on protein quality and antigenic recognition sites that ultimately affect the western blot results [8,15,16]. An optimal lysis buffer should efficiently extract the target proteins. In some cases the lysis buffer should possess the ability to maintain the integrity of protein as this is important for doing western blots on protein complexes separated by native gels [17,18]. Lysis buffer can be optimized by varying the concentration of detergents and other chemical components to solubilize target proteins and prevent protein precipitation [5,6]. One study suggested that the use of detergent octyglucoside is the best option to get high quality and high amount of integrin protein receptors (an integral membrane protein) compared to the non-ionic detergents nonidet-40 (NP-40) or Triton X-100 [19]. The use of sodium chloride (NaCl) or potassium chloride (KCl) at a concentration of 100 mM to 150 mM in homogenization buffers prevents protein aggregation [5]. Reducing agents such as dithiothreitol (DTT) are often utilized to help solubilize proteins as well as to keep some proteins in an active state (such as for native gel electrophoresis). These reducing agents often interfere with protein quantification methods utilized. Thus, the assay chosen for quantifying proteins must be compatible with concentration of reducing reagents used in the lysis buffer for protein extraction. Avoiding reducing agents in sample preparation and gels has also been shown to be beneficial in antibody detection of some conformationally sensitive epitopes. Some antibodies only recognize non-linear epitopes that require conformational integrity of the target protein. Since reducing agents are used to provide linear epitopes, non-denaturing PAGE systems are required for non-linear epitopes. The common reagents and their typical concentration range used in lysis buffer have been summarized in Table 1.
Table 1.
Commonly used reagents in lysis buffer and their concentration
| Common lysis buffers | Common reagents in lysis buffer | Typical concentration range | Role | References |
|---|---|---|---|---|
| NP-40 buffer, RIPA buffer, Tris-Triton buffer, Tris-HCl buffer | Tris-HCl | 50 mM-100 mM (pH 7.4 –8.0) | pH buffering of solution | [5,8,22] |
| NaCl | 50 mM− 150 mM | Cell membrane rupture and protein solubility | [5,8,22] | |
| Non-ionic detergents: NP40 Octyl Glucoside Triton X-100 Tween |
0.1–2% | Solubilization of non-polar insoluble proteins | [5,8,19,22,97] | |
| Ionic Detergents: SDS Sodium deoxycholate CTAB | 0.1–1% 0.1–0.5% 0.01–0.5% |
Coat proteins with negative charge and facilitates their separation on gels based on their molecular mass Membrane disruption Solubilization of poorly soluble proteins | [5,8] | |
| Zwitterionic CHAPS (SB3–10 and ASB-14) | 2% | Break protein-protein interactions | [98,99] | |
| Urea | 5–8M | Breaks non-covalent interactions and leads to protein denaturation | [5,99] | |
| EDTA | 1–5 mM | Chelate metal ions, reduce oxidation damage and inhibition of metalloproteases | [8] | |
| EGTA | Chelate metal ions | [5] | ||
| Glycerol | 5–10% | Stabilization | [5,97] | |
| DTT | 1–10 mM | Cleavage of disulfide bonds and protein denaturation | [5,97,99] | |
| 2-Mercaptoethanol ^ | 1–10 mM | Cleaves disulfide bonds and protein denaturation | [5,97] | |
| TCEP | 10–50 mM | Cleaves disulfide bonds destabilizes the secondary and tertiary structure | [97] | |
| Na3VO4 | 0.1– 2 mM | Tyrosine and alkaline phosphatase inhibition | [5,19] | |
| NaF | 50 mM | Serine/threonine phosphatase inhibition | [5] | |
| Protease inhibitor cocktail | Usually in prepackaged tablet or liquid forms | Inhibits proteolysis | [5,8] |
Abbreviations: Sodium dodecyl sulphate (SDS); Nonidet- 40 (NP-40); Radio immunoprecipitation assay (RIPA); Ethylene diamine tetra acetic acid (EDTA); Ethylene glycol tetra acetic acid (EGTA); Dithiothreitol (DTT); Sodium vanadate (Na3VO4); Sodium fluoride (NaF); cetyl trimethyl-ammonium bromide (CTAB) (CTAB); (tris(2-carboxyethyl)phosphine (TCEP).
Radioimmunoprecipitation assay (RIPA) buffer is a commonly used lysis buffer that utilizes increased concentration of salts and detergents to facilitate nuclear disruption and increase the yield of proteins in preserved conformations [6]. The RIPA buffer is one of the most commonly used buffers in protein extractions used for western blotting [16]. The constituents of RIPA buffer typically includes 150 mM NaCl, 0.1% SDS, 1% NP-40, or Triton X-100, 1mM ethylene diamine tetra acetic acid (EDTA), 50 mM Tris-HCl, 1% sodium deoxycholate, pH 7.4 −7.8 along with protease and phosphatase inhibitors [6]. Protease and phosphatase inhibitors are available as tablets from many companies including Thermo Fisher and Sigma-Aldrich. One major problem with using RIPA buffer is the many different formulations of this buffer and the lack of information about the advantages and disadvantages of each of these different fomulations. For example, RIPA buffer from Sigma-Aldrich (catalog # R0278) contains 150 mM NaCl, 1.0% IGEPAL® (CA-630), 0.5% sodium deoxycholate, 0.1% SDS, 50 mM Tris-HCl, pH 8.0, Thermo Scientific RIPA buffer (catalog # R0278) contains 150 mM NaCl, 1% NP-40, 1% sodium deoxycholate, 0.1% SDS, 25mM Tris-HCl pH 7.6, Millipore RIPA buffer (catalog #20–188) contains 150 mM NaCl, 1% NP-40, 0.25% deoxycholic acid, 1 mM EDTA, 50 mM Tris-HCl, pH 7.4, while Cell Signaling RIPA buffer (catalog # R0278) contains 150 mM NaCl, 1% NP-40, 1% sodium deoxycholate, 1 mM EDTA, 1 mM EGTA, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 0.1% SDS, 1 mM sodium vanadate (Na3VO4), 1 μg/ml leupeptin, 20 mM Tris-HCl pH 7.5. Sodium pyrophosphate has both chelating ability (binds calcium) and can act as a buffering agent, while β-glycerophosphate is utilized in some sample preparation buffers as a protein phosphatase inhibitor. The use of different RIPA buffers may yield higher levels of certain proteins of interest [20–22], but a comprehensive study of what RIPA buffer is optimal for which type of samples is needed.
Although use of RIPA buffer for lysis of HT-29 human colon adenocarcinoma cells yielded both solubilized cytoplasmic proteins and insoluble proteins (containing several histones and transcriptional factors) [6], the use of Laemmli sample buffer as the lysis buffer resulted in higher concentrations of apoptotic factor cleaved caspase 8 compared to RIPA buffer [6]. Others have successfully used Laemmli buffer as the lysis buffer but concerns about quantification and the presence of insoluble material in the lysates may limit the use of this method.
In a recent report, protein extraction from Trizol-precipitated brain tissue proteins allowed the analysis of both gene and protein expression from same sample simultaneously [21]. A Trizol based method is generally employed to isolate DNA and RNA, however, protein extraction from the Trizol-precipitated proteins is difficult due to insolubility of precipitated protein. The method gives a high yield of proteins with similar protein amounts as those isolated using RIPA buffer [21]. The lysis buffer was optimized by using high concentrations of EDTA, SDS, and Tris (20 mM EDTA, 140 mM NaCl, 5% SDS, 100 mM Tris, pH 8.0). This solubilization method enabled the analysis of post translational modifications of proteins [21].
After proteins are extracted from cells or tissues, the amount of protein in the lysate must be determined. Protein concentration is measured by different methods including the Bradford assay, UV absorbance (Nanodrop) method, bicinchoninic acid (BCA) method, and ortho-phthaladehyde assay [5]. Appropriate lysis buffer compatible with subsequent chosen protein assays must be used as blanks and in the standard curves to avoid interference by buffer components during protein quantification [19]. The presence of insoluble and soluble proteins in samples will also affect protein quantification.
4. Western Blotting
4.1. Problems related to western blotting
A major concern of western blotting is the lack of any consensus on what constitutes reproducibility. Although western blotting seems to be a straightforward and simple method for protein analysis in practice, it is an error-prone method due to its time-consuming multistep protocol [3,7]. Detection of low abundant proteins and post-translational modified proteins such as phosphorylation, acetylation, and methylation are difficult due to the requirement of relatively large sample amounts (usually >10 μg), as well as high quality validated antibodies against phosphorylated antigens used for western blotting [7,23]. Other problems include analysis of multiple proteins from a single sample run is often cumbersome, as it usually requires stripping of membranes to remove antibody and then again re-probing with second antibody for next target protein detection [24]. A recent method called multiple antigen detection (MAD) was developed to allow non-stripping detection of multiple antigens on a single blot [25]. To minimize nonspecific background in the MAD-immunoblotting procedure, the blot is incubated with secondary antibody and the membrane developed with chromogenic substrate before probing with the primary antibodies of interest [25]. While this method seems promising, an important aspect of western blotting, the quantification of these blots, was not well described [25]. Blots can also be cut into several pieces and used to detect targets within the molecular mass ranges of the cut membrane, but this limits information about what other targets the antibody may detect.
Detection of some very large molecular weight proteins (>500 kDa) can be problematic due to difficulty in their complete transfer from gels to membranes [26]. Our laboratory has found that the transfer of very large protein complexes (20S and 26S proteasomes, 800 kDa to over 2000 kDa) was more optimal with long (overnight) transfers using the Bio-Rad mini-Protean 3 “wet-transfer” system when compared to semi-dry blotters such as the Bio-Rad Trans-Blot Turbo™ [13,17]. However, we utilize the Trans-Blot Turbo™ to transfer proteins as large as 300 kDa in seven minutes. Moreover, improper sample preparation, proteolytic degradation of samples, lack of automation, problems with normalization of blots and quantitative analysis are other critical issues associated with western blotting procedure [7,23,24].
5. The antibody problem
Irrespective of how advanced or automated the methodology for western blotting gets, the main problem associated with western blotting, the quality of the antibodies used, still needs to be properly addressed. An ad hoc group of scientists called the International Working Group for Antibody Validation (IWGAV) suggested at least one of five areas is needed for validation of conventional and recombinant antibodies: (i) genetic strategies, (ii) orthogonal strategies, (iii) independent antibody strategies, (iv) expression of tagged proteins, and (v) immune-capture followed by mass spectrometry (MS) [27]. The group suggested that the use of more than one strategy would strengthen the conclusion.
Many researchers don’t realize that validating an antibody is different from characterizing an antibody. Importantly, validating an antibody doesn’t just involve determining the antibody’s specificity for its target but also involves determining its reproducibility; that is, demonstrating that the antibody’s properties are independent of lot-to-lot variations. Methods for validating antibodies for western blotting do not apply to other applications utilizing the same antibodies including immunohistochemistry and enzyme-linked immunosorbent (ELISA) applications.
