Abstract
Objective:
A repeated measures study was used to assess the effect of work tasks on select proinflammatory biomarkers in firefighters working at prescribed burns.
Methods:
Ten firefighters and two volunteers were monitored for particulate matter and carbon monoxide on workdays, January-July 2015. Before and after work-shift dried blood spots were analyzed for inflammatory mediators using the Meso Scale Discovery assay, while blood smears were used to assess leukocyte parameters.
Results:
Firefighters lighting with drip-torches had higher cross-work-shift increases in interleukin-8, C-reactive protein, and serum amyloid A compared to holding, a task involving management of fire boundaries. A positive association between interleukin-8 and segmented-neutrophil was observed.
Conclusion:
Results from this study suggest that intermittent occupational diesel exposures contribute to cross-work-shift changes in host systemic innate inflammation as indicated by elevated interleukin-8 levels and peripheral blood segmented-neutrophils.
INTRODUCTION
Wood smoke is composed of hundreds of pollutants, including respirable particulate matter (PM), carbon monoxide (CO), nitrogen and sulfur oxides, aldehydes, polycyclic aromatic hydrocarbons, free radicals, and others, some of which are carcinogenic components (e.g. dioxins and furans).1 In vitro, in vivo, clinical and epidemiological studies in humans suggest that various components in wood smoke and smoke emissions from other sources can affect the immune system.2-7 Acute exposure to particulate matter has been shown to induce a neutrophilic airway inflammatory response resulting in prolonged systemic inflammation in municipal firefighters. 8 Systemic inflammation is also well recognized as an important process in the pathogenesis of cardiovascular and other chronic diseases.9 After exposure, there is an increase in infiltrating immune cells at the site of insult as wells as shifts in select proinflammatory and anti-inflammatory mediators. For example, elevation in cytokines interleukin-6 (IL-6), interleukin-8 (IL-8), interleukin-1β (IL-1β), and tumor necrosis factor-α (TNF-α) are commonly observed during acute inflammation. After local inflammation ensues, the persistence of the initial antigenic insult can trigger a systemic response, which can be detected by screening inflammatory mediators in the peripheral blood.
A few human studies have reported inflammatory effects of wood smoke exposure, however the specific immune mechanisms associated with these observed health effects are still being explored. In two recent studies, IL-6 and IL-8 were used to assess systemic inflammation in wildland firefighters.5, 10 These results showed that peripheral blood cytokine levels increased after wood smoke exposure; however, a dose response relationship between specific wood smoke constituents and inflammatory responses has yet to be clearly established.5, 10
Results from our previous pilot study suggest that the work tasks performed by wildland firefighters at prescribed burns influence cross-work-shift (pre-work-shift to post-work-shift) changes in the inflammatory biomarker IL-8.10 Firefighters who lighted fires had the highest IL-8 response even though their PM and CO exposures were significantly lower than firefighters who performed holding, a task that involves maintaining the fire within pre-established boundaries.10 It is suspected that this finding could be due to other occupational exposures during firefighting such as from drip-torches which are used to light fires and fueled by a 3-part diesel 1-part gasoline mixture. Studies show that acute exposure to diesel exhaust induces increases in IL-8 levels,11, 12 and previous in vitro and animal studies suggest that the type of systemic response observed from particulate exposure may also depend on the type of combustion sources.3, 4
The purpose of the current study was to assess the effect of work tasks conducted during prescribed burning on inflammatory biomarkers collected from wildland firefighters, while also addressing some important limitations from our previous work.10 We prospectively powered our current study based on a primary health endpoint measured from this prior study. We collected before and after work-shift blood samples from firefighters on workdays when prescribed burns were not conducted and were able to account for exercise using pulmonary ventilation rate estimations, as a number of inflammatory biomarkers may increase after rigorous physical activity.13 In addition, data from our concurrent exposure study14, regarding personal PM specific light absorbing carbon (used as a surrogate for black carbon [BC], a constituent of incomplete combustion of diesel and biomass burning15) was used in this study to better understand how source of exposure (i.e. soot from drip-torches) may influence inflammatory responses in firefighters. Blood was collected to generate blood smears for 5-point leukocyte differentials and dried blood spots to measure inflammatory mediators such as cytokines, acute phase proteins, and cellular adhesion molecules in peripheral blood samples. Since IL-8 is a neutrophil chemokine, we predicted that blood smear leukocyte differentials would shift favoring an elevation in blood neutrophils. PM exposure samples were collected using a personal aerosol monitor capable of also collecting real-time accelerometry measurements that were used to estimate pulmonary ventilation rates and inhaled dose of PM.
We hypothesized that the effect of work task would be associated with cross-work-shift (before/pre to after/post-work-shift) changes in inflammatory biomarkers, and that cross-work-shift increases in inflammatory responses would be higher in firefighters igniting fires using drip-torches (“lighting”) compared to firefighters performing other work tasks. Morning-after work-shift samples were also collected to further characterize the time-course of inflammatory events post exposure. We also predicted that there would be changes in leukocyte populations across work-shifts after prescribed burning. To our knowledge, this is the first study to use blood smears to assess changes in systemic differential leukocyte cell populations following wood smoke exposure. This research is useful for understanding the mechanisms involved in acute inflammatory responses due to such exposures. In addition, internal dose of PM exposure (previously calculated from our concurrent exposure study14) and its relationship with inflammation was uniquely explored.
METHODS
Study Population
This study was approved by the University of Georgia Institutional Review Board. Written informed consent was obtained from each subject before participation. A total of twelve healthy subjects, currently non-smokers, not pregnant, and 18 years of age or older, enrolled in the study. This included ten firefighters employed by the United States Forest Service-Savannah River (USFS-SR), South Carolina and two volunteers certified to work on prescribed burns. Baseline questionnaires were administered along with daily work activity questionnaires to capture information pertaining to personal work history, length of firefighter career, health habits (i.e. exercise frequency, diet), disease history, allergy, medication, food, daily work tasks, and other factors that could be considered influences on exposure and/or on the inflammatory biomarkers of interest.
Study Design
A repeated measures design was used to collect samples from subjects working at prescribed burns and non-burn workdays during January-July 2015. We conducted personal monitoring to measure occupational exposure to particulate matter with an aerodynamic diameter of 2.5 microns and below (PM2.5) and CO. During the active prescribed burning season from January to March, personal exposure measurements were obtained from subjects working at four prescribed burn days and two non-burn days. Measurements were also collected on three burn days and one non-burn day from May to July during the summer burn season. Subjects served as their own controls. Dried blood spot samples were collected during each sample day throughout the study period and blood smears were collected on a subset of sampling days (May to July). Work tasks performed by the subjects were categorized into four groups, “Holding”, “Lighting”, and “Non-burn day—Exposures” and “Non-burn day—Office.” On burn days, subjects were employed in work tasks including holding where firefighters used fire engine trucks to monitor and maintain fire boundaries (also referred to as a “Holder”), and lighting either by hand using a drip-torch or by helicopter (both referred to as a “Lighter”). On non-burn workdays, subjects performed various tasks such as, patrolling areas where recent burns were conducted, field prep work, engine maintenance, etc. which were all classified either as non-burn day exposures (when subjects reported experiencing occupational exposures to vehicle exhaust, diesel exhaust and/or dust) or office work. Work tasks were self-reported by subjects and responses were cross-checked with a technician’s field notes. A primary work task was assigned accordingly if the subject had spent more than 50% of the duration of a work-shift conducting the task.
Exposure Monitoring
A detailed description of exposure monitoring methods has been reported previously.14 In brief, personal gravimetric PM2.5 samples were collected in the breathing zone of the subjects during the work-shifts using MicroPEMs (Research Triangle Park, NC, USA). In addition, real-time CO measurements were collected using Drager Pac III single gas monitors outfitted with CO sensors (DragerSafety Inc., Pittsburgh, PA, USA). Time-weighted average (TWA) concentrations were calculated for both PM2.5 (reported in μg/m3) and CO (reported in ppm). Estimated pulmonary ventilation rates and estimated dose of PM2.5 used in this study were previously calculated using subject-specific accelerometry data collected by the MicroPEMs and linear regression models.14
Gravimetric Analysis
The gravimetric analytic method has been described elsewhere14. Briefly, gravimetric PM2.5 samples, which were collected on pre-weighed 25-millimeter polytetrafluoroethylene (PTFE) membrane filters with a porosity of 3.0 micrometers (Pall Life Sciences, Ann Arbor, MI, USA), were weighed using the Cahn C-35 microbalance (sensitivity of ±1.0 μg; Thermo Electron, Waltham, MA, USA) and stored in a −20ºC freezer until further analyses.
