Abstract
More than 10 years have passed since the “Berlin Patient” was presumed to have been cured of his HIV-1 infection when he received allogenic stem cell transplants from a CCR5 Δ32 homozygous donor in addition to chemotherapy and radiation to treat his acute myelocytic leukemia. This event stimulated great hope and a massive research effort toward developing a more generalizable strategy for achieving a cure of HIV-1 infection. Much has been learned, but little therapeutic progress has been made. We review the lessons learned and the challenges that lay ahead for the field, with new potential approaches that can be taken to advance our ability to eliminate active infection in an individual.
Background
In the life cycle of HIV-1, pro-viral DNA becomes integrated into the host cell genome, and these cells remain latently infected, even when viral replication is suppressed with antiretrovirals. When antiretroviral therapy (ART) is discontinued, viral replication resumes.
These latently infected cells, largely memory CD4 T lymphocytes, persist, perhaps for a life-time, as they were inherently designed to do.[1] Although the CD4 T lymphocyte is the major “reservoir” of latent infection, other cells such as the monocyte/macrophage series, may have some importance, particularly in the central nervous system (CNS).[2]
Anatomic considerations may be important in that certain tissues may be more impenetrable to immune responses and drugs. For example, CD8 cytolytic T lymphocytes do not have ready access to the B cell follicles of lymph nodes, where T follicular helper cells laden with latent virus reside.[3] Other possible sanctuaries include the brain, gastrointestinal lymphatic tissue, and genito-urinary tract. Various antiretroviral drugs achieve lower levels in lymph nodes and other tissues than in blood.[4]
Definitions
To review definitions, two types of cure of HIV-1 infection have been envisioned. An eradication cure would entail the complete elimination of all replication-competent HIV-1 DNA and RNA in blood and tissues. This would be difficult to establish definitively because of diagnostic limitations detailed below. Short of making that determination, an achieved remission from HIV-1 disease, a functional cure, would be demonstrated by the sustained absence of viral replication, as represented by assays of plasma HIV-1 RNA, off ART. Conceivably, this could be accomplished without the complete elimination of replication competent virus, and would be aided, or even fully effected, by the induction of more effective immune responses to the virus. In this situation, an important consideration would be the potential continued risk for HIV-related clinical disease from the effects of residual immune activation and inflammation despite control of HIV-1 replication, as noted in natural “elite controllers” of HIV-1 infection.[5]
The Cure Experience
The possibility of curative treatment was energized by the case of the “Berlin patient” an HIV-infected individual who received stem cell transplants from a CCR5 Δ32 homozygous donor after radiation and chemotherapy for his acute myelocytic leukemia. This patient has maintained undetectable virus in his blood and tissues for more than 10 years after ART was stopped (although recently he has been taking antiretrovirals for pre-exposure prophylaxis to prevent a new infection). Importantly, the Berlin patient has manifested some degree of graft vs. host disease. How much that has contributed to maintaining the apparent absence of active HIV-1 infection is unclear.[6,7]
Two individuals in Boston with HIV infection and lymphoma, themselves heterozygous for the CCR5 Δ32 polymorphism, received stem cell transplants from donors without the CCR5 Δ32 polymorphism and had evidence of graft vs. host disease. Continuing ART after the transplant, both individuals remained undetectable for HIV RNA and DNA in blood (and rectal lymph tissue in one), had negative viral outgrowth assays, and lost HIV antibody seropositivity 4.3 years later in one individual and 2.6 years in the other. Nevertheless, when ART was stopped, viremia returned.[8]
Limitations of the Laboratory Assays
These cases illustrate the point that our current laboratory assays are not adequate for determining the loss of the latent HIV-1 reservoir. A major limitation of HIV-1 cure research is the absence of a clinically validated assay that reliably, with good reproducibility, measures the size of the latent cell reservoir; one that is comparable to the plasma HIV-1 RNA assays (“viral load”) that are available to measure active viral replication and have become the mainstay of the clinical and investigational assessment of the activity of disease and response to treatment. Assays measuring cell-associated viral DNA and RNA overestimate the size of the reservoir, because most of what is measured are defective, non-replicating viral genetic elements. Assays measuring inducible virus or viral elements underestimate the size of the reservoir, because the efficiency of induction may not be complete. Recently developed whole genome assays have been employed to measure intact viral genomes as surrogates of replication competent virus, but these assays have not been clinically validated yet.[9] Furthermore, ascertaining the complete elimination of replicative virus is limited by the ready, safe accessibility of all potential tissues that might harbor virus. Thus, a pause in ART remains the best currently available tool to assess whether the intervention being tested has done anything clinically meaningful to effect a cure, however defined. This pause should be monitored with blood antiretroviral drug testing to ensure the reliability of the virological findings.