Numerous examples of the problems associated with improperly validated antibodies exist. One example is a laboratory at Mount Sinai Hospital in Toronto, Canada, that was using a commercial kit to detect their protein target CUZD1, which they hypothesized, could be used as a marker for pancreatic cancer. After two years of using kits, hundreds of thousands of dollars in cost, and thousands of patient samples they realized that the antibody provided in the kit did not interact with CUZD1 and was binding to a different protein, CA125 [28,29]. Larger scale studies done in 2008 showed that fewer than half of approx. 6000 commercial antibodies recognized only their targets [30]. More in depth studies showed that only 6 of 53 published preclinical studies could be replicated [31]. Hence believing the “validation” done by commercial companies can often lead to false results. Although we suggest that all manufacturers should properly validate all antibodies and include lot specific data, this is unlikely to happen due to the high production rate of new uncharacterized antibodies and the desperation of many researchers to find antibodies for specific targets. Most researchers don’t currently think about validating antibodies they obtain commercially and rely on the reputation of the company or previous publications using these antibodies. However, as previously shown by our group, the most utilized antibodies are not always the best quality antibody for specific targets [32]. To help increase the number of quality antibodies used in western blotting, national agencies such as NIH and journals should start requiring basic validation data for antibodies that are critical for conclusions of the manuscript. A Western Blotting Minimal Reporting Standard (WBMRS) that should be included in all manuscripts has been suggested by our group to help with the reproducibility of western blotting [32]. Unfortunately, currently researchers need to independently validate key antibodies because changes in the western blotting equipment used, the temperature, the incubation times, the buffers used, or the concentrations of antibodies used may result in different results from what is provided by the antibody manufacturer.
Although polyclonal antibodies are cheaper and easier to make, monoclonal antibodies are advantageous because the specificity of each batch of monoclonal from the same hybridoma is the same. Polyclonal antibodies for the same immunogen raised in different rabbits show batch to batch variations, sometimes major variations, mainly due to differences in the mix of antibodies generated by the animal. Although monoclonal antibodies are superior to polyclonal antibodies with respect to being able to generate the same antibody in different batches, monoclonal antibodies also have problems. Sometimes hybridoma cell lines can be contaminated and not grow after storage or may die off. One possibility to overcome some of the limitations of monoclonal and polyclonal antibodies is the use of recombinant antibodies. Several projects are currently underway to improve the quantity of renewable validated antibodies available. One project at Johns Hopkins University, is creating a large number of monoclonal antibodies while another project at the University of Chicago is generating recombinant antibodies [33].
5.1. Recombinant antibodies
Recombinant antibodies have many advantages including the ability to engineer the antigen binding site, the ability to rapidly produce many variants to a specific antigen and the ability to quickly screen these variants [34]. However, the main advantage of recombinant antibodies is that it can be defined by its sequence, and researchers anywhere would be able to create the same recombinant antibodies, significantly reducing the lot-to-lot variability. If a batch of recombinant antibody is contaminated or lost, new ones can be created if the antibody sequence is known [34]. However, like all antibodies, validation of recombinant antibodies is needed before it should be used for western blotting.
6. The “housekeeping” protein problem
Another concern in the western blotting field is that the most commonly used normalization technique for getting semi-quantitative data is the use of “house-keeping” proteins. Several reports from multiple laboratories including our laboratory suggest that most if not all housekeeping proteins are not equally expressed in the same tissue under all experimental conditions as previously assumed [7,15,35]. The Journal of Biological Chemistry instructions for authors suggest that quantification normalization of signal intensity to total protein loading (using a total protein stain such as Ponceau S) is preferred [7,36]. If “house-keeping” proteins are used for normalization, evidence must be provided to show that the expression of the proteins is not affected by the experimental conditions. The best methods currently available for normalization using total protein staining utilize either Ponceau S or Bio-Rad’s Stains-free methods [37,38].
7. Improvement in western blotting over last decade
Over last two decades, substantial improvements in the basic western blot technique have been reported. Modifications in the preparative phase (lysis buffers, homogenization), blotting (blotting reagents, apparatus, and procedures) and detection methods have all been reported. These modifications improve the time required, the reliability, and the reproducibility of the western blot. Several more sensitive alternative methods such as single cell-resolution western blot, far-western blotting, diffusion blotting, automated microfluid western blotting, western blotting using capillary electrophoresis and microchip electrophoresis have all been developed to overcome problems associated with western blotting. The most recent technologies to improve the sensitivity and reproducibility of western blotting are discussed below.
7.1. Capillary and microchip electrophoresis (MCE)
Major limitations associated with western blot include its time-consuming nature, the requirement of a relatively large amount of sample (usually 10–20 μg/assay), and they usually detection of only one protein at a time. Moreover, it requires detection of housekeeping proteins or total proteins on the membrane to normalize the target bands detected [23]. To address these issues, a hybrid between capillary electrophoresis for SDS-PAGE and conventional blotting for the western part, referred to as capillary and microchip electrophoresis (MCE) based western blotting was developed by Jin et. al. (2016) [24]. Compared to conventional western blotting technique, MCE has a higher sensitivity with better resolution that allows measurement of multiple target proteins from a single cell lysate sample. This approach eliminates blocking steps and requires shorter analysis time (approx. 8 min for electrophoretic resolution). MCE allows the performance of parallel multiplexed assays of a group of proteins using a small sample amount. In brief, multiple injections of the same protein samples are loaded in separate tracks on a microchip and are captured on a PVDF membrane for immunoassay. Using only 400 ng of Jurkat cell lysate sample MCE was applied to measure 11 different proteins including ERK1/2, MEK1/2 AKT, STAT3, phosphor-ERK1, phospho-ERK2, and β-tubulin [24]. Several of these protein targets show very little difference in molecular weight but were detected by MCE. This method is still being developed and is unlikely to be available for the average researcher within the next few years. Further improvements to this technique would allow significant improvements in multiplexing protein detection.
7.11. Automated microfluidic protein immunoblotting and single cell-resolution western blotting
Another automated microfluidic protein immunoblotting technique was developed to save time, avoid multiple assay steps and limit equipment and reagents requirements. This automated protein immunoblotting is a programmable controlled technique (i.e. voltage control and pressure) that combines PAGE with blotting in one device [39]. The technique allows the integrated assay steps (PAGE, transfer and in-gel blotting) to be viewed using an epifluorescence microscope equipped with a charge-coupled device camera. In this method photo patterning (photochemical etching) of polyacrylamide gels is done on microfluid glass devices that act as a platform to integrate the multiple assay steps. The method is rapid and was employed to detect free prostate specific antigen in human seminal fluid sample in less than 5 min. This method is economical and lowers the consumption of reagents as the glass chips that are used are reusable after simple chemical treatments. The technique is still in process of development to further improve sensitivity and enable protein quantitation [39].
Single cell-resolution western blotting was developed to detect individual cell-to- cell variations in protein expression between cells [40]. The microdevice used for this assay consists of a thin layered polyacrylamide gel with micro wells. The micro wells in the gel layer are loaded with single cell protein samples and are lysed chemically in each well to obtain single cell lysate to be resolved on PAGE. Subsequently, the proteins are immobilized on the same gel using ultraviolet (UV) light and are probed with antibodies for immunoblotting. Multiplexing of a single cell is achieved by incubating immobilized proteins with different antibodies using stripping/ re-probing protocols. From a single cell, multiplexed data (more than 10 proteins) was obtained within a time of 4–6 hrs. The microdevice used can be manipulated for the gels having either uniform or gradient pore-size, and proteins of similar or different molecular mass can be analyzed at the same time. For example, the use of a gradient polyacrylamide gel allowed target proteins ranging from 25 to 289 kDa molecular mass to be detected in breast cancer cells [40]. The highly sensitive single cell-resolution immunoblotting has also been applied to study protein variations in stem cell signaling and differentiation [41]. A recent commercial product called Milo™ (ProteinSimple) is the world’s first Single-Cell Western platform. Milo won the Scientists’ Choice Awards Best New Life Sciences Product of 2016. Milo™ is capable of measuring protein expression (up to 4 proteins per cell simultaneously) in around 1000 single cells in about 4 hours.
7.12. Simple Western
The first reported use of the imaged capillary IEF (iclEF)-based method for western blots was by O’Neill et al. [42]. A few years later, a microchip capillary electrophoresis (CE)-SDS combined with immunoprecipitation was utilized as a western blot alternative [43]. In 2010 a new instrument, Simple Western™, which utilized similar technology to the latter method, but separated proteins based upon size instead of charge was reported [44]. Scientists from Merck Research Laboratories showed that quantitation of Simple Western™ western blots using vaccine sample preparations had a coefficient of variation (CV) of < 10% [45]. Quantitative western blotting was found to be associated with an error of >35% standard deviation [46]. Investigation of the source(s) of the error suggested that the antibody interaction and the 5-bromo-4-chloro-3-indolylphosphate (BCIP) and nitro blue tetrazolium (NBT) color development reagents were the major sources of the error [46].
Simple Western developed by ProteinSimple (San Jose, California) is based on CE-SDS where the separated proteins are attached to the wall of capillary by a proprietary photo (ultraviolet) activated chemical crosslink [47]. Each capillary contains separation gel matrix and stacking matrix proteins are separated based upon size using high voltage. Subsequent blotting is done automatically by washing the capillary to remove the gel matrix, then incubating and washing the capillary with primary and secondary antibodies conjugated with horseradish peroxidase. The horseradish peroxide is detected by its signal generated upon exposure to luminol and an electropherogram trace or a virtual gel image displayed. The system combines immunoassay, detection, and data analysis in one machine [48]. For assay the prepared protein samples together with required antibodies and a molecular weight marker are loaded in the plate prefilled with separation matrix and buffers. Once the assay begins all steps ranging from loading and separation of sample protein, immobilization of samples in the capillary, immunodetection by primary and secondary antibodies, and quantitation are automated. The advantages of the Simple Western technique includes: relatively fast procedure, requires small sample amounts, can perform analysis using 24–90 different antibodies simultaneously, can carry out standard curve quantification similar to ones done using high pressure liquid chromatography (HPLC), and potentially improves reproducibility [44,45,49,50]. The technique was recently employed to measure mitogen-activated protein kinase (MAPK) signaling proteins in the sex-determining stage of gonadal development [51]. Although this technique has been available for about 10 years, the number of publication using this technology has significantly increased over the last few years [45,52–54]. The disadvantages include the need for specialized reagents, and the small number of independent comparisons comparing this system to the classical western blotting method [54].