Particulate Differentiation
PM2.5 on sample filters were analyzed for light absorbing carbon. Analysis was performed by a method described elsewhere in detail.14 Briefly, the PTFE membrane filters were analyzed for reflectance using the Evans Electroselenium Limited (EEL) smoke stain reflectometer (Model 43D by Diffusion Systems Ltd, London, United Kingdom). Absorption coefficients were determined using an adjusted equation provided in ISO 983516.14 Absorption coefficients are reported in 10−5m-1. A mass absorption efficiency (×10−5m2/μg) was also calculated by dividing the absorption coefficients by the PM2.5 concentrations to quantify absorption (surrogate for BC) per unit concentration of total PM2.5.14
Dried Blood Spot Collection and Multiplex Assay Analysis
Whole blood samples were collected from subjects immediately before a work-shift, immediately after a work-shift, and the following morning after a work-shift. Time of blood collection was recorded. A detailed collection protocol was described previously.10 Briefly, single-use, permanently retracting sterile lancets (BD Genie 366582, 1.5 mm blades by 2.0 mm depth; Becton, Dickinson and Company, Franklin Lakes, NJ, USA) were used to prick the subject’s sterilized finger and drops of whole blood were collected on Whatman 903 Protein Saver Cards (GE Healthcare Life Sciences/Whatman, Piscataway, NJ, USA). Cards were allowed to dry overnight and subsequently packaged in low-gas permeable plastic bags with desiccants (AGM Container Controls, Inc., Tucson, AZ, USA) and humidity indicator cards (3M, Austin, TX, USA). Samples were transported and stored in a −20°C freezer until immunoassays were performed. Dried blood samples were analyzed using the Meso Scale Discovery multiplex assay system to analyze for IL-1β, IL-6, IL-8, TNF-α, and C-reactive protein (CRP), serum amyloid A (SAA), cellular adhesion molecules (inter-cellular adhesion molecule 1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1) in two separate kits, respectively.10 IL-1β, IL-6, IL-8, and TNF-α were reported in pg/mL while CRP, SAA, ICAM-1, and VCAM-1 were reported in ng/ml. For all assays both reliability (intra plate variability) and reproducibility (inter plate variability) were tested and the coefficient of variation was between 5-10%. The above proinflammatory biomarkers are herein referred to as proinflammatory mediators.
Whole Blood Smears and Enumeration
In addition to dried blood spot samples, blood smears were made from finger sticks by placing one drop of whole blood (approximately 10-15μL) on a glass slide to allow for leukocyte differential counts. On few occasions, replicate blood smears were made if an adequate smear was not performed the first time. The slides were allowed to air dry and were stained with Wright Giemsa stain (Sigma-Aldrich, St. Louis, MO, USA). For each slide, 200 leukocytes were manually enumerated for the examination of a 5-point differential (neutrophils-segmented and -band, lymphocytes, monocytes, eosinophils and basophils). Cells were examined in a blinded fashion under 40x magnification using an Olympus CX31 Light Microscope (Olympus Optical Co., LTD) and were expressed as percent (%) out of 200 (unless otherwise noted). Smears were also examined for the presence of any abnormal red blood cell, platelets, or leukocytes. Overall staining quality of the blood smear and distribution of cells was examined at low power (10x magnification) and noted comments were recorded.
Statistical Analyses
All statistical analyses were conducted using Microsoft Excel 2016 or SAS v.9.4 (SAS Institute, Cary, NC, USA). Statistical significance for all analyses was set at p < 0.05 and adjusted using the Bonferroni method when doing multiple comparisons. Primary analyses consisted of using linear mixed-effects models to test the effect of work task on changes in proinflammatory mediators and leukocytes.
Initially, descriptive statistical analyses were performed. Histograms, scatter plots, and matrices of scatterplots of the residuals from fitted models were created to assess heteroskedasticity and normality of the data. Log-transformations were applied to inflammatory mediators, TWA gravimetric PM2.5, CO, absorption coefficients, and estimated inhaled dose of PM2.5 to normalize these variables. Mass absorption efficiencies were arcsine-square root transformed to achieve normality. All transformed variables were back transformed before reporting results. Under a log-transformation, back-transformed estimates can be interpreted as geometric means. Some proinflammatory mediator concentrations fell below the lower limit of detection (LLOD) (in the case of 33 of 218 [~15%] IL-1β sample concentrations; LLOD: 0.18 pg/mL). The LLOD is reported herein as the calculated concentration of the signal that is 2.5 standard deviations over the zero calibrator. In such instances, values were replaced with the LLOD divided by the square root of two. All IL-6 and TNF-α concentrations fell below their respective LLODs (0.26 and 0.37 pg/mL, respectively) and therefore were not statistically analyzed. In addition, matrices of scatterplots showed one point-outlier across all inflammatory mediator variables (the outlier was well below the absolute median ± 3σ for all proinflammatory mediators) and therefore was removed from the data before final statistical analyses. Leukocyte cell counts on replicate smears were averaged and the average used as the final value. Percentage of white blood cell types were transformed by taking the log odds of the value (p/1-p) to achieve normality. If zero values were present, a value of 0.025 (half of the lowest observed value above zero percent) was added to the proportion before transformation and the same value subtracted after back transformation.
For the proinflammatory mediators, we tested whether the difference between the means of log transformed post-work-shift and pre-work-shift inflammatory mediators was significantly different from zero, or equivalently when results were back-transformed, that the post- over pre-work-shift ratio was significantly different from one. Cross-work-shift changes are defined herein as between log post-work-shift minus log pre-work-shift changes (post-work-shift/pre-work-shift ratios). Linear mixed models were fit to test whether there were cross-work-shift changes in percent leukocyte cell type using the differences in log odds values. We tested that the log odds post-work-shift minus the log odds pre-work-shift leukocyte cell type was significantly different from zero, or when results were back transformed, that the odds ratio was significantly different from one. Morning-after minus pre-work-shift paired samples were also compared as outlined above for both proinflammatory mediators and leukocytes.
Work task was used as the primary explanatory variable of interest in all linear mixed models. Non-burn day work tasks were collapsed into one category (“Non-burn day activities”) due to small sample sizes in subcategories for cross-work-shift changes in leukocyte cell percentages. Random subject and date effects were included in the models to account for longitudinal within-subject correlation among the data. Other outcome variables were included individually in the linear mixed effect model to test whether they were associated with cross-work-shift differences in proinflammatory biomarkers. These included TWA PM2.5 and CO, absorption coefficient, mass absorption efficiency, and estimated inhaled dose of PM2.5. We tested for other possible cofounding factors such as gender, years of service as a firefighter, days between blood sample collections and last prescribed burn, age, body mass index (BMI), ventilation rate (surrogate for physical exertion/exercise), medication use, and allergies. Covariates were evaluated based on the forward elimination procedure. Only significant covariates were included in the final model. We also explored the relationships between proinflammatory mediators and leukocyte cell type, as we were specifically interested in assessing the relationship between IL-8 and segmented-neutrophils. In addition, we explored the possibility of a dose-response relationship between cross-work-shift proinflammatory biomarker changes and estimated dose of PM2.5.