Reducing the Latent Viral Reservoir
For a clinically meaningful cure, does the elimination of replication-competent pro-viral genome need to be complete? Early treatment with ART soon after exposure, whether it be within hours of childbirth to an infected mother or within 1–2 days of sexual exposure, would seem to minimize the size of the pro-viral reservoir established, but viremia returns in most individuals when ART is discontinued.[10,11] On the other hand, in individuals treated with long-term ART either soon after initial infection or during chronic infection, two studies found that post-treatment control of viremia when ART is stopped is associated with lower HIV DNA levels.[12,13] Another study in persons treated during chronic infection found an association with lower cell-associated RNA, not with lower HIV DNA.[14]
Efforts to reverse integration of the HIV genome in latently infected cells of HIV-infected persons receiving ART have only attained a modest level of efficiency in clinical studies, not enough to affect the size of the pro-viral DNA pool when administered alone.[15–18] Ex vivo data suggest that a targeted immune response must be on the ready to eliminate latently infected cells when they are induced to express HIV antigens.[19]
Unless the efficiency of latency reversal agents, alone or in combination, is improved, alternative strategies to reduce the latent pro-viral DNA burden will be needed. Several genome-based approaches are being explored. Promising candidates include those that ablate, permanently inactivate or silence essential components of the integrated HIV-1 genome or host cell genetic elements critical for enabling HIV-1 infection of cells. In studies to-date, autologous CD4 T lymphocytes or stem cells from HIV-infected persons are modified ex vivo to make these cells resistant to HIV-1 infection and subsequently re-infused into the same individuals with the goal of creating a population of protected cells, perhaps ultimately replacing those latently infected. The latter scenario would probably need an additional immunological strategy component that supports the targeted killing of the infected cells. In one uncontrolled clinical study of the ex vivo modification of CCR5 on CD4 T lymphocytes with zinc finger nuclease technology, total CD4 T lymphocyte counts increased and the CCR5 gene-modified cells persisted, declining at a slower rate than other CD4 T cells during an interruption of ART.[20]
Promising as this might be, the more efficient delivery of gene modifying technologies would occur through direct in vivo administration utilizing viral vectors, nanoparticles or other carrier constructs. This method would allow for widespread distribution to the cells and tissues to be targeted, albeit at greater risk for systemic toxicity. Replication incompetent lentiviral vectors are nonpathogenic and can efficiently deliver large amounts of genetic material that is stably expressed in targeted cells.[21,22] The risk of immunogenicity is low. However, they can integrate into the host cell genome, and there is a theoretical risk of insertional mutagenesis. Non-integrating lentiviruses are available, but they are less efficient at gene delivery. Non-pathogenic adeno-associated viruses (AAVs) are non-integrating; the durable, stable gene expression occurs on episomes of targeted cells.[23,24] An advantage of AAVs is that their carbohydrate-binding capsid sequences can be modified to optimize the desired cell-type and tissue tropism as well as influence penetration across the blood-brain barrier. Unfortunately, anti-AAV immune responses readily occur after administration, and pre-existing immunity is not uncommon. Thus, additional administrations of a specific AAV vector strain may lose effectiveness. The use of multiple different AAV vector strains, natural and engineered, or the combination of AAV delivery with nanoparticle or another enhanced delivery technique, may allow for repeat administrations and enable the targeting of different cells and tissues.