7.2. DigiWest
Several technologies are vying to replace traditional western blotting. One method, a high-throughput western blotting procedure called DigiWest, is a modified version of the western blot method which increases the throughput of western blotting [55]. The DigiWest method separates proteins by electrophoresis and transfers proteins to blotting membrane as usually done for western blots. The next step is to biotinylate (using amino-reactive biotinylation reagents) proteins on the membrane and cut the membrane into 96 strips to create 96 different molecular weight fractions that are on the membrane. The proteins on these strips are eluted into wells in 96 well plates that contain neutravidin-coated Luminex beads. Each well contains a different distinct bead set with different color codes. After pooling the beads, the components of the entire lane are now reconstructed with different color codes corresponding to the molecular weight of the immobilized proteins. Using a small aliquot of the bead pool, new wells are then incubated with antibodies overnight and then phycoerythrin-labelled secondary antibodies added to detect the primary antibodies used. Detection of the phycoerythrin is done using a Luminex FLEXMAP 3D instrument. The advantages of this method are the increased throughput of the samples, lower amounts of lysate require/target detection, and lower amounts of antibody required. The disadvantages of this method include the modification of the target proteins by biotinylation, the need for specialized reagents and equipment, the initial startup costs, concerns about the translational of the digital DigiWest result to western blots mimics, and specifics about certain procedures such as elution of proteins from PVDF membranes which has proven to be difficult.
7.3. Micro-loader
Several modifications of the standard technique have resulted in improved sensitivity of PAGE and the western blot assay. One such method involves a funnel-like structure sample micro-loader device to load samples. The device is attached to the top of polyacrylamide gel and filled with 4% stacking gel solution through the outlet of the tips via capillary action. The protein in a sample that travels through the transfer pipette is concentrated by electrophoresis. The incorporation of micro-loader device in gels improved both protein separation and resolution. This technique was able to detect number of proteins and phosphoproteins in each sample by loading only 1.5 μg of protein per lane. This technique has the advantage of being relatively simple to do and would be very useful to measure protein expression and phosphorylation in samples that are limited, rare and difficult to obtain, as it require very small amount of loading samples for signal detection [1].
7.4. Thin-film direct coating with suction-western blotting
High consumption of expensive antibodies and operational time are current drawbacks of western blotting. Thin-film direct coating with suction (TDCS) is a capillary-tube based approach designed to reduce antibody consumption and time required for western blotting [56]. This is a highly sensitive and rapid detection method for quantitative analysis of multiple antigen-antibody interactions. The operational time for TCDS is much shorter (about 5 min) than conventional western blotting with increased signal-to-noise ratio. The TCDS method gave a high dynamic detection range for purified recombinant glutathione-S-transferase (GST) proteins (90–6000 ng). In this method proteins are resolved on SDS-PAGE by electrophoresis and are transferred to PVDF membrane for 1 hour using 10 mM 3-(Cyclohexylamino)-1-propanesulfonic acid (CAPS) transfer buffer (pH 11) with 15% methanol. The PVDF membrane is placed on a stage and allowed to air dry for 10 min. Thereafter, the primary antibody solution (0.1 −0.2 μl) is added to the coater (super light-and-slim slot die coater or capillary tube) mounted on a translational stage for coating the membrane via capillary force/liquid pump. The coating process is less than one minute and is programmable. The gap between the coater and the PVDF membrane is controlled. The PVDF membrane is then incubated for 2–10 min followed by subsequent washing steps for less than one minute with suction (~30 cm Hg) and again coated with secondary antibody using a similar coating process [56]. Thus, this technique is fast and enables quantitative analysis of multiple protein interactions. This technique is also still in the experimental stage and it is likely that it would be a few years before other laboratories utilize it.
7.5. Improvement in techniques to transfer proteins from gel to membrane in western blotting
The different approaches that have been employed by various research groups to transfer resolved proteins from SDS-PAGE gels to PVDF or nitrocellulose membranes is summarized in Table 2.
Table 2.
Different western blot methods to transfer proteins from gel to membrane
| Methods | Brief Description | Publication s using this method * | Notes | References |
|---|---|---|---|---|
| Vacuum blotting | This technique is based on a suction force of a pump to transfer proteins from a gel to a nitrocellulose membrane connected to a slab dryer system. | [100–104] | Efficiently transferred hemolymph proteins and homogenates. | [4,105] |
| Centrifuge blotting | Protein is eluted and transferred to PVDF membrane by centrifugation instead of electro blotting. SDS-PAGE gel with protein of interest was immersed in 1M KCl for 2 min. The clear protein bands were then cut and centrifuged at 3000 × g for 1 hr along with PVDF and dialysis membranes after soaking them in the eluent. | [106,107] | Dialysis membrane was used to retain non- immobilized proteins which was placed below PVDF membrane | [4] |
| Multiple tissue blotting | Premade human multiple tissues western (MTW) blots allow detection of tissue-specific protein expression. Whole human tissues proteins are extracted, fractionated by SDS-PAGE gels, transferred to PVDF membranes and are incubated with antibodies specific to target protein. | [108] | Allows western blotting of human tissues by laboratories that do not usually have access to this tissue. | [109] |
| Electroblotting of proteins to Teflon tape and membranes for N and C terminal sequence analysis | Proteins are electro transfer to low-density Teflon tape and GORE-TEX expanded polytetrafluorethylene membranes. These membranes were soaked with absolute ethanol before electrotransfer. Following transfer blots were stained with 0.005 % sulforhodamine B in 30 % methanol for 10 min to visualize proteins. | [110] | Teflon blots were found to be suitable for amino acid analysis, in situ proteolytic digestion, N- and C- terminal sequencing and are inert to the chemistry used on C-terminal sequencers. | [4] |
| Two-step transfer of low and high molecular -weight proteins | This is a two-step method for transfer of high- molecular-weight (greater than 400,000) and low molecular-weight (less than 20,000) proteins to nitrocellulose membranes from polyacrylamide gels. In first step low-molecular-weight proteins are eluted for 1 hr at a low current density. Followed by the second step of prolonged electro-transfer at high current density for 16–20 hr. To enhance protein transfer SDS at a 0.01 % concentration was added to transfer buffer. In addition, after electrotransfer the nitrocellulose membrane was air-dried to minimize protein loss during subsequent processing. | [111–114] | This method is suitable for all kind of polyacrylamide gel systems as well as for proteins obtained from various cell types. | [4,109,111] |
| Transfer of high and low molecular weight proteins using heat | The high and low molecular weight proteins are efficiently transferred to nitrocellulose membrane with the use of normal transfer buffer that was heated to 70–75 °C. The electrotransfer was done for 10 min/ 7% SDS-PAGE (0.75mm) gels, 15 min/10% and 12.5% gels (0.75mm) gels and 20 min/7%, 10% and 12.5% (1.5 mm). | [26,115,116] | Rapid and efficiently transfer both low and high molecular weight proteins. Heating increased the gel permeability allowing easy transfer of proteins trapped in gel matrix. |
[26] |
| Semi-dry Electroblotting of peptides and proteins from acid-urea polyacrylamide gels | Used low pH PAGE system by adding acidic urea to resolve various proteins and peptides that otherwise would not able to be resolved by SDS-PAGE. Proteins are transferred to PVDF membranes at 115 mA and 5 V for 15 min in a transfer solution that contains 5 % acetic acid. | [117] | Faster and convenient method used which can be used for DNA and RNA electroblotting as well. | [4] |
| Transfer of silver- stained proteins from polyacrylamide gels to PVDF membranes | Silver-stained proteins are efficiently transferred to PVDF from a polyacrylamide gel by rinsing the gel in 2× SDS Laemmli buffer prior to transfer. | [118,119] | Some silver stained proteins were found to be transferred directly without the rinsing of gel in Laemmli buffer. | [4,120] |
| Enhanced protein recovery using square wave alternating voltage after electro transfer | In this method, the combination of square wave alternative voltage (SWAV) and CAPS buffer has been used to transfer protein from gels previously soaked in deionized water and equilibrated two times in the cathodic blotting buffer for 5 min. | [121] | Compared to standard blotting technique 65 % more protein was recovered using this technique. | [4,122] |
| PEG-mediated immunoblotting transfer | Proteins separated on SDS-PAGE were soaked for 2 hr in a 30 % PEG 2000 buffer to fix the proteins. This was followed by electrotransfer of proteins from the gel to PVDF membrane for 24 –48 h at 200 mA/120 V in a −20 °C freezer. | [123,124] | PEG 1000, 1500 & 2000 increased the sensitivity of immunoblotting by 10–100 fold. However, PEG less than 1000 had no have remarkable effect. | [4,125] |
| Acid electroblotting on activated glass | Proteins were electrotransferred to activated glass instead of nitrocellulose or PVDF to obtain sub picomolar levels of proteins. The activated glass fiber paper support was found to be extremely stable for sequencing conditions. To activate glass various treatments were employed: (a) Acid Etching: Whatman GF/C or GF/F sheets were treated with TFA in a Petri dish for 1 h at room temperature and then dried completely. TFA treatment can adsorb about 7—10 μg/cm 2 of proteins. (b) Acid Blotting: After SDS PAGE the gels were incubated in 0.5 % acetic acid containing 0.5 % NP-40 for 10 min at room temperature. At low pH, the positive charged proteins were electro transferred to glass fiber paper. |
[23,126] | Both nitrocellulose and nylon membranes are not suitable for sequencing chemistry. The underlying mechanism for protein adsorption on activated glass fiber was due to the ionic interaction of the positively charged proteins with the negative charges on the glass fiber sheet. |
[4,23] |
| Wet transblot | In this method, the nitrocellulose or PVDF membrane is sandwiched between the gel, filter papers and a support pad facing anode. The transfer sandwich is secured in a cassette and is submerged inside a Trans-blot buffer tank with platinum wire electrodes and filled with cold transfer buffer. Finally, electrotransfer is carried out to transfer proteins from gel to membrane. | >1000 | Good transfer efficiency, but requires large volume of transfer buffer with methanol. Transfer is carried out by placing an ice pack within the transfer tank or by placing the whole apparatus in a cold room. | [109] |
| Fast transfer (Rapid electroblotting) | This is a recent technique developed by Thermo Scientific for rapid transfer of proteins from gel to membrane using the Pierce™ G2 Fast Blotter. The technique utilizes the combination of high-ionic strength transfer buffer and a high current power supply (10 fold or more) that increases the transfer efficiency like wet or semidry transfer and can be achieved in 5–10 min. |
None | Efficiently transfer of low, medium and high molecular weight proteins in just 10 min. | [127] |
| Trans-Blot Turbo Transfer | Highly efficient and rapid method for protein transfer that allows transfer of protein in just 3 min. The Trans-Blot Turbo Transfer utilizes an optimized buffer, membrane, filter paper and the Trans-Blot Turbo blotting apparatus. | >200 | Transfer mini gels in 3 min Higher transfer efficiency and high throughput |
[128] |
other than the original paper describing the method. Abbreviations: polyvinylidene difluoride (PVDF); 3-(cyclohexylamino)-1-propane- sulfonique acid (CAPS); polyethylene glycol (PEG); trifluoracetic acid (TFA); Nonidet P-40 (NP-40); matrix-assisted laser desorption/ionization (MALDI); enzyme-linked immunosorbent assay (ELISA); hINF (human interferon)-γ.