RESULTS
Descriptive Characteristics
A total of twelve subjects (nine men and three women) enrolled in the study. No subject reported using any form of respiratory protection during the study. Four (33%) of the subjects reported having allergies and four (33%) reported quitting smoking although several years prior. The mean age of the participating subjects was 33 ± 5.4 years, with a range from 26 to 43 years. The average BMI was 27.0 ± 4.8 kg/m2. The average acres burned per day were 280 (Range: 38-1000 acres) and the length of work-shifts on prescribed burn days averaged 4.5 hr (Range: 1.9-9.4 hr). Non-burn day work-shifts averaged 6.2 hr (Range: 3.9-7.8 hr). Subjects were classified by years of experience as a wildland firefighter while the two volunteers, although certified to work on prescribed burns were non-career volunteer firefighters (<1 year of experience). Career firefighters reported 3.25 to 22 years of experience as wildland firefighters. Days between blood sample collection and last prescribed burn ranged from 1 to over 30 days.
A total of fifty-four paired dried blood spot cross-work-shift (pre- and post- work-shift) samples were collected from the twelve subjects on seven prescribed burn days during the study. Twenty-one paired dried blood spot cross-work-shift (pre- and post- work-shift) samples were collected from eight subjects during three non-burn workdays throughout the study period. Additionally, twenty-person day blood smear samples were collected from ten subjects (2 women, 8 men) on three burn days and eight blood smear samples were collected on one non-burn workday from each of the eight subjects (1 woman, 7 men). During the study, some subjects did not report to work the following day, which resulted in fewer morning-after work-shift samples. Unadjusted means of pre-, post-, and morning-after-work-shift inflammatory mediator concentrations and 5-point differential leukocyte cells expressed as percentages collected on burn and non-burn workdays are presented in Tables 1 and 2, respectively.
Table 1.
Unadjusted Means of Proinflammatory Mediator Concentrations by Day Type and Time of Sample Collection.
| Day Type | Time of Sample | Avg Time |
Proinflammatoiy Mediators |
|||||
|---|---|---|---|---|---|---|---|---|
| IL-1b (pg/mL) |
IL-8 (pg/mL) |
CRP (ng/mL) |
SAA (ng/mL) |
ICAM-1 (ng/mL) |
VCAM-1 (ng/mL) |
|||
| Pre-Work Shift (n=54) | 08:14 | |||||||
| Arithmetic Means (95% CL) | 1.72 (1.12, 2.32) | 2.84 (2.58, 3.11) | 24.00 (15.75, 32.25) | 38.13 (25.37, 50.88) | 6.42 (5.33, 6.02) | 11.14 (10.44, 11.83) | ||
| Geometric Means (95% CL) | 0.89 (0.65, 1.23) | 2.72 (2.50, 2.95) | 12.13 (8.87, 16.59) | 22.27 (16.74, 29.63) | 6.21 (5.79, 6.66) | 10.85 (10.18, 11.56) | ||
| Ranges | (0.13, 11.03) | (1.42, 7.67) | (2.15, 129.71) | (3.05, 260.99) | (3.97, 11.67) | (6.52, 16.52) | ||
| Post-Work Shift (n=54) | 15:35 | |||||||
| Burn | Arithmetic Means (95% CL) | 1.30 (0.75, 1.85) | 3.51 (3.11, 3.92) | 24.53 (15.66, 33.41) | 39.97 (25.76, 54.18) | 6.76 (6.22, 7.30) | 11.55 (10.86, 12.24) | |
| Geometric Means (95% CL) | 0.60 (0.43, 0.84) | 3.09 (2.55, 3.74) | 12.20 (8.90, 16.72) | 22.33 (16.51, 30.19) | 6.48 (5.98, 7.02) | 11.27 (10.59, 11.99) | ||
| Ranges | (0.13, 12.68) | (0.04, 9.18) | (2.07, 159.72) | (2.38, 300.67) | (2.49, 12.15) | (6.40, 17.49) | ||
| Morning-After (n=50) | 08:27 | |||||||
| Arithmetic Means (95% CL) | 1.21 (0.68, 1.74) | 2.83 (2.57, 3.09) | 26.70 (18.06, 35.34) | 37.52 (26.68, 48.36) | 6.37 (5.82, 6.92) | 10.73 (10.05, 11.41) | ||
| Geometric Means (95% CL) | 0.65 (0.48, 0.89) | 2.68 (2.42, 2.96) | 14.39 (10.43, 19.85) | 23.49 (17.55, 31.45) | 6.14 (5.69, 6.62) | 10.46 (9.80, 11.17) | ||
| Ranges | (0.13, 12.20) | (0.59, 6.20) | (1.87, 111.86) | (3.58, 190.73) | (3.83, 12.77) | (6.76, 15.53) | ||
| Pre-Work Shift (n=21) | 09:14 | |||||||
| Arithmetic Means (95% CL) | 0.79 (0.47, 1.12) | 2.72 (2.46, 2.97) | 19.09 (8.49, 29.69) | 27.24 (16.48, 38.00) | 6.25 (5.44, 7.07) | 10.75 (9.69, 11.80) | ||
| Geometric Means (95% CL) | 0.55 (0.36, 0.84) | 2.66 (2.42, 2.93) | 10.02 (5.99, 16.76) | 17.87 (11.40, 28.02) | 6.04 (5.34, 6.82) | 10.51 (9.51, 11.61) | ||
| Ranges | (0.10, 2.99) | (1.89, 3.74) | (2.60, 75.28) | (3.32, 78.64) | (3.83, 10.66) | (7.22, 15.10) | ||
| Post-Work Shift (n=21) | 16:26 | |||||||
| Non-bum | Arithmetic Means (95% CL) | 1.24 (0.68, 1.81) | 3.11 (2.84, 3.38) | 19.45 (8.90, 30.00) | 28.48 (17.48, 39.48) | 6.69 (5.89, 7.49) | 11.50 (10.39, 12.61) | |
| Geometric Means (95% CL) | 0.73 (0.43, 1.23) | 3.06 (2.81, 3.33) | 10.36 (6.21, 17.30) | 18.68 (11.85, 29.45) | 6.50 (5.83, 7.24) | 11.25 (10.21, 12.41) | ||
| Ranges | (0.13, 4.36) | (2.23, 4.47) | (2.46, 75.40) | (3.18, 76.18) | (4.50, 11.06) | (7.91, 15.86) | ||
| Morning-After (n=18) | 09:15 | |||||||
| Arithmetic Means (95% CL) | 0.94 (0.41, 1.46) | 2.66 (2.33, 2.99) | 24.70 (12.02, 37.38) | 49.41 (21.56, 77.26) | 6.41 (5.51, 7.31) | 10.28 (9.23, 11.33) | ||
| Geometric Means (95% CL) | 0.58 (0.36, 0.95) | 2.59 (2.30, 2.91) | 13.63 (7.60, 24.42) | 25.26 (13.62, 46.84) | 6.20 (5.44, 7.06) | 10.08 (9.11, 11.16) | ||
| Ranges | (0.13, 4.10) | (1.70, 4.46) | (2.53, 78.58) | (4.37, 174.49) | (4.09, 10.56) | (7.35, 13.94) | ||
Abbreviations: n, person-day samples; 95% CL, lower and upper confidence levels. Note: Samples were collected on seven burn days and three non-burn days.
Table 2.
Unadjusted Arithmetic Means of Five Point Differential Percent of White Blood Cells by Day Type and Time of Sample Collection.
| Day Type | Time of Sample | Avg Time |
% Neutrophils- Segmented |
% Neutrophils- Band |
% Lymphocytes | % Monocytes | % Eosinophils | % Basophils |
|---|---|---|---|---|---|---|---|---|
| Avg ± SEM | Avg ± SEM | Avg ± SEM | Avg ± SEM | Avg ± SEM | Avg ± SEM | |||
| Pre-Work Shift (n=20) | 07:57 | 47 ± 3 | 0.2 ± 0.1 | 41 ± 3 | 7 ± 0.5 | 4 ± 1 | 1 ± 0.2 | |
| Bum | Post-Work Shift (n=20) | 14:19 | 57 ± 2 | 1 ± 0.1 | 33 ± 2 | 6 ± 1 | 3 ± 1 | 1 ± 0.3 |
| Morning A fter (n=18) | 08:44 | 46 ± 2 | 0.3 ± 0.1 | 42 ± 2 | 7 ± 1 | 4 ± 1 | 1 ± 0.2 | |
| Pre-Work Shift (n=8) | 09:07 | 44 ± 5 | 0.3 ± 0.2 | 44 ± 5 | 7 ± 1 | 3 ± 1 | 1 ± 0.2 | |
| Non-Bum | Post-Work Shift (n=8) | 16:04 | 48 ± 4 | 0.1 ± 0.1 | 42 ± 4 | 6 ± 1 | 3 ± 1 | 1 ± 0.1 |
| Morning After (n = 7) | 09:37 | 44 ± 5 | 0.3 ± 0.2 | 45 ± 6 | 7 ± 1 | 3 ± 1 | 1 ± 0.2 |
Abbreviations: Avg, average; SEM, standard error of the mean; n, person days; Note: Samples were collected on 3 burn days and 1 non-burn day.