The CRISPR (clustered regularly interspaced short palindromic repeats) technology is derived from a bacterial host defense system that consists of RNA complements of DNA sequences that contain elements of the bacteriophages that have infected the bacterium previously.[25] The bacterium can then recognize DNA from a subsequent infection with a similar virus, and use CRISPR-associated proteins, Cas, to recognize and cleave the newly invading viral DNA. The CRISPR-Cas complex can be adapted using specific “guide RNAs” (gRNAs) in a new potent technology to cleave a specific targeted site on a cell gene and disrupt the function of that gene, or to introduce new genetic components at that site. Several laboratories have successfully employed this technology to introduce mutations in the non-coding HIV-1 LTR promoter regions as well as the coding sequences for various viral proteins, causing permanent inactivation of viral gene expression and replication in cell cultures and small animal models.[26–32] Simultaneous use of multiple gRNAs for targeting and editing various regions within the viral genome has led to the removal of large intervening segments of viral DNA and reduces the risk of mutant “escape” virus emerging.[33–35]
Other safety concerns, including off-target effects and other potentially undesired changes in chromosome and cell homeostasis caused by the presence of CRISPR/Cas, need close attention in the design and implementation of this strategy for targeting the viral genome.[36] As noted above, effective delivery of the CRISPR/Cas construct to the sites of virus latency presents another challenge under intense investigation.[37] Recent studies have shown widespread distribution of a non-integrating AAV vector in mouse models harboring the HIV-1 genome, with efficient editing of the viral DNA in various sites including lymphoid organs.[38,39] Nanoparticles and extracellular vesicles are alternative promising methods for targeted delivery of CRISPR/Cas.[40–42] Regardless of the method of delivery, one important issue relates to the genetic variations seen in the patient-derived HIV-1 sequence and how that would affect the creation of sets of universal gRNA’s for this therapeutic strategy. Indeed, as the technology advances, one may begin to personalize the strategy for elimination of replication competent viral quasispecies present in the patient.[43] Nevertheless, it remains to be seen whether the CRISPR/Cas technology, alone or in combination with other strategies, can eliminate replication competent virus in chronically infected non-human primates and humans.
Immunological Strategies
Virus-targeting strategies are unlikely to completely eliminate all latent viral elements on their own. Potent immunological targeting of residual cells harboring latent virus will be needed to provide synergy and contain the potential re-emergence of replication-competent virus from hidden sanctuaries (Fig.1). There is widespread belief that cells harboring latent viral genomes do not express viral antigens and avoid being subject to immune recognition and attack, but whether partial or complete expression of viral antigens occurs intermittently has not been fully explored. HIV-1 vaccines designed to improve HIV-specific immune responses in individuals already infected with HIV-1 have yet to demonstrate substantial potency at controlling viremia, although there have been some hints of activity.[44]
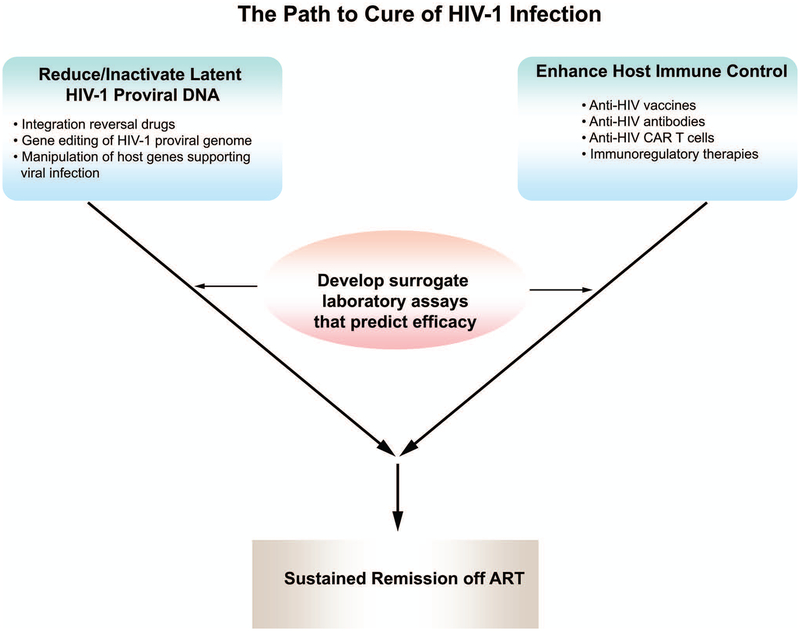
Figure 1.