7.6. Improvements in transfer methods
One of the utilized changes in western blotting technology is the development of faster methods to transfer proteins from gels to membranes. These include the Trans-Blot Turbo (3–7 minutes transfers, Bio-Rad, USA), the iBlot® dry blotting system (5–7 minute transfers, Thermo Fisher Scientific, USA), and the Pierce™ Power Blot Cassette (5–10 minute transfers, Pierce, USA). The use of these new methods significantly reduces the transfer time from 1 hour or overnight to 10 minutes or less. My laboratory has not used wet transfer methods for over two years now.
7.7. Improvements in Polyacrylamide gels
Many companies have introduced pre-made gels with better protein separation abilities, longer shelf life (typically one year, but we have found that they can last several months longer than the expiry date), faster running times, and are cheaper than pre-made gels just 5 years ago.
7.8. Improvements in retention of peptide hormones on blotting membranes
The retention of insulin on PVDF membranes was found to be low [57]. Western blotting of the insulin and proinsulin was found to be significantly improved by treating the PVDF blotting membrane with 0.2% glutaraldehyde in Tris buffer saline containing Tween 20 (TBST) for 15 minutes. The glutaraldehyde fixation prevented or reduced the amount of insulin lost from the membrane during western blotting. Utilization of a citrate retrieval buffer (10 mM citric acid, 1 mM EDTA, 0.05% Tween 20, pH 6.0) slightly enhanced the signal of both insulin and proinsulin [57].
7.9. Diffusion blotting
Diffusion blotting is simple and fast method to develop multiple blots from single precast SDS-PAGE gels on plastic support. Through diffusion blotting, multiple proteins can be tested on identical blots made from same gel. It was demonstrated that through diffusion blotting about 10 % of protein was transferred in 3 min, 20% of protein was transferred after 20 min, and 45–65 % was transferred after 3 hours. Compared to electroblotting, diffusion blotting may increase the amount of both high and low molecular weight proteins that are efficiently transferred to the membranes. This method allows complete transfer of proteins from ultrathin gels of 0.1 mm-0.2 mm size on plastic support, which are otherwise difficult to separate using standard electroblotting methods [58].
7.10. Western blot analysis using secondary antibody-detecting molecular weight marker
In general, most molecular weight markers to determine the size of specific proteins in immunoblotting are proteins conjugated with dyes. However, use of dyes for protein conjugation often modulate the electro mobility of proteins and may affect accurate molecular weight determination [59,60]. Molecular weight markers conjugated with dyes are usually calibrated with unstained standards and many unstained protein ladders are available but they are invisible when blots are being imaged. To overcome this issue, new molecular weight standards that are dye free and auto-detectable such as green fluorescent protein (GFP) ladder [61], Protein A/G-based protein ladder [62] and Easy See Western marker (from Spark Biologicals Technology) [63], have been developed. However, one limitation with the use of this class of markers is that they must be used under non-denaturing conditions for precise molecular weight determination. Another class of denaturable markers were developed such as mega-Tag ladder [64], hex histidine tagged and S-tagged marker (9). However, these markers need tag-specific primary antibodies for detection and are not suitable for tag-free protein measurements. A new mouse and rabbit linear epitopes (M&R LE) molecular weight standard for SDS-PAGE and immunoblotting was developed to provide easy and precise analysis of proteins size [59]. The M&R LE marker contains linear antibody-binding epitopes derived from heavy chain constant regions of mouse and rat immunoglobulin G. Compared to conventional protein markers, M&R LE marker is dye free, auto-detectable, and can be recognized by both horseradish peroxidase-conjugated mouse and rat secondary antibodies in denaturing conditions, and can be used as a positive control for secondary antibodies [59]. Another concept using antibody-binding epitopes, the MagicMark™ XP western protein standard, was developed by Invitrogen.
MagicMark™ contains 9 recombinant proteins, each of which contains an IgG binding site that can interact with the primary or secondary antibody used for target protein detection. The IgG binding site on the recombinant proteins can interact with many host species including human, mouse, and rat, allowing direct visualization of the standard on the western blot.
7.10. Co-detection of target and total protein by Cydye labeling and fluorescent ECL plex immunoblotting
Western blotting is commonly applied to one-dimensional gel electrophoresis to detect target proteins using antigen-antibody interactions. However, application of immunoblotting to two-dimensional gel electrophoresis (2-DE) which can identify thousands of proteins on one gel is complicated due to the problem in identification of spot correlation (reproducibility) between immunoblots [65]. To counteract this problem an ECL Plex CyDye immunoblot detection system that combines differential gel electrophoresis (DIGE) labeling with ECL Plex CyDye conjugated secondary antibodies was developed. This approach allows simultaneous immune-detection of specific target proteins as well as the total protein expression. After labeling protein samples with cyanine CyDye reagents, before they are resolved on one-dimensional and 2-D gel electrophoresis, the samples are run on the gel and the separated proteins immobilized into membranes. The immobilized proteins are incubated with specific primary antibodies and fluor-labeled ECL Plex CyDye secondary antibodies. The proteins are then visualized using a fluorescent imager at specific wavelengths [66]. Although this process has high sensitivity the modification of proteins by the CyDye may affect antibody-antigen interactions.
7.11. Micro-Western
The micro-Western is an efficient next generation western blot technique employed for confirmatory diagnosis of purified gp120 and p24 HIV proteins in human serum [67]. Compared to conventional western blotting, Micro-Western is an automated advanced microchip technique with up to 5-plex protein detection in a single microchannel, 54-plex channel throughput, requires total 10–80 min operational time and is economic (103 fold less antibody and buffer consumption than basic western blot). Although not yet commonly available, the Micro-Western is likely a suitable choice to exploit as a diagnostic tool for cancer biology and infectious diseases [67].
7.12. iBind™ Western System
A relatively inexpensive semi-automated western blotting system, the iBind™ Western System (Thermo Fisher Scientific), utilizes sequential lateral flow technology (capillary action) to distribute antibodies and reagents that are placed in different compartments. The advantages of this system include the low cost, the lower amount of antibody needed, and once the reagents are loaded the relatively small system does the primary and secondary antibody incubations unassisted. The concerns about this system include the low through-put of the device as well as the need for specialized materials such as a Bind card.
7.13. BlotCycler™
The BlotCycler™ (Precision Biosystems) performs complete automation of all membrane processing steps and allows the processing of up to twelve membranes simultaneously. Some advantages of this system are lower antibody requirements than conventional blots, the primary antibody is automatically saved, and the increased throughput. Non-manufacturer comparisons about how the BlotCycler™ compares to conventional western blotting technique is limited.
7.14. SNAP i.d.® 2.0 system
The inexpensive SNAP i.d.® 2.0 system (MilliporeSigma) uses a vacuum-based technology and a flow distributor to thrust reagents through the membrane in an evenly distributed manner. The main advantage of this system is the reduced time needed for blocking, antibody incubations and washing (about 30 minutes). In our hands, this system worked well but the signal to noise ratio of the bands detected were not as good as the classical western blotting method (unpublished results).
8. Relationship between improved western blot methods and its clinical implications
Western blotting is a valuable tool to studies ranging from regulatory signaling processes to confirmatory serum diagnosis of HIV [68–70]. The evolution of western blot technique from identification of a specific protein in a complex mixture to the direct detection of protein in a single cell allows this technique to be an important analytical tool for clinical research. An advanced single cell western blotting technique was employed to study stem cell signaling and differentiation as well as drug response in tumor cells [69]. Through single cell western blotting it was possible to analyze cell-to-cell variations in approximately 2000 cells simultaneously within complex populations of cells [71]. With the integration of intact cell imaging, the technique allows the identification of protein expression changes of a single drug resistant tumor cell and its isoforms among heterogeneous population of tumor cells in human glioblastoma cells treated with chemotherapeutic daunomycin [69]. Identification of upregulated multidrug resistant protein, P-glycoprotein in living glioblastoma subpopulations was indicative of an active drug eflux pump as an underlying mechanism for drug resistance [69,71]. With the application of 2-DE gel separation together with spotting of protein by peptide mass fingerprint, the analysis of clinically relevant Helicobacter pylori (H. pylori) in related gastric disease conditions (chronic gastritis, duodenal ulcer) was possible [72]. The database of H. pylori (low expressed and membrane proteins) was created through the application of one-dimensional or 2-DE/MALDI-mass spectrometry techniques [72]. In a similar manner, the Simple Western technique was employed for the analysis of 15-valent pneumococcal vaccine PCV15-CRM197 [73]. Due to its high sensitivity and automation, the Simple Western method may be extended to analyze serotypes of other polysaccharide protein conjugate vaccines [73].
Western blotting is commonly used for the clinical diagnosis of various parasitic and fungal diseases including echinococcosis [74], toxoplasmosis [75], and aspergillosis [76]. In a recent study, the assay was successfully used for the reliable serodiagnosis of Farmer’s lung disease (FLD), a pulmonary disorder caused by inhalation of antigenic particles [77]. Thus, this technique can be exploited for rapid routine diagnosis of FLD in clinics [77]. Similarly, for immunodiagnostic of tuberculosis meningitis which is a chronic disease of central nervous system different molecular and immunological methods were used for clinical diagnosis of the disease. However, each of these techniques has their own limitations [78]. To overcome diagnostic issues of lower sensitivity and specificity, the immunoreactivity to Mycobacterium tuberculosis antigens was performed by western blotting [78]. Furthermore, western blotting was performed for the early and sensitive diagnosis of congenital toxoplasmosis [79] and was employed for rapid and sensitive serological diagnosis of a serious infectious disease paracoccidioidomycosis (PCM) [80]. Using immunoblotting, a new subgroup of human lymphotropic retroviruses (HTLV), was detected in patients with the acquired immunodeficiency syndrome (AIDS) [81]. Antigens of HTLV-III, specifically detected by antibodies in serum from AIDS or pre-AIDS patients [81]. Western blotting has also been used as a test for variant Creutzfeldt-Jakob Disease [82], some forms of Lyme disease [83] and is sometimes used as a confirmatory test for Hepatitis B [84] and Herpes Type 2 [85] infections. Western blots have also been used to confirm feline immunodeficiency status in cats [86].
Recently, a commercial Aspergillus western blotting IgG kit was developed by LD Bio Diagnostics (France) to carry out immunoblotting for the clinical diagnosis of chronic aspergillosis. The commercial kit was found to be sensitive and can analyze hundreds of samples from patients with aspergillus disease [87]. Thus, the clinical applications of western blotting technique will continue to progress as further advancements are made to improve sensitivity and reproducibility of the western blot.