Work Task Exposures
Adjusted geometric means of gravimetric PM2.5 and TWA CO concentrations and 95% confidence limits (95% CL), respectively, by work tasks were 338 (174, 654) μg/m3 and 2.0 (0.7, 5.1) ppm for holding, 240 (134, 430) μg/m3 and 0.7 (0.3, 1.7) ppm for lighting, 24 (10, 61) μg/m3 and 0.004 (0.001, 0.016) ppm for non-burn workday self-reported exposures, and 55 (21, 144) μg/m3 and 0.003 (0.001, 0.012) ppm for non-burn workday office.14 While PM2.5 exposure concentrations did not differ significantly between holding and lighting (p=0.1743), there was marginally significant evidence that firefighters conducting lighting had 36% higher inhaled total PM2.5 compared to when they were holding (p=0.0751; 1310 [561, 3054] μg; 841 [344, 2054] μg, respectively).14 Lighting had nearly a three-fold higher mean absorption coefficient (surrogate for black carbon) compared to holding (p=0.0005; 60.7 [34.5, 107.0] ×10−5m−1; 22.3 [11.6, 43.1] ×10−5m−1, respectively).14 No difference was observed between non-burn day exposures and office tasks (p=0.9807; 0.9 [0.4, 2.3] ×10−5m−1, and 0.9 [0.4, 2.4] ×10−5m−1, respectively) on non-burn days.14 Lighting had over five times higher mean mass absorption efficiency compared to holding (p<0.0001; 0.32 [0.24, 0.40] ×10−5m2/μg, and 0.06 [0.02, 0.12] ×10−5m2/μg, respectively), while the mean mass absorption efficiency was 1.8 times higher for non-burn workday exposures compared to non-burn office tasks, although the difference was not statistically significant (p=0.2034; 0.07 [0.02, 0.15] ×10−5m2/μg, and 0.4 [0.004, 0.11] ×10−5m2/μg, respectively).14
Proinflammatory Mediators
Adjusted cross-work-shift (pre to post) changes of proinflammatory mediators according to work tasks performed on burn and non-burn workdays are presented in Figure 1a. Likewise, adjusted pre to morning-after (pre to MA) cross-work-shift differences of proinflammatory mediators according to work tasks are presented in Figure 1b.
Figure 1 (a). Adjusted Geometric Mean Cross-work-shift (Pre to Post) Changes in Proinflammatory Mediators according to Work Task.
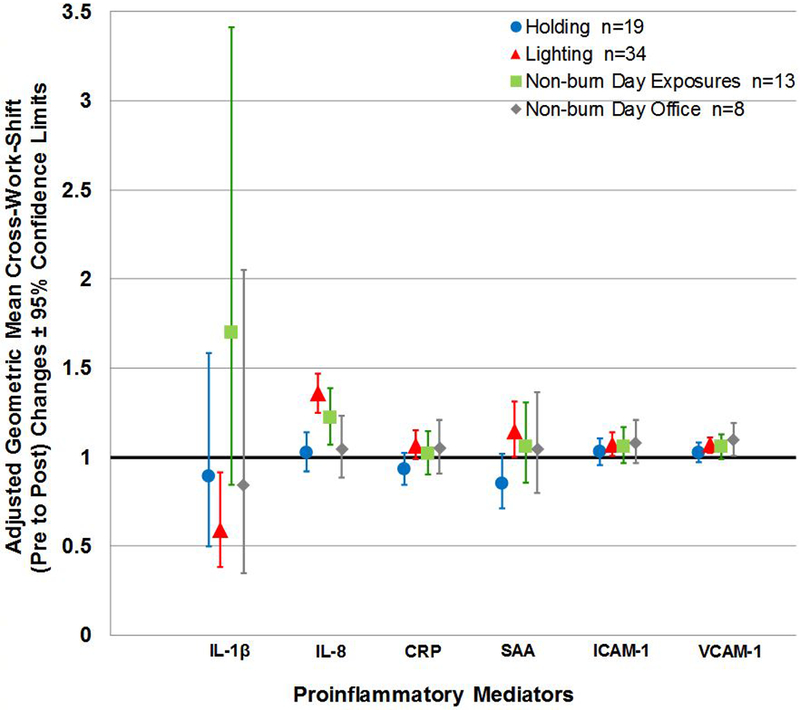
Note: n= person-day pre-post paired samples; Cross-work-shift changes are reported as post-work-shift/pre-work-shift ratios. Where 95% confidence limits do not cross the x-axis, proinflammatory mediator cross-work-shift changes are statistically different from 1 (p-values < 0.05, specific p-values not reported herein).
Figure 1 (b). Adjusted Geometric Mean Pre to Morning-after Cross-work-shift Changes in Proinflammatory Mediators according to Work Task.
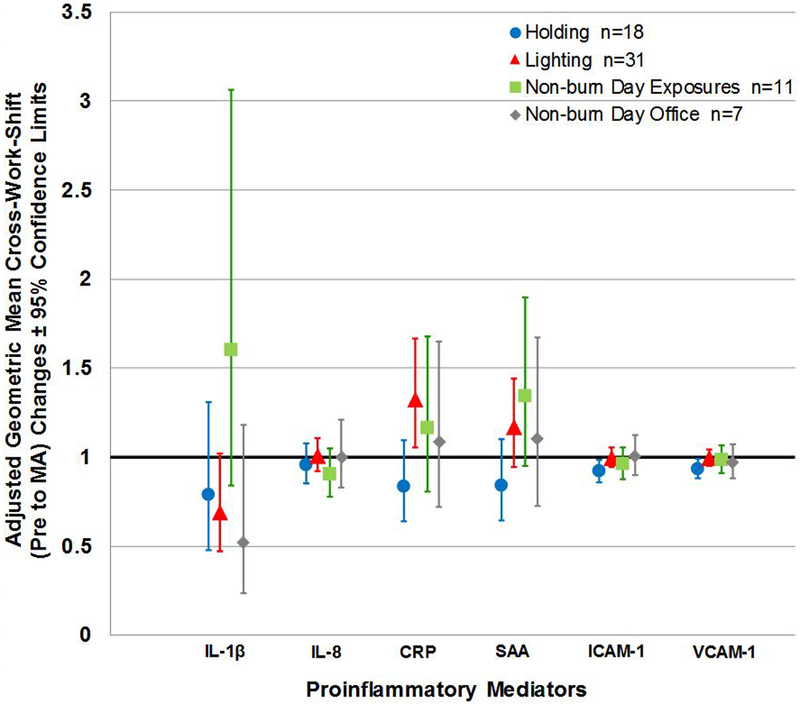
Note: n= person-day pre-morning-after paired samples; Cross-work-shift changes are reported as pre-work-shift/morning-after-work-shift ratios. Where 95% confidence limits do not cross the x-axis, proinflammatory mediator cross-work-shift changes are statistically different from 1 (p-values < 0.05, specific p-values not reported herein). Abbreviations: MA, morning-after.