Illustration of the combined strategy of reducing the latent proviral reservoir together with enhancing immune activity against HIV-1 to achieve a sustained remission of active infection off antiretroviral therapy (ART). The several approaches to targeting the latent proviral genome, as well as measures to improve host immune control of viral replication, are listed. To expedite the development of both types of cure strategies, laboratory assays that measure the size of the latent proviral reservoir and assays that measure anti-HIV host immune responses proven to contribute to reducing the latent proviral reservoir or to controlling viral replication in the absence of ART need to be developed and clinically validated as predictive of sustained remission of active infection.
Most therapeutic HIV-1 vaccines, whether protein-, peptide-, or DNA-based, have been based on consensus HIV-1 antigens. Newer strategies are exploring conserved epitopes that, during natural infection, elicit subdominant immune responses that are overshadowed by dominant immune repsones to variable epitopes to which the virus readily escapes.[45] Acknowledging the wide genetic variability of HIV-1 in the infected population, providing pulsed exposure to autologius viral antigens with brief pauses of ART after long-term ART suppression of virus, has demonstrated evidence of containing viral replication,[46] but attempts to build on this finding with studies of autologous viral antigens presented on autologous dendritic cells have been disappointing.[47–49]
As with the laboratory assays measuring the replication competent HIV-1 latent cell reservoir, there is no clinically reliable laboratory surrogate of improved host control of viral replication as a result of an immunological intervention that can predict the viral kinetics observed (compared to those in controls) during a subsequent antiretroviral drug interruption or measure an improved immunological effect at enhancing elimination of the latent cell reservoir. There is an urgent need to develop and clinically validate such assays.
A newer generation of more potent broadly active neutralizing antibodies against HIV-1 have been generated from infected persons. Initial clinical studies have demonstrated persistent antiviral suppressive activity when substituted as maintenance therapy in HIV-infected individuals receiving ART.[50,51] Because of some degree of baseline and treatment-emergent resistance, these antibodies need to be given in combination. To effect a cure of HIV-1 infection by targeting latently infected cells that are induced to present HIV-1 antigens, it will probably be necessary to engineer the Fc activities of these antibodies or other antibodies to engage natural killer (NK) cell or phagocytic cell functions.[52] With the same goal, other bispecific and trispecific antibodies are being designed to combine anti-HIV envelope specificity with cytolytic cell binding specificity.[53] Finally, genetically modified chimeric antigen receptor (CAR) CD8 T lymphocytes are being engineered with MHC-independent receptors capable of binding HIV-1 envelope such as CD4 or anti-envelope antibodies. Coupled to an intracellular signaling molecule activated upon binding, these cytolytic cells would serve to target HIV-1 antigen expressing cells.[54]
An anti-α4β7 antibody, an inhibitor of cell trafficking to the gastrointestinal tract, induced long-term remission of SIV infection off ART in a non-human primate (NHP) model,[55] but these promising results were not repeated in follow-up NHP and human studies.[56,57]
Finally, an alternative immunological strategy to contribute to a cure of HIV-1 infection involves the reversal of immune cell exhaustion and the re-stimulation of effective HIV-directed responses with checkpoint inhibitors and anti-regulatory T cell therapies.[58] These broadly active techniques risk off-target adverse autoimmune reactions in individuals otherwise faring well on suppressive ART.[59]
Conclusions
A much greater understanding of both the promise and the difficulties of the path toward curing HIV-1 infection has become apparent. Much has yet to be learned. From the current vantage point, it would appear that the most effective strategy is likely to combine the genetic inactivation of latent viral genomes with efficient HIV-directed immune attack. That said, if a cure of HIV-1 infection is achieved, questions will remain as to the residual immune damage left in its wake and its reversibility.[60,61]
Acknowledgments
The authors wish to thank past and present members of the Department of Neuroscience and the Center for Neurovirology for their continued support and sharing of ideas. This work was made possible by the Comprehensive NeuroAIDS Center grant (P30MH092177) awarded by NIH to KK.