9. Conclusion
Significant advancements have been made to solve various issues related to western blotting. Kits (Thermo Scientific Pierce Fast Western Kits) and streamlined procedures (Bio-Rad’s V3 Western Workflow™) are available to make western blotting faster and more quantitative. Advances in gel preparation, enhanced chemilumescence reagents, fluorescent secondary antibodies, and chemiluminescence and fluorescence imagers have all occurred at a rapid pace over the last five years as companies realize the large amount of money spent on the western blotting technique every year. Conventional western blots can be completed within one hour using fast western kits. While these advances are significant, the major advances in the last five years is the introduction of non-traditional western blotting concepts, mainly based upon microfluidic systems and capillary electrophoresis. Microfluidic systems drastically reduce both the time require for western blotting as well as the amount of antibodies and reagents required. These systems also are easier to adapt to systems which allow high throughput.
While the future looks better than it has ever looked for western blotting, several non-equipment related problems still need to be addressed. The choice of primary antibody and its user validation by positive and negative controls is critical to avoid non-specific binding of proteins and subsequent inaccurate western blot results. The optimal choice of homogenization buffer for sample preparation is not known for many samples and untrained or inexperienced researchers often lack a basic understanding of the technique complicating data collection and often resulting in misinterpretation of western blot results.
10. Five-year view
Over next five years, it is likely that the expansion and improvement in the available western blot equipment, blotting reagents, software, digital detection systems and quantification methods will increase at a faster rate than the previous years based upon the number of new western blot products released in the last two years. Many companies are investing in next generation western blotting products. Licor was awarded a small business innovation research (SBIR) grant from the US federal government to create a new automated microfluid direct blotting technique [88].
Further improvements and innovation in multiplexing to study protein expression will continue with the development of new fluorochromes. Additional enhancements to commercially available digital detection systems will continue and prices will continue to fall as the cost of the high resolution and low light condition cameras decrease. The use of polyclonal antibodies will decrease relative to monoclonal antibodies. The reduction in availability of polyclonal antibodies to many targets was partly due to the inability of Santa Cruz Biotech (USA) to sell polyclonal antibodies following the loss of its license under the Animal Welfare Act to use rabbits or goats [89]. Santa Cruz Biotech was one of the largest suppliers of polyclonal antibodies, however, the company is now focusing on generating a large number of monoclonal antibodies.
Substantial progress will be made to enhance the sensitivity and specificity of antibodies for unique phosphorylation sites. Efforts are being made to improve the efficacy of monoclonal antibodies. By modulating the antigen-binding fragment (Fab) and crystallizable fragment (Fc) region of monoclonal antibodies, bispecific antibodies with a wide range therapeutic applications will be developed [90,91]. These bispecific antibodies are artificial molecules that recognize two different epitopes either on the same or on different antigens and are still in a process to development [92]. The use and availability of recombinant antibodies will significantly increase as recombinant antibodies to complex post-translational modifications such as tri-methylated lysine residues of histone H3 have already been successful and used to study histone post-translational modifications [93].
Important advancements in the automated techniques like Simple Western and microfluidic protein immunoblotting will be made by introducing variations in the separation matrix, capillary flow, capillary materials, and automated imaging and analysis software. The amount of sample (reduced from micrograms levels to nanograms levels) and antibody needed for western blotting will continue to decrease because of new equipment as well as improvements in ECL reagents and detection systems. With the availability of more reliable, higher sensitivity, and faster western blot assays, the analysis of individual cells from different patients is already possible and will be adopted by more laboratories to investigate different diseases.
Western blotting will continue to be an important analytical tool to provide mechanistic insights into the molecular processes associated with various metabolic disorders and will continue to help in designing novel therapeutic approaches against different disorders. Improvements in the western blot technique over the next five years will result in significantly more clinical studies being conducted using this technique. In the next decade small, high throughput automated western blotting devices that contain automated analysis software are likely to challenge ELISA’s for diagnosis of some human infections. Western blotting is already a powerful method for detection of viruses in human serum such as the zika virus [94].
11. Expert Commentary
Although the western blotting procedure is extensively used in most basic and some clinical research settings and is a very sensitive technique, it is prone to errors, if important aspects of the method are ignored. Procedural discrepancies, lack of expertise, and poor interpretations of data all generate inaccurate results. To continue to improve the technique many improvements are needed including the proper characterization of different lysis buffer and development of improved lysis buffers to enhance the quality and quantity of sample proteins obtained from cells or tissues.
A major concern with western blotting is associated with the quantification step. Most semi-quantification of targets relies on loading controls to normalize the results. However, all loading controls seem to be altered under some experimental conditions. Often, when incorrect results are obtained the problem usually is due to loading control and the target protein band intensities are not within the same linear range. Using tubulin as a loading control to determine protein expression of a specific gene in mice the target protein was found to be significantly reduced in the mutant group relative to the control group [95]. However, this result was due to more sample amount being loaded for the wild type group, as there was no difference in the protein expression between the two groups when same amount of sample was loaded [95]. The expression of housekeeping proteins also varies with type of tissues and different experimental conditions. Under such conditions relative quantification of protein expression is misleading. To address these problems, the use of a total protein stain as an alternate loading control is suggested. The protein loading steps of western blot method should be optimized to ensure linear dynamic range of sample protein and loading control. However, quantification of protein levels from different samples on different blots is still challenging, and should be avoided if possible.
Problems are still encountered when blotting high molecular weight proteins. Due to their larger size (>200 KDa) these proteins are poorly resolved on gels and are sometimes not efficiently transferred. The use of tris-acetate (3–8 %) gels and addition of 0.05 % SDS in the transfer buffer is a suitable choice to achieve efficient resolution and transfer of larger proteins. It is known that many proteins exist as several isoforms and can appeared as corresponding multiple bands in western blots [96]. In general, it is common to crop the extra bands on the blot and present only the band of interest for a research article, sometimes giving the misinterpretations of the presence of only single form of protein. On other hand, the information related to the isoform-specificity of the antibodies used is most often not well documented thus, negatively affecting the characterization of a given protein of interest.
Because of problems with batch to batch reliability of polyclonal antibodies the use of monoclonal antibodies should be preferred, if both polyclonal and monoclonal antibodies are available. The western blotting technique will continue to be improved and will offer novel possibilities for research and clinical diagnosis of diseases. However, to advance at a faster rate, major journals and organizations such as the National Institute of Health should require better reporting and documentation of western Blotting performed in manuscript. This can easily be done by accepting a standardized method for reporting the western blotting technique used.
Table 3.
An overview of western blotting procedure, key issues and possible solutions to address these issues.
| Steps in Western blotting | Major issues | Possible Solutions | References |
|---|---|---|---|
|
Sample preparation a) Protein extraction and purification |
Frequent freeze/thaw cycles of tissue samples. Time gap between tissue collection and its freezing. Use of inappropriate lysis buffer. Poor homogenization techniques. Erroneous sample preparation and proteolytic degradation. |
Optimize lysis buffer (detergent, salt, EDTA concentrations, pH) to prevent proteolytic degradation and high yield of target proteins. A suitable homogenization method must be chosen that can efficiently release the intracellular contents of the cell. Homogenization technique should be optimized for isolation of target proteins. To avoid endogenous proteolytic degradation samples should be quickly harvested in ice cold buffer (neutral pH) and immediately frozen in liquid nitrogen and stored at −80 °C until use. Multiple freeze/thaw cycles of protein samples must be avoided. |
[3,5–7,19,129] |
| b) Measurement of protein concentration | Incorrect protein quantification due to incompatibility of buffer components. Reducing agents such as DTT often interfere with protein quantification methods. |
Use protein quantification methods that are compatible with concentration of reducing reagents used in the lysis buffer. Appropriate lysis buffer must be used as blanks and in the standard curves to account for interference by buffer components. |
[3,19] |
| c) Sample loading amount | Protein of interest is not within its linear detection range. Detection of low abundant proteins. Detection of post-translational modified proteins. |
The amount of protein loaded on gels should be optimized to ensure linear dynamic range of sample protein and loading control in the blot. Use of advanced automated techniques such as capillary electrophoresis based western blotting methods may be useful in detecting low abundant and post-translationally modified proteins. |
[7,23,24,40] |
|
Gel electrophoresis Polyacrylamide gel |
SDS-PAGE gels are not properly casted. Proteins are not properly resolved on gels. | Proper sample separation on SDS gels depends on protein samples containing SDS, DTT or 2-mercaptoethanol. Samples must be denatured by heating prior to loading. Optimization of separation conditions for target proteins (percentage of acrylamide, bis-acrylamide ratio) as well as amount of protein loaded on the gel for detection must be optimal. Routine check of pH of buffers used for electrophoresis is useful. |
[3,19] |
|
Blotting Transfer conditions and membrane |
Poor and insufficient protein transfer. Low transfer efficiency of high and low molecular weight protein. Use of low quality membrane for transfer process. High background staining. Inefficient binding of protein to membrane. |
Low molecular weight proteins can be efficiently transferred with the use of high quality nitrocellulose and PVDF membrane. Transfer conditions for both high and low molecular weight proteins must be optimized. Background fluorescence can be optimized using different combinations of blocking solutions and antibody concentrations. Use fresh transfer buffer and pre-stained markers to ensure optimize transfer. | [19,26] |
| Blocking | High amount of non-specific proteins on membrane and high background. | Optimize blocking time and solutions. Increase the percentage of non-fat dry milk or BSA used for blocking and antibody solutions. | [7,19] |
|
Labelling Primary antibody |
Multiple bands recognized by antibody tor the target protein. No bands or taint bands. Lot-to-lot variability in antibody. Lack to proper antibody vacation. |
Optimize primary antibody dilutions, incubation time and temperature, and number to washes in washing butter. To minimize background, and non-specificity reduce primary antibody concentration and the amount to total protein loaded on gel. Validate all antibodies unless previously validate with lot specific data. Use to monoclonal antibodies over polyclonal antibodies is advantageous tor higher specificity to target protein. Use to recombinant antibodies has many advantages including the ability to engineer the antigen binding site, the ability to rapidly produce many variants to a specific antigen. Use positive and negative controls. |
[3,7,19] |
| Secondary antibody | Non-specific bands, high background, ghost bands. | Reduce secondary antibody concentration. | [3,7,19] |
| Detection and imaging | Saturate band signal. Signal too high or too low. |
Use shorter exposure time. Optimize incubation time and amount to enhanced chemiluminescent reagent. Use digital imagers over film-based processors tor higher sensitivity, and better linearity. Use fluorescent probes tor western blot. |
[7,19] |
|
Quantification Densitometry analysis to target protein using afferent software |
Inaccurate quantification to protein to interest, poor image quality, and signal saturation. | Optimize quantification using well established antibodies and sample lysates. Use two different software tor quantification. Use software that shows signal saturation. |
[7,15,19,35,129] |
| Normalization of housekeeping protein | Loading control and the target protein band intensities are not within the same linear range. The expression to housekeeping proteins can sometimes vary with type to tissue and aitterent experimental conditions. |
The linear dynamic range tor the total protein, the housekeeping protein ana the target protein needs to be determined and used within this range tor accurate western blots. Use ot a total protein stain as an alternate loading control (Ponceau S or Bio-Rad’s Stains-tree methods). |
[15,19,37,38] |
Abbreviations: polyvinylidene difluoride (PVUF); dithiothreitol (DTT); Sodium dodecyl sulphate (SUS); polyacrylamide gel electrophoresis (PAGE).