Overall, we found the IL-8 cross-work-shift (pre to post) changes depended on work tasks conducted on both non-burn and burn days (p=0.0004). As anticipated, IL-8 cross-work-shift (pre to post) changes were significantly different between firefighters lighting compared to those who were holding (p<0.0001), with lighters having over 1.3 times higher mean cross-work-shift increase in IL-8 compared to holders (1.36 [1.25, 1.47] and 1.02 [0.92, 1.14], respectively) (Figure 1a). Although not statistically different (p=0.1737), lighters had observably higher IL-8 mean cross-work-shift (pre to post) increase compared to non-burn day activities that included self-reported exposures (Figure 1a). In addition to IL-8, we found that SAA cross-work-shift (pre to post) changes marginally depended on work task (p=0.0611). Lighters had a SAA cross-work-shift (pre to post) increase that was 1.3 times that of the holders (p=0.0074) (Figure 1a). Although no overall work task effect was seen in CRP cross-work-shift (pre to post) changes (p=0.1036), lighters had 1.1 times higher CRP cross-work-shift (pre to post) increase compared to holders (p=0.0153) (Figure 1a). We observed the highest cross-work-shift (pre to post) increase in IL-1β for non-burn day exposure tasks compared to all other work tasks (p=0.0487) (Figure 1a). Lastly, we observed no significant cross-work-shift (pre to post) differences between work tasks in ICAM-1 or VCAM-1.
We also found that the adjusted geometric mean of CRP pre to morning-after work-shift changes depended on work tasks (p=0.0009) with lighters having a 1.6 times higher work-shift increase compared to holders (Figure 1b). In addition, corresponding increase in SAA appeared to depend on work tasks (p=0.0403; Bonferroni adjusted p-value < 0.0167); pre to morning-after work-shift increases were 1.4 times higher for lighters compared to holders (Figure 1b). However, we did not find an overall effect of work task on pre- to morning-after work-shift changes in the remaining proinflammatory mediators.
5-point Leukocyte Differentials
The unadjusted arithmetic 5-point differential percentages of white blood cell type are presented for non-career (volunteer) and career firefighters across the sampling times in Figures 2a and b, respectively. Reference values17 are also provided. Interestingly, eosinophils were elevated in several career wildland firefighters who had no reported history of allergies (Figure 2b) whereas the two career firefighter subjects with reported seasonal allergies had eosinophil levels within the human population reference range.
Figure 2 (a). Five-point Differential Percentage of White Blood Cell Type by Non-Career Volunteer Firefighter Subjects and Sampling Time.
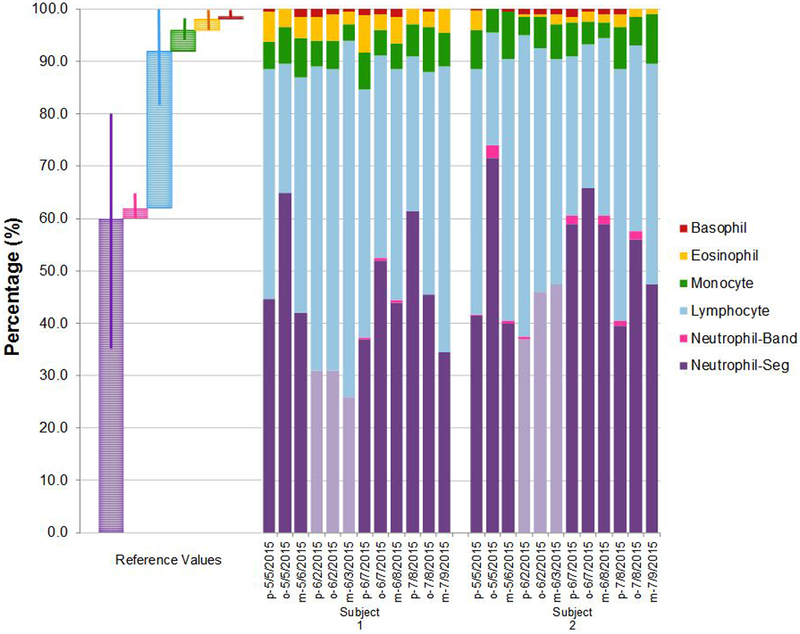
Note: Subject numbers 1 and 2 were non-career volunteer firefighters. Pre-work-shift, post-work-shift, and morning-after work-shift samples were taken and correspond to “p”, “o”, and “m”, respectively. Non-burn workday samples are colored as light purple columns. Reference mean % values are provided along with corresponding range % values (Reference range represents 95% of the general population). Reference values and range % values were retrieved from Williams, James, and Roberts, 2014.17
Figure 2 (b). Five-point Differential Percentage of White Blood Cell Type by Career Firefighter Subjects and Sampling Time.
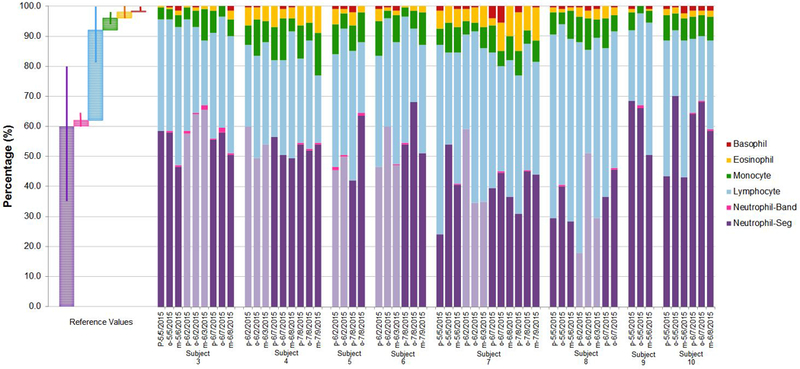
Note: Subject numbers 3-10 were career firefighters. Pre-work-shift, post-work-shift, and morning-after work-shift samples were taken and correspond to “p”, “o”, and “m”, respectively. Non-burn workday samples are colored as light purple columns. Reference mean % values are provided along with corresponding range % values (Reference range represents 95% of the general population). Reference values and range % values were retrieved from Williams, James, and Roberts, 2014.17
Adjusted logit-transformed mean cross-work-shift (pre to post) leukocyte changes by work tasks are provided in Figure 3a. Although not statistically significant (p-values not reported herein), results indicate that firefighters who conducted lighting had cross-work-shift increase in segmented neutrophils and decrease in lymphocytes compared to other work tasks (Figure 3a). Figure 3b visually depicts the pre- to morning-after work-shift changes according to work tasks while controlling for medication use.
Figure 3 (a). Adjusted Odds Ratios of Cross-Work-shift (Pre to Post) Changes according to Work Tasks across Five-point Differential Percentages of White Blood Cell Type.
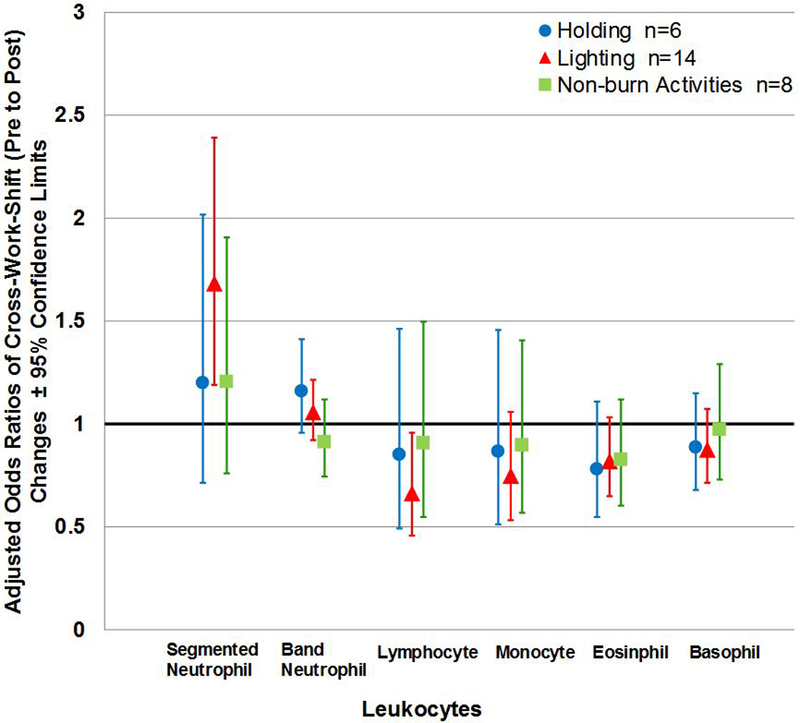
Note: Non-burn day—Exposure and Office were collapsed in to one category; n= person-day pre-post paired samples; Cross-work-shift changes are reported as post-work-shift/pre-work-shift ratios. Where 95% confidence limits do not cross the x-axis, cross-work-shift changes in leukocytes are statistically different from 1 (p-values < 0.05, specific p-values not reported herein). No p-value derived from the linear mixed models were statistically significant.