Footnotes
Conflict of interest: KK is a named inventos on patents that cover the viral gene editing technology that is the subject of this journal article. In addition to the foregoing interests, KK is a co-founder, board member, scientific advisor, and holds equity in Excision Biotherapeutics, a biotech start-up that has licensed the viral gene editing technology from Temple University for commercial development and clinical trials. The authors declare that this work was produced solely by the authors and that no other individuals or entities influenced any aspect of the work. No other entities provided funds for the work. The authors further declare that they have received no financial compensation from any other third parties for any aspect of the published work.
References
- 1.Finzi D, Blankson J, Siliciano J, Margolick JB, Chadwick K, Pierson T, et al. Latent infection of CD4+ T cells provides a mechanism for lifelong persistence of HIV-1, even in patients on effective combination therapy. Nat Med. 1999; 5:512–7. [DOI] [PubMed] [Google Scholar]
- 2.Koenig S, Gendelman HE, Orenstein JM, Dal Canto MC, Pezeshkpour GH, Yungbluth M, et al. Detection of AIDS virus in macrophages in brain tissue from AIDS patients with encephalopathy. Science. 1986; 233:1089–93. [DOI] [PubMed] [Google Scholar]
- 3.Bronnimann MP, Skinner PJ, Connick E. The B-Cell follicle in HIV Infection: barrier to a cure. Front Immunol. 2018; 9:20. doi: 10.3389/fimmu.2018.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fletcher CV, Staskus K, Wietgrefe SW, Rothenberger M, Reilly C, Chipman JG, et al. Persistent HIV-1 replication is associated with lower antiretroviral drug concentrations in lymphatic tissue. Proc Natl Acad Sci U S A. 2014; 111:2307–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hunt PW, Brenchley J, Sinclair E, McCune JM, Roland M, Page-Shafer K, et al. Relationship between T cell activation and CD4+ T cell count in HIV-seropositive individuals with undetectable plasma HIV RNA levels in the absence of therapy. J Infect Dis. 2008;197:126–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hütter G, Nowak D, Mossner M, Ganepola S, Müssig A, Allers K, et al. Long-term control of HIV by CCR5 Delta32/Delta32 stem-cell transplantation. N Engl J Med. 2009; 360:692–8. [DOI] [PubMed] [Google Scholar]
- 7.Brown TR. The Conference on Cell & Gene Therapy for HIV Cure. Fred Hutchinson Cancer Research Center; Seattle, Washington USA: August 17–18, 2017. [Google Scholar]
- 8.Henrich TJ, Hanhauser E, Marty FM, Sirignano MN, Keating S, Lee TH, et al. Antiretroviral-free HIV-1 remission and viral rebound after allogeneic stem cell transplantation: report of 2 cases. Ann Intern Med. 2014; 161:319–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bruner K, Murray AJ, Ho Y-C, Laird G, Wang Z, Kwon KJ, et al. Novel Paradigm for measuring HIV-1 reservoir allows quantitation of intact proviruses. 25th Conference on Retroviruses and Opportunistic Infections Boston, MA March 2018 Abstract 151. [Google Scholar]
- 10.Luzuriaga K, Gay H, Ziemniak C, Sanborn KB, Somasundaran M, Rainwater-Lovett K, et al. Viremic relapse after HIV-1 remission in a perinatally infected child. N Engl J Med. 2015; 372:786–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Colby DJ, Trautmann L, Pinyakorn S, Leyre L, Pagliuzza A, Kroon E, et al. Rapid HIV RNA rebound after antiretroviral treatment interruption in persons durably suppressed in Fiebig 1 acute HIV infection. Nat Med. 2018; 24:923–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williams JP, Hurst J, Stohr W, Robinson N, Brown H, Fisher M, et al. HIV-1 DNA predicts disease progression and post-treatment virological control. Elife. 2014; 3:e03821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Assoumou L, Weiss L, Piketty C, Burgard M, Melard A, Girard PM, et al. A low HIV-DNA level in PBMCs at antiretroviral treatment interruption predicts a higher probability of maintaining viral control. AIDS. 2015;29 (E-pub). [DOI] [PubMed] [Google Scholar]