11. Key issues.
The availability of validated antibodies to target proteins is the main limitation for increasing the reliability of western blots. Several major initiatives are currently underway to produce better quality monoclonal and recombinant antibodies.
Each step of western blot protocol is crucial and has potential for variations and errors to occur. For reliable and reproducible western blot results care should be taken to use an optimized and standardized protocol.
Most laboratories that employ western blotting do not put significant emphasis on sample preparation. Sample extraction and purification step has a substantial impact on the quantification and interpretations of western blot results and should be optimized before use.
The development of new transblotting machines, improved imagers, ECL reagents and other components have all contributed to increased sensitivity and faster western blotting using the classical technique. However, throughput is still an issue.
The development of highly sensitive, automated, and advanced techniques such as single cell-resolution western blotting, Micro-Western, and Simple Western all reduce sample and antibody amounts required, and reduce the time needed to get results.
A lack of detailed non-manufacturer studies comparing the various automated methods available with the classical western blotting method is limiting the use of these automated techniques in academic research.
Due to the problems associated with housekeeping proteins, total protein staining is recommended as a better method for normalization of the western blot results.
To obtain better reproducibility between laboratories, standardized methods are needed for reporting western blotting results.
Acknowledgments
Funding
This paper received funding from the U.S. National Institute of Health and the American Heart Association.
Footnotes
Declaration of interest
A.V. Gomes has received funding from the U.S. National Institutes of Health and the American Heart Association and acted as a beta tester of Bio Rad imagers and analysis software. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interests
- 1.Vallejo-Illarramendi A, Marciano DK, Reichardt LF. A novel method that improves sensitivity of protein detection in PAGE and Western blot. Electrophoresis, 2013;34(8), 1148–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.He M, Herr AE. Polyacrylamide gel photopatterning enables automated protein immunoblotting in a two-dimensional microdevice. J Am Chem Soc, 2010;132(8), 2512–2513. [DOI] [PubMed] [Google Scholar]
- 3.Murphy RM, Lamb GD. Important considerations for protein analyses using antibody based techniques: down-sizing Western blotting up-sizes outcomes. J Physiol, 2013;591(23), 5823–5831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kurien BT, Scofield RH. Other notable protein blotting methods: a brief review. Methods Mol Biol, 2015;1312, 487–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bass JJ, Wilkinson DJ, Rankin D et al. An overview of technical considerations for Western blotting applications to physiological research. Scand J Med Sci Sports, 2017;27(1), 4–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. •.Janes KA. An analysis of critical factors for quantitative immunoblotting. Sci Signal, 2015;8(371), rs2 An important review which discusses the importance of sample preparation, protocol implementation, detection scheme, and the normalization approach on the quantitative performance of immunoblotting. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. •.Ghosh R, Gilda JE, Gomes AV. The necessity of and strategies for improving confidence in the accuracy of western blots. Expert Rev Proteomics, 2014;11(5), 549–560. The paper discusses the key issues that are associated with the western blotting technique and essential strategies needed for reliable and reproducible western blot results. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. •.Taylor SC, Posch A. The design of a quantitative western blot experiment. Biomed Res Int, 2014;2014, 361590 This manuscript highlights the key issues related to data analysis and describes important techniques to obtain good quality quantitative western blot results. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Glatter T, Ahrne E, Schmidt A. Comparison of Different Sample Preparation Protocols Reveals Lysis Buffer-Specific Extraction Biases in Gram-Negative Bacteria and Human Cells. J Proteome Res, 2015;14(11), 4472–4485. [DOI] [PubMed] [Google Scholar]
- 10.Murphy RM, Mollica JP, Lamb GD. Plasma membrane removal in rat skeletal muscle fibers reveals caveolin-3 hot-spots at the necks of transverse tubules. Exp Cell Res, 2009;315(6), 1015–1028. [DOI] [PubMed] [Google Scholar]
- 11.De Cecco L, Musella V, Veneroni S et al. Impact of biospecimens handling on biomarker research in breast cancer. BMC Cancer, 2009;9, 409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simpson RJ. Homogenization of mammalian tissue. Cold Spring Harb Protoc, 2010;2010(7), pdb prot5455. [DOI] [PubMed] [Google Scholar]
- 13.Cui Z, Hwang SM, Gomes AV. Identification of the immunoproteasome as a novel regulator of skeletal muscle differentiation. Mol Cell Biol, 2014;34(1), 96–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roman B, Gonzalez-Verdejo CI, Pena F, Nadal S, Gomez P. Evaluation of different pulverisation methods for RNA extraction in squash fruit: lyophilisation, cryogenic mill and mortar grinding. Phytochem Anal, 2012;23(6), 622–626. [DOI] [PubMed] [Google Scholar]
- 15. •.Ferguson RE, Carroll HP, Harris A, Maher ER, Selby PJ, Banks RE. Housekeeping proteins: a preliminary study illustrating some limitations as useful references in protein expression studies. Proteomics, 2005;5(2), 566–571. One of the first significant studies to show that housekeeping the limitations of several housekeeping proteins. [DOI] [PubMed] [Google Scholar]
- 16.Structural Genomics C, China Structural Genomics C, Northeast Structural Genomics C et al. Protein production and purification. Nat Methods, 2008;5(2), 135–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ghosh R, Hwang SM, Cui Z, Gilda JE, Gomes AV. Different effects of the nonsteroidal anti-inflammatory drugs meclofenamate sodium and naproxen sodium on proteasome activity in cardiac cells. J Mol Cell Cardiol, 2016;94, 131–144. [DOI] [PubMed] [Google Scholar]
- 18.Ghosh R, Goswami SK, Feitoza LF, Hammock B, Gomes AV. Diclofenac induces proteasome and mitochondrial dysfunction in murine cardiomyocytes and hearts. Int J Cardiol, 2016;223, 923–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.MacPhee DJ. Methodological considerations for improving Western blot analysis. J Pharmacol Toxicol Methods, 2010;61(2), 171–177. [DOI] [PubMed] [Google Scholar]
- 20.Williams SJ, White BG, MacPhee DJ. Expression of alpha5 integrin (Itga5) is elevated in the rat myometrium during late pregnancy and labor: implications for development of a mechanical syncytium. Biol Reprod, 2005;72(5), 1114–1124. [DOI] [PubMed] [Google Scholar]
- 21.Kopec AM, Rivera PD, Lacagnina MJ, Hanamsagar R, Bilbo SD. Optimized solubilization of TRIzol-precipitated protein permits Western blotting analysis to maximize data available from brain tissue. J Neurosci Methods, 2017;280, 64–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Butler TM, Elustondo PA, Hannigan GE, MacPhee DJ. Integrin-linked kinase can facilitate syncytialization and hormonal differentiation of the human trophoblast-derived BeWo cell line. Reprod Biol Endocrinol, 2009;7, 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aebersold RH, Pipes GD, Nika H, Hood LE, Kent SB. Covalent immobilization of proteins for high-sensitivity sequence analysis: electroblotting onto chemically activated glass from sodium dodecyl sulfate-polyacrylamide gels. Biochemistry, 1988;27(18), 6860–6867. [DOI] [PubMed] [Google Scholar]
- 24.Jin S, Furtaw MD, Chen H et al. Multiplexed Western Blotting Using Microchip Electrophoresis. Anal Chem, 2016;88(13), 6703–6710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krajewski SS, Tsukamoto MM, Huang X, Krajewski SB. Nonstripping “Rainbow” and Multiple Antigen Detection (MAD) Western Blotting. Methods Mol Biol, 2015;1314, 287–301. [DOI] [PubMed] [Google Scholar]
- 26.Kurien BT, Scofield RH. Western blotting of high and low molecular weight proteins using heat. Methods Mol Biol, 2015;1312, 247–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Uhlen M, Bandrowski A, Carr S et al. A proposal for validation of antibodies. Nat Methods, 2016;13(10), 823–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. ••.Prassas I, Brinc D, Farkona S et al. False biomarker discovery due to reactivity of a commercial ELISA for CUZD1 with cancer antigen CA125. Clin Chem, 2014;60(2), 381–388. Manuscript showed that a commercially available kit to detect CUZD1 actually detected a different protein CA125. Highlights the importance of validating the antibody for an key target protein. [DOI] [PubMed] [Google Scholar]
- 29.Prassas I, Diamandis EP. Translational researchers beware! Unreliable commercial immunoassays (ELISAs) can jeopardize your research. Clin Chem Lab Med, 2014;52(6), 765–766. [DOI] [PubMed] [Google Scholar]
- 30.Berglund L, Bjorling E, Oksvold P et al. A genecentric Human Protein Atlas for expression profiles based on antibodies. Mol Cell Proteomics, 2008;7(10), 2019–2027. [DOI] [PubMed] [Google Scholar]
- 31.Begley CG, Ellis LM. Drug development: Raise standards for preclinical cancer research. Nature, 2012;483(7391), 531–533. [DOI] [PubMed] [Google Scholar]
- 32.Gilda JE, Ghosh R, Cheah JX, et al. Western Blotting Inaccuracies with Unverified Antibodies: Need for a Western Blotting Minimal Reporting Standard (WBMRS). PLoS One, 2015;10(8), e0135392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marx V Calling the next generation of affinity reagents. Nat Methods, 2013;10(9), 829–833. [DOI] [PubMed] [Google Scholar]
- 34.Bradbury A, Pluckthun A. Reproducibility: Standardize antibodies used in research. Nature, 2015;518(7537), 27–29. [DOI] [PubMed] [Google Scholar]
- 35.Petrak J, Ivanek R, Toman O et al. Deja vu in proteomics. A hit parade of repeatedly identified differentially expressed proteins. Proteomics, 2008;8(9), 1744–1749. [DOI] [PubMed] [Google Scholar]
- 36.Immunoblots. Journal of Biological Chemistry. Cited Sept 2017. Available from http://www.jbc.org/site/misc/ifora.xhtml#blots [Google Scholar]
- 37.Gilda JE, Gomes AV. Stain-Free total protein staining is a superior loading control to beta-actin for Western blots. Anal Biochem, 2013;440(2), 186–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gilda JE, Gomes AV. Western blotting using in-gel protein labeling as a normalization control: stain-free technology. Methods Mol Biol, 2015;1295, 381–391. [DOI] [PubMed] [Google Scholar]
- 39.He M, Herr AE. Automated microfluidic protein immunoblotting. Nature protocols, 2010;5(11), 1844–1856. [DOI] [PubMed] [Google Scholar]
- 40.Duncombe TA, Kang CC, Maity S et al. Hydrogel Pore-Size Modulation for Enhanced Single-Cell Western Blotting. Adv Mater, 2016;28(2), 327–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. •.Kang CC, Yamauchi KA, Vlassakis J, Sinkala E, Duncombe TA, Herr AE. Single cell-resolution western blotting. Nature protocols, 2016;11(8), 1508–1530. The paper describes a novel automated and an advanced alternate technique for western blotting to measure cell-to-cell variations in protein expression in individual cells. The technique has applications in many areas of basic and clincal research. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.O’Neill RA, Bhamidipati A, Bi X et al. Isoelectric focusing technology quantifies protein signaling in 25 cells. Proc Natl Acad Sci U S A, 2006;103(44), 16153–16158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wenz C, Rufer A. Microchip CGE linked to immunoprecipitation as an alternative to Western blotting. Electrophoresis, 2009;30(24), 4264–4269. [DOI] [PubMed] [Google Scholar]
- 44.Chen JQ, Heldman MR, Herrmann MA et al. Absolute quantitation of endogenous proteins with precision and accuracy using a capillary Western system. Anal Biochem, 2013;442(1), 97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rustandi RR, Loughney JW, Hamm M et al. Qualitative and quantitative evaluation of Simon, a new CE-based automated Western blot system as applied to vaccine development. Electrophoresis, 2012;33(17), 2790–2797. [DOI] [PubMed] [Google Scholar]
- 46.Koller A, Watzig H. Precision and variance components in quantitative gel electrophoresis. Electrophoresis, 2005;26(12), 2470–2475. [DOI] [PubMed] [Google Scholar]
- 47.The Simple Western Overview. ProteinSimple (2011–2014). Accessed Sep 2017. Available from: www.proteinsimple.com.