Figure 3 (b). Adjusted Odds Ratios of Pre to Morning-after Work-shift Changes according to Work Tasks across Five-point Differential Percentages of White Blood Cell Type.
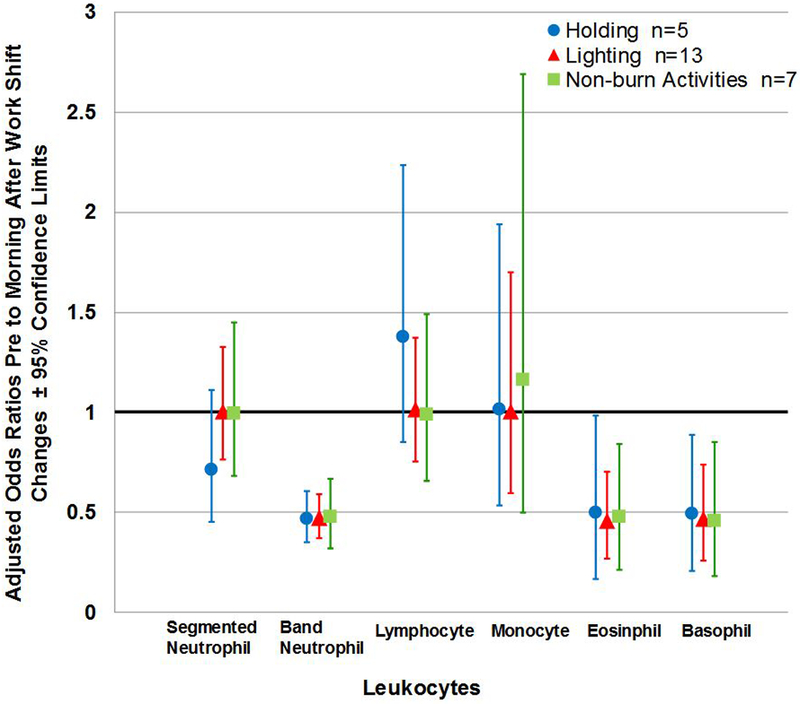
Note: Non-burn day—Exposure and Office were collapsed in to one category; n= person-day pre-morning-after paired samples; Cross-work-shift changes are reported as pre-work-shift/morning-after-work-shift ratios. Where 95% confidence limits do not cross the x-axis, cross-work-shift changes in leukocytes are statistically different from 1 (p-values < 0.05, specific p-values not reported herein). No p-value derived from the linear mixed models were statistically significant. Model controls for medication use.
Additionally, we found that adjusted logit-transformed mean cross-work (pre to post) shift changes in band neutrophil cells appeared to be higher on burn day tasks compared to non-burn day (p-values=0.0810). All other adjusted mean cross-work-shift changes in the reaming leukocyte cell types were not statistically different (data not shown).
Other Covariates and Significant Correlations
A significant effect of medication use (use within prior 24 hours) on pre to morning-after cross-work-shift changes in segmented neutrophils and lymphocytes was found (p=0.0044), with those who used medication having 1.8 times lower pre to morning-after cross-work-shift increases in segmented-neutrophils compared to those who did not take medication (1.07 [0.87, 1.31] n=8, and 0.60 [0.45, 0.81], n=17, respectively). A reciprocal relationship was found between medication use and pre to morning-after cross-work-shift changes in lymphocytes (Medication: 1.70 [1.13, 2.55]; and No medication: 0.92 [0.67, 1.27]). The remaining tested covariates had no significant confounding effects (i.e. gender, years of service as a firefighter, days between blood sample collections and last prescribed burns, age, body mass index (BMI), ventilation rate [surrogate for physical exertion/exercise], and allergies) (results not presented herein).
We found a statistically significant positive correlation between IL-8 and segmented neutrophils (p=0.0179) (Figure 4). Additionally, a statistically significant positive correlation was observed between log-transformed cross-work (pre to post) shift changes in IL-8 and arcsine-square root transformed mass absorption efficiency (p=0.0080). Figure 5 depicts a scatter plot of the exposure-response relationship, using IL-8 as a representative proinflammatory mediator. Likewise, log-transformed cross-work (pre to post) shift changes in CRP and SAA had significant positive relationships with arcsine-square root transformed mass absorption efficiency (p=0.0574; and p=0.0312, respectively). No significant relationships were found between cross-work-shift (pre to post; and pre to morning-after) changes in proinflammatory biomarkers and the remaining outcomes variables (results not shown).
Figure 4. Correlation Between Logit-transformed Cross-work-shift (Pre to Post) Changes in Segmented Neutrophils and Log-transformed IL-8 Changes.
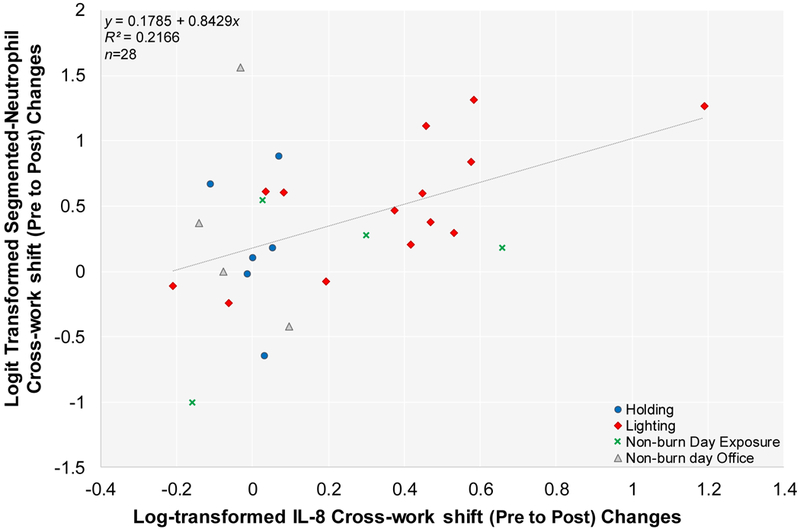
Person days are indicated as n. Linear mixed effect model results showed statistical significant positive correlation (p=0.0179). Note: Cross-work-shift changes are reported as post-work-shift/pre-work-shift ratios.
Figure 5. Correlation Between Log-transformed Cross-work-shift (Pre to Post) Changes in IL-8 and Arcsine-square root Transformed Mass Absorption Efficiency.
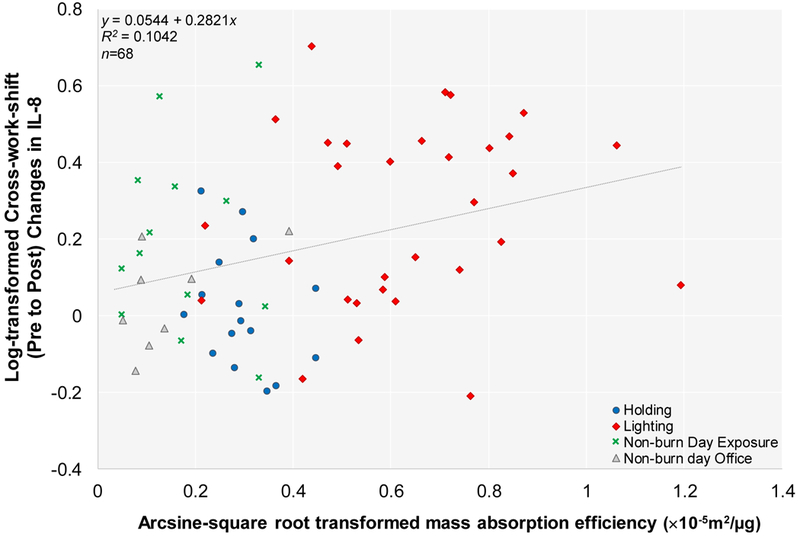
Person days are indicated as n. Linear mixed effect model results showed statistical significant positive correlation (p=0.0080). Note: Cross-work-shift changes are reported as post-work-shift/pre-work-shift ratios.