- 14.Li JZ, Etemad B, Ahmed H, Aga E, Bosch RJ, Mellors JW, et al. The size of the expressed HIV reservoir predicts timing of viral rebound after treatment interruption. AIDS. 2016; 30:343–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Archin NM, Bateson R, Tripathy MK, Crooks AM, Yang KH, Dahl NP, et al. HIV-1 expression within resting CD4+ T cells after multiple doses of vorinostat. J Infect Dis. 2014; 210:728–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elliott JH, Wightman F, Solomon A, Ghneim K, Ahlers J, Cameron MJ, et al. Activation of HIV transcription with short-course vorinostat in HIV-infected patients on suppressive antiretroviral therapy. PLoS Pathog 2014; 10:e1004473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rasmussen TA, Tolstrup M, Brinkmann CR, Olesen R, Erikstrup C, Solomon A, et al. Panobinostat, a histone deacetylase inhibitor, for latent-virus reactivation in HIV-infected patients on suppressive antiretroviral therapy: a phase 1/2, single group, clinical trial. Lancet HIV 2014; 1:e13–e21. [DOI] [PubMed] [Google Scholar]
- 18.Elliott JH, McMahon JH, Chang CC, Lee SA, Hartogensis W, Bumpus N, et al. Short-term administration of disulfiram for reversal of latent HIV infection: a phase 2 dose-escalation study. Lancet HIV. 2015; 2:e520–e529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shan L, Deng K, Shroff NS, Durand CM, Rabi SA, Yang HC, et al. Stimulation of HIV-1-specific cytolytic T lymphocytes facilitates elimination of latent viral reservoir after virus reactivation. Immunity 2012;36:491–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tebas P, Stein D, Tang WW, Frank I, Wang SQ, Lee G, et al. Gene editing of CCR5 in autologous CD4 T cells of persons infected with HIV. N Engl J Med. 2014;370: 901–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.White M, Whittaker R, Gandara C, Stoll EA. A guide to approaching regulatory considerations for lentiviral-mediated gene therapies. Hum Gene Ther Methods. 2017; 28:163–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao Y, Stepto H, Schneider CK. Development of the first World Health Organization lentiviral vector standard: toward the production control and standardization of lentivirus-based gene therapy products. Hum Gene Ther Methods. 2017; 28:205–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saraiva J, Nobre RJ, Pereira de Almeida L. Gene therapy for the CNS using AAVs: the impact of systemic delivery by AAV9. J Control Release. 2016; 241:94–109. [DOI] [PubMed] [Google Scholar]
- 24.Naso MF, Tomkowicz B, Perry WL, Strohl WR. Adeno-associated virus (AAV) as a vector for gene therapy. BioDrugs. 2017; 31:317–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, Charpentier E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science. 