- 48.Harris VM. Protein detection by Simple Western analysis. Methods Mol Biol, 2015;1312, 465–468. [DOI] [PubMed] [Google Scholar]
- 49.Brumbaugh K, Liao WC, Houchins JP, Cooper J, Stoesz S. Phosphosite-Specific Antibodies: A Brief Update on Generation and Applications. Methods Mol Biol, 2017;1554, 1–40. [DOI] [PubMed] [Google Scholar]
- 50.Loughney JW, Ha S, Rustandi RR. Quantitation of CRM197 using imaged capillary isoelectric focusing with fluorescence detection and capillary Western. Anal Biochem,2017; 534, 19–23. [DOI] [PubMed] [Google Scholar]
- 51.Siggers P, Carre GA, Bogani D et al. A novel mouse Fgfr2 mutant, hobbyhorse (hob), exhibits complete XY gonadal sex reversal. PLoS One, 2014;9(6), e100447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rustandi RR, Hamm M, Lancaster C, Loughney JW. Applications of an Automated and Quantitative CE-Based Size and Charge Western Blot for Therapeutic Proteins and Vaccines. Methods Mol Biol, 2016;1466, 197–217. [DOI] [PubMed] [Google Scholar]
- 53.Rustandi RR, Hamm M, Loughney JW, Ha S. Detection of ADP ribosylation in PARP-1 and bacterial toxins using a capillary-based western system. Electrophoresis, 2015;36(21–22), 2798–2804. [DOI] [PubMed] [Google Scholar]
- 54.Yeh HY, Serrano KV, Acosta AS, Buhr RJ. Production of recombinant Salmonella flagellar protein, FlgK, and its uses in detection of anti-Salmonella antibodies in chickens by automated capillary immunoassay. J Microbiol Methods, 2016;122, 27–32. [DOI] [PubMed] [Google Scholar]
- 55.Treindl F, Ruprecht B, Beiter Y et al. A bead-based western for high-throughput cellular signal transduction analyses. Nat Commun, 2016;7, 12852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu CY, Lu DC, Jiang YW, Yen YK, Chang SC, Wang AB. Easy and Fast Western Blotting by Thin-Film Direct Coating with Suction. Anal Chem, 2016;88(12), 6349–6356. [DOI] [PubMed] [Google Scholar]
- 57.Okita N, Higami Y, Fukai F et al. Modified Western blotting for insulin and other diabetes-associated peptide hormones. Sci Rep, 2017;7(1), 6949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Olsen I, Wiker HG. Diffusion blotting: a rapid and simple method for production of multiple blots from a single gel. Methods Mol Biol, 2015;1312, 73–76. [DOI] [PubMed] [Google Scholar]
- 59.Lin WW, Chen IJ, Cheng TC et al. A Secondary Antibody-Detecting Molecular Weight Marker with Mouse and Rabbit IgG Fc Linear Epitopes for Western Blot Analysis. PLoS One, 2016;11(8), e0160418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tsang VC, Hancock K, Simons AR . Calibration of prestained protein molecular weight standards for use in the “Western” or enzyme-linked immunoelectrotransfer blot techniques. Anal Biochem, 1984;143(2), 304–307. [DOI] [PubMed] [Google Scholar]
- 61.Chang M, Hsu HY, Lee HJ. Dye-free protein molecular weight markers. Electrophoresis, 2005;26(16), 3062–3068. [DOI] [PubMed] [Google Scholar]
- 62.Bischof DF, Hardmeier S, Fairhead M, Ihssen J, Thony-Meyer L. Heme ladder, a direct molecular weight marker for immunoblot analysis. Anal Biochem, 2011;409(2), 213–219. [DOI] [PubMed] [Google Scholar]
- 63.Zhang YM, Geng L, Huang DW. Generate Western blot protein marker from a single construct. Anal Biochem, 2009;390(2), 206–208. [DOI] [PubMed] [Google Scholar]
- 64.Kao CH, Cheng CM, Chuang KH et al. A regularly spaced and self-revealing protein ladder for anti-tag Western blot analysis. Anal Biochem, 2012;431(1), 1–3. [DOI] [PubMed] [Google Scholar]
- 65.Gorg A, Weiss W, Dunn MJ. Current two-dimensional electrophoresis technology for proteomics. Proteomics, 2004;4(12), 3665–3685. [DOI] [PubMed] [Google Scholar]
- 66.Scaife C, McManus CA, Donoghue PM, Dunn MJ. Co-detection of Target and Total Protein by CyDye Labeling and Fluorescent ECL Plex Immunoblotting in a Standard Proteomics Workflow. Methods Mol Biol, 2015;1314, 139–149. [DOI] [PubMed] [Google Scholar]
- 67.Herr AJHaAE. 10 minute Western blotting with 54-plex throughput for clinical confirmatory HIV diagnosis in human serum. 16th International Conference on Miniaturized Systems for Chemistry and Life Sciences 978–0-9798064–5-2μTAS 2012/$20©12CBMS-0001 October 28 - November 1, 2012, Okinawa, Japan. [Google Scholar]
- 68.Zulfiqar HF, Javed A, Sumbal et al. HIV Diagnosis and Treatment through Advanced Technologies. Frontiers in public health, 2017;5, 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kang CC, Lin JM, Xu Z, Kumar S, Herr AE. Single-cell Western blotting after whole-cell imaging to assess cancer chemotherapeutic response. Anal Chem, 2014;86(20), 10429–10436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Torian LV, Forgione LA, Punsalang AE, Pirillo RE, Oleszko WR. Comparison of Multispot EIA with Western blot for confirmatory serodiagnosis of HIV. J Clin Virol, 2011;52 Suppl 1, S41–44. [DOI] [PubMed] [Google Scholar]
- 71.Hughes AJ, Spelke DP, Xu Z, Kang CC, Schaffer DV, Herr AE. Single-cell western blotting. Nat Methods, 2014; 11(7), 749–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bernardini G, Figura N, Ponzetto A, Marzocchi B, Santucci A. Application of proteomics to the study of Helicobacter pylori and implications for the clinic. Expert Rev Proteomics, 2017;14(6), 477–490. [DOI] [PubMed] [Google Scholar]
- 73.Hamm M, Ha S, Rustandi RR. Automated capillary Western dot blot method for the identity of a 15-valent pneumococcal conjugate vaccine. Anal Biochem, 2015;478, 33–39. [DOI] [PubMed] [Google Scholar]
- 74.Aslan M, Yuksel P, Polat E et al. The diagnostic value of Western blot method in patients with cystic echinococcosis. New Microbiol, 2011,34(2), 173–177. [PubMed] [Google Scholar]
- 75.Magi B, Migliorini L. Western blotting for the diagnosis of congenital toxoplasmosis. New Microbiol, 2011,34(1), 93–95. [PubMed] [Google Scholar]
- 76.Stopiglia CD, Arechavala A, Carissimi M et al. Standardization and characterization of antigens for the diagnosis of aspergillosis. Can J Microbiol, 2012;58(4), 455–462. [DOI] [PubMed] [Google Scholar]
- 77.Rognon B, Reboux G, Roussel S et al. Western blotting as a tool for the serodiagnosis of farmer’s lung disease: validation with Lichtheimia corymbifera protein extracts. J Med Microbiol, 2015;64(Pt 4), 359–368. [DOI] [PubMed] [Google Scholar]
- 78.Patil S, Giribhattanavar P, Patil M, Kumar K. Immunoconfirmation of central nervous system tuberculosis by blotting: A study of 300 cases. Int J Mycobacteriol, 2015;4(2), 124–130. [DOI] [PubMed] [Google Scholar]
- 79.Capobiango JD, Monica TC, Ferreira FP et al. Evaluation of the Western blotting method for the diagnosis of congenital toxoplasmosis. J Pediatr (Rio J), 2016;92(6), 616–623. [DOI] [PubMed] [Google Scholar]
- 80.Perenha-Viana MC, Gonzales IA, Brockelt SR, Machado LN, Svidzinski TI. Serological diagnosis of paracoccidioidomycosis through a Western blot technique. Clin Vaccine Immunol, 2012;19(4), 616–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schupbach J, Popovic M, Gilden RV, Gonda MA, Sarngadharan MG, Gallo RC. Serological analysis of a subgroup of human T-lymphotropic retroviruses (HTLV-III) associated with AIDS. Science, 1984;224(4648), 503–505. [DOI] [PubMed] [Google Scholar]
- 82.Head MW, Yull HM, Ritchie DL et al. Variably protease-sensitive prionopathy in the UK: a retrospective review 1991–2008. Brain, 2013;136(Pt 4), 1102–1115. [DOI] [PubMed] [Google Scholar]
- 83.Waddell LA, Greig J, Mascarenhas M, Harding S, Lindsay R, Ogden N. The Accuracy of Diagnostic Tests for Lyme Disease in Humans, A Systematic Review and Meta-Analysis of North American Research. PLoS One, 2016;11(12), e0168613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Duriez M, Mandouri Y, Lekbaby B et al. Alternative splicing of Hepatitis B virus: a novel virus/host interaction altering liver immunity. J Hepatol, 2017. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Neal JD, Tobian AA, Laeyendecker O et al. Performance of the Euroline Western blot assay in the detection of herpes simplex virus type 2 antibody in Uganda, China and the USA. Int J STD AIDS, 2011;22(6), 342–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Egberink HF, Lutz H, Horzinek MC. Use of western blot and radioimmunoprecipitation for diagnosis of feline leukemia and feline immunodeficiency virus infections. J Am Vet Med Assoc, 1991; 199(10), 1339–1342. [PubMed] [Google Scholar]
- 87.Oliva A, Flori P, Hennequin C et al. Evaluation of the Aspergillus Western blot IgG kit for diagnosis of chronic aspergillosis. J Clin Microbiol, 2015;53(1), 248–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Li-cor Biosciences I Next Generation Western for high-throughput and highcontent protein analysis. Avaialble at: https://sbirsource.com/sbir/awards/167330-next-generation-western-for-high-throughput-and-high-content-protein-analysis).