DISCUSSION
Concurrent exposure to wood smoke and diesel particles may be seen at prescribed burns where firefighters perform work tasks involving ignition of understory growth using diesel-gasoline fueled drip-torches. Wood smoke exposure by inhalation is thought to induce systemic inflammation through various cascading biological pathways involving oxidative stress in the airways and the induction of a local inflammatory response, which if prolonged can lead to systemic changes measured in the vasculature.1 Primary soluble inflammatory mediators such as cytokines and chemokines can be released during an innate response and can thereby induce other immune mediators and trigger activation, proliferation, differentiation, or migration of leukocytes. Specifically, IL-8 is involved in the recruitment of neutrophils to the site of insult.18 During response to an acute insult (i.e. the lung), neutrophils demarginate in the blood stream and increase in number. Diesel exposures can illicit similar responses.11, 12 Moreover, previous animal and human chamber studies have reported significant increased levels of CRP and ICAM-1 following diesel exhaust particle exposure.19
Results from our previous pilot study suggest that wildland firefighter work task influences cross-work-shift increases in inflammatory biomarker, IL-8.10 Firefighters who lighted fires had the highest IL-8 response even though their PM exposures were significantly lower than firefighters who performed holding a task involving the maintenance of fire within pre-established boundaries.10 Acute phase protein, CRP, was also found to be elevated in more than 75% of cross-work (pre to post) shift blood samples. We proposed that the impact of different smoke constituents (i.e. wood smoke vs. diesel exposure) could influence proinflammatory responses seen in the wildland firefighters. In 2015, we set out to investigate the effect of work tasks on select proinflammatory markers in firefighters working at prescribed burns.
We found that lighters had the highest IL-8 cross-work-shift (pre to post) response compared to other work tasks performed on burn and non-burn workdays. These results are consistent with our previous findings in 2011 showing that IL-8 cross-work-shift (pre to post) differences were significantly higher in lighters compared to holders (p<0.0001 [current study], and p=0.0122 [Hejl et al.]10, respectively). The lower cross-work-shift (pre to post) changes in IL-8 may be due to lower exposures and shorter work-shifts in the current study compared to the 2011 study (1.36 [1.25, 1.47]; n=34 [current study], and 1.70 [1.35, 2.13]; n=13 [Hejl et al.]10). Personal exposures to PM2.5 and CO of the wildland firefighters working at prescribed burns during the 2011 study were 2.5 and 4.3 times higher than those observed in the current study, respectively (2011 study: 650 [510, 828] μg/m3; n=22, and 3.6 [2.6, 5.0] ppm; n= 23, from four burn days, respectively).10 Work-shift hours at the fire-line (during active prescribe burning) in our current study14 averaged 4.5 hours as opposed to 6.2 hours in 201110.
We found that firefighters that served as lighters had the highest SAA and CRP cross-work-shift (pre to post) changes compared to firefighters holding. The even greater increase in SAA and CRP from pre- to morning-after work-shift changes suggests there was a delay in select proinflammatory responses post work task related exposures. This is explainable as both CRP and SAA acute phase proteins are produced downstream of the release of cytokines and thus, likely to undergo a delayed systemic release.20 In contrast, IL-8 immune response morning-after concentrations appeared to return to pre-work-shift blood levels as indicated by the pre-work-shift/morning-after work-shift ratio of 1.00 (0.92, 1.10).
The observed strong correlation between IL-8 and peripheral blood segmented neutrophils would suggest that acute diesel and wood smoke exposure evoke systemic innate inflammation in the host. Although we did not find significant correlations between inhaled dose of PM2.5 exposure and cross-work-shift changes in the proinflammatory biomarkers, we found a positive significant correlation between IL-8 and mass absorption efficiency (Figure 5). Similarly, strong positive correlations of mass absorption efficiency were also seen with CRP and SAA, respectively. Such findings suggest that intermittent diesel soot exposures may contribute to more pronounced cross-work-shift changes in host systemic inflammation as indicated by elevated IL-8 levels and segmented-neutrophil number. Additionally, increased band neutrophil cell populations seen in firefighters conducting burn day work-related tasks (holding and lighting) as compared to collective non-burn work tasks is suggestive of a systemic effect of occupational exposures to smoke emissions. Band neutrophils are immature segmented-neutrophils in that they are an intermediary step prior to complete maturation of segmented-neutrophils. An increase in band neutrophils can often indicate that the bone marrow has been signaled to release more leukocytes during an insult.18
Interestingly, we found a significant increase in IL-8 cross-work-shift (pre to post) for non-burn day work tasks involving occupational exposures related to combustion smoke. These results indicate that some occupational exposures on non-burn days might also elicit an immune response. Work tasks involving fire engine maintenance and field prep work using bulldozers is often performed on such days. Although there was an anomalous result regarding cross-work-shift changes in IL-1β with lower concentrations for holders and lighters compared to non-burn day exposures, results from in vitro studies suggest that IL-1β may not be involved in the pathway for the inflammatory response induced by combustion derived smoke particles.4, 21
In the present study, the leukocyte cell differentials of the study participants were within the reference range of the general population, however there appeared to be a numerically higher neutrophil number in our study population compared to the general population average.17 Additionally, eosinophil counts were numerically higher for several career wildland firefighter subjects with no known history of allergies or parasitic infections. Immune functional assays from future firefighter blood samples could be employed to evaluate their sensitivity to the different burn products. For instance, radioallergosorbent (RAS) tests could be used to measure changes in Immunoglobulin E (IgE) levels to select allergenic antigen profiles as a result of diesel and/or wood smoke exposure. Non-career volunteer firefighters had a greater increase in neutrophils on burn days compared to career wildland firefighters, indicating naivety of their immune system to the exposures. However, we did not see a significant firefighter career length effect on cross-shift changes in neutrophils or IL-8 in our linear mixed effect models, possibly due to the small sample size of this study.
Our study’s findings are consistent with results from previous studies looking at the effects of smoke (diesel/wood smoke) emission components on systemic inflammation. One study on seasonal forest firefighters showed significant cross-work (pre to post) shift increases in serum IL-8 on days when the firefighters worked at wildfires compared to days when they did not (Estimated PM3.5 exposures: peak levels of 2.8 mg/m3 and 6-hours of levels less than 1 mg/m3 during a given work-shift).5 A human chamber study found that healthy volunteers had increased neutrophils in peripheral blood samples after only 1-hour exposure to diluted vehicle-produced diesel particles (exposure standardized by maintaining PM10 concentration at 300 μg/m3).19 Additionally, various in vivo models have shown that exposure to wood smoke particles induces airway inflammation characterized by an increase in cytokines and infiltration of immune cells, especially neutrophils.22–25 A clinical study that exposed young healthy individuals to wood smoke particles saw an increase in systemic neutrophils.6 Along with varying combustion conditions, such combined wood smoke and diesel particles may act additively or perhaps even synergistically in their overall toxic effect.26
The concentrations for IL-6 and TNF-α were observed to be below their respective LLODs. It is possible that the sensitivity of the multiplex instrument may have been a factor. For future studies, additional blood volume may be needed to measure these cytokines, as another study found significant changes in serum blood levels of IL-6 in firefighters after wood smoke exposure.5
Limitations
Although sample size was small, firefighters served as their own controls in this study improving the ability to detect differences. However, sample size may be increased, and control non-exposed subjects may be included in future studies for comparisons. We were also unable to capture time spent (i.e. > 50% of the day) during a specific work task on non-burn workdays in this current study resulting in possible exposure misclassification on non-burn days. This study primarily assessed the effect of acute occupational exposures on firefighter health. Future studies are needed to evaluate the chronic effects of occupational exposures among wildland firefighters.
CONCLUSION
Healthy seasonal wildland firefighters conducting lighting at prescribed burns had significantly higher cross-work-shift changes in three proinflammatory mediators—IL-8, CRP, and SAA—compared to holding. No significant correlation was found between internal dose of PM2.5 exposure and inflammatory biomarkers; however, we did observe a significant exposure-response relationship between a surrogate black carbon measure and inflammation. To our knowledge, our study is the first to include blood smears to assess changes in differential white blood cell counts following occupational smoke exposure. This technique may serve as a useful and cost-effective supportive tool for the assessment of acute inflammatory responses due to related occupational smoke exposures. In conclusion, results from this study suggest that intermittent diesel exposures may contribute to more pronounced cross-work-shift changes in systemic inflammation among wildland firefighters during prescribed burns as indicated by elevated IL-8 levels and peripheral blood segmented-neutrophil number.
Acknowledgements
Funding and support is by the National Institute of Occupational Safety and Health Education Research Center (NIOSH/ERC) Small Project/Pilot Study Grants via the University of Alabama at Birmingham (UAB) (Grant no.: 5T42OH008436-10) and the Interdisciplinary Toxicology Program at the University of Georgia. We would like to sincerely thank William Crolly, Chris Hobson, Paul Varnedoe, John Blake, and the USFS-Savannah River crew and subjects who participated in the study.
Footnotes
Disclaimer
The article has been reviewed and approved for publication by the U.S. Environmental Protection Agency. Approval does not signify that the contents necessarily reflect the views and policies of the Agency.
REFERENCES
- 1.Adetona O, Reinhardt TE, Domitrovich J, Broyles G, Adetona AM, Kleinman MT, et al. Review of the health effects of wildland fire smoke on wildland firefighters and the public. Inhalation Toxicology. 2016;28(3):95–139. [DOI] [PubMed] [Google Scholar]
- 2.Donaldson K, Mills N, MacNee W, Robinson S, Newby D. Role of inflammation in cardiopulmonary health effects of PM. Toxicology and applied pharmacology. 2005;207(2):483–8. [DOI] [PubMed] [Google Scholar]
- 3.Kocbach A, Herseth JI, Låg M, Refsnes M, Schwarze PE. Particles from wood smoke and traffic induce differential pro-inflammatory response patterns in co-cultures. Toxicology and applied pharmacology. 2008;232(2):317–26. [DOI] [PubMed] [Google Scholar]
- 4.Kocbach A, Namork E, Schwarze PE. Pro-inflammatory potential of wood smoke and traffic-derived particles in a monocytic cell line. Toxicology. 2008;247(2):123–32. [DOI] [PubMed] [Google Scholar]
- 5.Swiston JR, Davidson W, Attridge S, Li GT, Brauer M, van Eeden SF. Wood smoke exposure induces a pulmonary and systemic inflammatory response in firefighters. European Respiratory Journal. 2008;32(1):129–38. [DOI] [PubMed] [Google Scholar]
- 6.Ghio AJ, Soukup JM, Case M, Dailey LA, Richards J, Berntsen J, et al. Exposure to wood smoke particles produces inflammation in healthy volunteers. Occupational and environmental medicine. 2012;69(3):170–5. [DOI] [PubMed] [Google Scholar]
- 7.Ferguson MD, Semmens EO, Dumke C, Quindry JC, Ward TJ. Measured Pulmonary and Systemic Markers of Inflammation and Oxidative Stress Following Wildland Firefighter Simulations. Journal of Occupational and Environmental Medicine. 2016;58(4):407–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Greven FE, Krop EJ, Spithoven JJ, Burger N, Rooyackers JM, Kerstjens HA, et al. Acute respiratory effects in firefighters. American journal of industrial medicine. 2012;55(1):54–62. [DOI] [PubMed] [Google Scholar]
- 9.Van Eeden SF, Sin DD. Chronic obstructive pulmonary disease: a chronic systemic inflammatory disease. Respiration. 2007;75(2):224–38. [DOI] [PubMed] [Google Scholar]
- 10.Hejl AM, Adetona O, Diaz-Sanchez D, Carter JD, Commodore AA, Rathbun SL, et al. Inflammatory effects of woodsmoke exposure among wildland firefighters working at prescribed burns at the Savannah River Site, SC. Journal of occupational and environmental hygiene. 2013;10(4):173–80. [DOI] [PubMed] [Google Scholar]
- 11.Riedl M, Diaz-Sanchez D. Biology of diesel exhaust effects on respiratory function. Journal of Allergy and Clinical Immunology. 2005;115(2):221–8. [DOI] [PubMed] [Google Scholar]
- 12.Salvi SS, Nordenhall C, Blomberg A, Rudell B, Pourazar J, Kelly FJ, et al. Acute exposure to diesel exhaust increases IL-8 and GRO-α production in healthy human airways. American journal of respiratory and critical care medicine. 2000;161(2):550–7. [DOI] [PubMed] [Google Scholar]
- 13.Pedersen BK, Steensberg A, Fischer C, Keller C, Ostrowski K, Schjerling P. Exercise and cytokines with particular focus on muscle derived IL-6. Exercise immunology review. 2001;7:18–31. [PubMed] [Google Scholar]
- 14.Adetona AM AO, Chartier RT, Paulsen M, Simpson C, Rathbun SL, & Naeher LP. Differences in Fine Particulates and Estimated Pulmonary Ventilation Rate with Respect to Work Task of Wildland Firefighters: A Repeated Measures Study. Submitted to Journal of Exposure Science and Environmental Epidemiology. In Prep. [Google Scholar]
- 15.U.S. Environmental Protection Agency. Black Carbon. 2016. Available from: http://www3.epa.gov/blackcarbon/basic.html
- 16.ISO I 9835, Ambient air–Determination of a black smoke index. Geneva: International Organization for Standardization; 1993. [Google Scholar]
- 17.Roberts SM, James RC, Williams PL. Principles of toxicology: environmental and industrial applications: John Wiley & Sons; 2014. [Google Scholar]
- 18.Harmening DM, Harmening EDM. Clinical hematology and fundamentals of hemostasis: FA Davis Company; 2009. [Google Scholar]
- 19.Salvi S, Blomberg A, Rudell B, Kelly F, Sandstrom T, Holgate ST, et al. Acute inflammatory responses in the airways and peripheral blood after short-term exposure to diesel exhaust in healthy human volunteers. American journal of respiratory and critical care medicine. 1999;159(3):702–9. [DOI] [PubMed] [Google Scholar]
- 20.van Eeden SF, Yeung A, Quinlam K, Hogg JC. Systemic response to ambient particulate matter: relevance to chronic obstructive pulmonary disease. Proceedings of the American Thoracic Society. 2005;2(1):61–7. [DOI] [PubMed] [Google Scholar]
- 21.Le Prieur E, Vaz E, Bion A, Dionnet F, Morin J-P. Toxicity of diesel engine exhausts in an in vitro model of lung slices in biphasic organotypic culture: induction of a proinflammatory and apoptotic response. Archives of toxicology. 2000;74(8):460–6. [DOI] [PubMed] [Google Scholar]
- 22.Wegesser TC, Pinkerton KE, Last JA. California wildfires of 2008: coarse and fine particulate matter toxicity. Environmental health perspectives. 2009;117(6):893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Danielsen PH, Loft S, Jacobsen NR, Jensen KA, Autrup H, Ravanat J-L, et al. Oxidative stress, inflammation and DNA damage in rats after intratracheal instillation or oral exposure to ambient air and wood smoke particulate matter. Toxicological Sciences. 2010:kfq290. [DOI] [PubMed] [Google Scholar]
- 24.Zhu F, Qiu X, Wang J, Jin Y, Sun Y, Lv T, et al. A rat model of smoke inhalation injury. Inhalation toxicology. 2012;24(6):356–64. [DOI] [PubMed] [Google Scholar]
- 25.Williams KM, Franzi LM, Last JA. Cell-specific oxidative stress and cytotoxicity after wildfire coarse particulate matter instillation into mouse lung. Toxicology and applied pharmacology. 2013;266(1):48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dilger M, Orasche J, Zimmermann R, Paur H-R, Diabaté S, Weiss C. Toxicity of wood smoke particles in human A549 lung epithelial cells: the role of PAHs, soot and zinc. Archives of toxicology. 2016:1–16. [DOI] [PubMed] [Google Scholar]