- 89.Reardon S US government issues historic $3.5-million fine over animal welfare. Nature, 2016. Available at: https://www.nature.com/news/us-government-issues-historic-3-5-million-fine-over-animal-welfare-1.19958.
- 90.Cai B, Wang M, Zhu X et al. The Fab Fragment of a Humanized Anti-Toll Like Receptor 4 (TLR4) Monoclonal Antibody Reduces the Lipopolysaccharide Response via TLR4 in Mouse Macrophage. Int J Mol Sci, 2015;16(10), 25502–25515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Brinkmann U, Kontermann RE. The making of bispecific antibodies. MAbs, 2017;9(2), 182–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Eigenbrot C, Fuh G. Two-in-One antibodies with dual action Fabs. Curr Opin Chem Biol, 2013;17(3), 400–405. [DOI] [PubMed] [Google Scholar]
- 93.Hattori T, Taft JM, Swist KM et al. Recombinant antibodies to histone post-translational modifications. Nat Methods, 2013;10(10), 992–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wikan N, Suputtamongkol Y, Yoksan S, Smith DR, Auewarakul P. Immunological evidence of Zika virus transmission in Thailand. Asian Pac J Trop Med, 2016;9(2), 141–144. [DOI] [PubMed] [Google Scholar]
- 95.Bell G Quantifying western blots: none more black. BMC Biol, 2016;14(1), 116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Liu X, Wang Y, Yang W, Guan Z, Yu W, Liao DJ. Protein multiplicity can lead to misconduct in western blotting and misinterpretation of immunohistochemical staining results, creating much conflicting data. Prog Histochem Cytochem, 2016;51(3–4), 51–58. [DOI] [PubMed] [Google Scholar]
- 97.Wu H, de Gannes MK, Luchetti G, Pilsner JR. Rapid method for the isolation of mammalian sperm DNA. BioTechniques, 2015;58(6), 293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bhairi Srirama M., Detergents CM A guide to the properties and uses of detergents in biology and biochemistry. EMD Biosciences, San Diego, CA, 2007. Avaialble at: http://sevierlab.vet.cornell.edu/resources/CALBIOCHEM-DetergentsIV.pdf [Google Scholar]
- 99.Posch A Sample preparation guidelines for two-dimensional electrophoresis. Arch Physiol Biochem, 2014;120(5), 192–197. [DOI] [PubMed] [Google Scholar]
- 100.Freeman JW, Chatterjee A, Busch H. Discrimination of epitopes identified by monoclonal antibodies by competitive binding to nitrocellulose bound antigens. Journal of immunological methods, 1985;78(2), 259–265. [DOI] [PubMed] [Google Scholar]
- 101.Baillod P, Affolter B, Kurt GH, Pflugshaupt R. Multimeric analysis of von Willebrand factor by vertical sodium dodecyl sulphate agarose gel electrophoresis, vacuum blotting technology and sensitive visualization by alkaline phosphatase anti-alkaline phosphatase complex. Thrombosis research, 1992;66(6), 745–755. [DOI] [PubMed] [Google Scholar]
- 102.Spurr-Michaud S, Argueso P, Gipson I. Assay of mucins in human tear fluid. Experimental eye research, 2007;84(5), 939–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hicks SJ, Carrington SD, Kaswan RL, Adam S, Bara J, Corfield AP. Demonstration of discrete secreted and membrane-bound ocular mucins in the dog. Experimental eye research, 1997;64(4), 597–607. [DOI] [PubMed] [Google Scholar]
- 104.Keeton TP, Francis BR, Maaty WS, Bulla LA Jr, Effects of midgut-protein- preparative and ligand binding procedures on the toxin binding characteristics of BT-R1, a common high-affinity receptor in Manduca sexta for Cry1A Bacillus thuringiensis toxins. Applied and environmental microbiology, 1998;64(6), 2158–2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Peferoen M, Huybrechts R, Loof AD. Vacuum-blotting: a new simple and efficient transfer of proteins from sodium dodecyl sulfate—polyacrylamide gels to nitrocellulose. FEBS Letters, 1982;145(2). [Google Scholar]
- 106.Hermansen LF, Pedersen O, Sletten K. Centrifuge-blotting of proteins after separation by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Electrophoresis, 1993;14(12), 1302–1306. [DOI] [PubMed] [Google Scholar]
- 107.Hermansen LF, Juul J, Sletten K. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis and centrifuge blotting: preparation of polypeptides for amino-terminal sequence analysis. Electrophoresis, 1996;17(4), 720–722. [DOI] [PubMed] [Google Scholar]
- 108.Kain SR, Mai K, Sinai P. Human multiple tissue western blots: a new immunological tool for the analysis of tissue-specific protein expression. BioTechniques, 1994;17(5), 982–987. [PubMed] [Google Scholar]
- 109.Kurien BT, Scofield RH. Western blotting. Methods, 2006;38(4), 283–293. [DOI] [PubMed] [Google Scholar]
- 110.Burkhart WA, Moyer MB, Bailey JM, Miller CG. Electroblotting of proteins to Teflon tape and membranes for N- and C-terminal sequence analysis. Anal Biochem, 1996;236(2), 364–367. [DOI] [PubMed] [Google Scholar]
- 111.Otter T, King SM, Witman GB. A two-step procedure for efficient electrotransfer of both high-molecular-weight (greater than 400,000) and low-molecular-weight (less than 20,000) proteins. Anal Biochem, 1987;162(2), 370–377). [DOI] [PubMed] [Google Scholar]
- 112.Bolt MW, Mahoney PA. High-efficiency blotting of proteins of diverse sizes following sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Anal Biochem, 1997;247(2), 185–192. [DOI] [PubMed] [Google Scholar]
- 113.Gibson W Protease-facilitated transfer of high-molecular-weight proteins during electrotransfer to nitrocellulose. Anal Biochem, 1981; 118(1), 1–3. [DOI] [PubMed] [Google Scholar]
- 114.Elkon KB, Jankowski PW, Chu JL. Blotting intact immunoglobulins and other high-molecular-weight proteins after composite agarose-polyacrylamide gel electrophoresis. Anal Biochem, 1984;140(1), 208–213. [DOI] [PubMed] [Google Scholar]
- 115.Kurien BT, Scofield RH. Ultrarapid electrophoretic transfer of high and low molecular weight proteins using heat. Methods Mol Biol, 2009;536, 181–190. [DOI] [PubMed] [Google Scholar]
- 116.Kurien BT, Scofield RH. Heat-mediated, ultra-rapid electrophoretic transfer of high and low molecular weight proteins to nitrocellulose membranes. Journal of immunological methods, 2002;266(1–2), 127–133. [DOI] [PubMed] [Google Scholar]
- 117.Wang MS, Pang JS, Selsted ME. Semidry electroblotting of peptides and proteins from acid-urea polyacrylamide gels. Anal Biochem, 1997;253(2), 225–230. [DOI] [PubMed] [Google Scholar]
- 118.Sasse J, Gallagher SR. Detection of proteins on blot transfer membranes. Current protocols in immunology, Chapter 8, 2008;Unit 8 10B. [DOI] [PubMed] [Google Scholar]
- 119.Harper S, Speicher DW. Detection of proteins on blot membranes. Current protocols in protein science, Chapter 10,2001; Unit 10 18. [DOI] [PubMed] [Google Scholar]
- 120.Wise GE, Lin F. Transfer of silver-stained proteins from polyacrylamide gels to polyvinylidene difluoride membranes. Journal of biochemical and biophysical methods, 1991;22(3), 223–231. [DOI] [PubMed] [Google Scholar]
- 121.Kurien BT, Scofield RH. A brief review of other notable protein blotting methods. Methods Mol Biol, 2009;536, 367–384. [DOI] [PubMed] [Google Scholar]
- 122.Bienvenut WV, Deon C, Sanchez JC, Hochstrasser DF. Enhanced protein recovery after electrotransfer using square wave alternating voltage. Anal Biochem, 2002;307(2), 297–303. [DOI] [PubMed] [Google Scholar]
- 123.Jadwin JA, Mayer BJ, Machida K. Detection and quantification of protein-protein interactions by far-western blotting. Methods Mol Biol,2015;1312, 379–398. [DOI] [PubMed] [Google Scholar]
- 124.Ye F, Smith PB, Wu C, Chiu DT. Ultrasensitive detection of proteins on Western blots with semiconducting polymer dots. Macromolecular rapid communications, 2013;34(9), 785–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zeng C, Suzuki Y, Alpert E. Polyethylene glycol significantly enhances the transfer of membrane immunoblotting. Anal Biochem, 1990;189(2), 197–201. [DOI] [PubMed] [Google Scholar]
- 126.Aebersold RH, Teplow DB, Hood LE, Kent SB. Electroblotting onto activated glass. High efficiency preparation of proteins from analytical sodium dodecyl sulfate-polyacrylamide gels for direct sequence analysis. The Journal of biological chemistry, 1986;261(9), 4229–4238. [PubMed] [Google Scholar]
- 127.Kilmer Greg, Webb Brian, Dworecki Boguslawa R, Hommema Eric, & SS, Rangaraj P. Optimized method for rapid protein electroblotting. Nature Methods 2013;10. [Google Scholar]
- 128.Bio-Rad. Trans-Blot® Turbo™ Transfer System. Accessed Sep 2017. Available from: http://www.bio-rad.com/en-cn/product/semi-dry-rapid-blotting-systems/trans-blot-turbo-transfer-system)).
- 129.Taylor SC, Berkelman T, Yadav G, Hammond M. A defined methodology for reliable quantification of Western blot data. Mol Biotechnol, 2013; 55(3), 217–226. [DOI] [PMC free article] [PubMed] [Google Scholar]