2012; 337:816–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hu W, Kaminski R, Yang F, Zhang Y, Cosentino L, Li F, et al. RNA-directed gene editing specifically eradicates latent and prevents new HIV-1 infection. Proc Natl Acad Sci USA 2014; 111:11461–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaminski R, Chen Y, Fischer T, Tedaldi E, Napoli A, Zhang Y, et al. Elimination of HIV-1 genomes from human T-lymphoid cells by CRISPR/Cas9 gene editing. Sci Rep 2016; 6:22555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lebbink RJ, de Jong DC, Wolters F, Kruse EM, van Ham PM, Wiertz EJ, Nijhuis M. A combinational CRISPR/Cas9 gene editing approach can halt HIV replication and prevent viral escape. Sci Rep 2017; 7:41968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liao HK, Gu Y, Diaz A, Marlett J, Takahashi Y, Li M, et al. Use of CRISPR/Cas9 system as an intracellular defense against HIV-1 infection in human cells. Nat Commun 2015; 6:6413. [DOI] [PubMed] [Google Scholar]
- 30.Yin C, Zhang T, Li F, Yang F, Putatunda R, Young WB, et al. Functional screening of guide RNAs targeting the regulatory and structural HIV-1 viral genome for a cure of AIDS. AIDS 2016; 30:1163–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang Z and Nair M. A CRISPR/Cas9 guidance RNA screen platform for HIV provirus disruption and HIV/AIDS gene therapy in astrocytes. Sci Rep 2017; 7:5955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Silva Feelixge HS, Jerome KR. Excision of latent HIV-1 from infected cells in vivo. Molec Ther. 2017; 25:1062–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang G, Zhao N, Berkhout B, Das AT. CRISPR-Cas9 can inhibit HIV-1 replication by NHEJ repair facilitates virus escape. Mol Ther 2016; 24:522–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Z, Pan Q, Gendron P, Zhu W, Guo F, Cen S, et al. CRISPR/Cas9-derived mutations both inhibit HIV-1 replication and accelerate viral escape. Cell Rep 2016; 15:481–89. [DOI] [PubMed] [Google Scholar]
- 35.White MK, Hu W, Khalili K Gene editing approaches against viral infections and strategy to prevent occurrence of viral escape. PloS Pathogens 2016; 12:e1005953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dampier W, Sullivan NT, Chung C-H, Mell JC, Nonnemacher MR, Wigdahl B. Designing broad spectrum anti-HIV-1 gRNAs to target patient-derived variants. Sci Rep 2017; 7:14413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yin H, Song C-Q, Dorkin JR, Zhu LJ, Li Y, Wu Q, et al. Therapeutic genome editing by combined viral and non-viral delivery of CRISPR system components in vivo. Nat Biotechnol. 2016; 34:328–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaminski R, Bella R, Yin C, Otte J, Ferrante P, Gendelman HE, et al. Excision of HIV-1 DNA by gene editing: proof-of-concept in vivo study. Gene Ther 2016; 23:696. [DOI] [PubMed] [Google Scholar]
- 39.Yin C, Zhang T, Li F, Yang F, Putatunda R, Young WB, et al. 2016. Functional screening of guide RNAs targeting the regulatory and structural HIV-1 viral genome for a cure of AIDS. AIDS. 2016: 30:1163–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Finn JD, Smith AR, Patel MC, Shaw L, Youniss MR, van Hetern J, et al. A single administration of CRISPR/Cas9 lipid nanoparticles achieves robust and persistent in vivo genome editing. Cell Rep 2018; 22:2227–35. [DOI] [PubMed] [Google Scholar]
- 41.Glass Z, Li Y, Xu Q. Nanoparticles for CRISPR-Cas9 delivery. Nat Bio Med Eng 2017. 1:854–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yin H, Song CQ, Dorkin JR, Zhu LJ, Li Y, Wu Q, et al. Therapeutic genome editing by combined viral and non-viral delivery of CRISPR system components in vivo. Nat Biotech 2016; 34:328–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dampier W, Sullivan NT, Mell JC, Pirrone V, Ehrlich GD, Chung CH, et al. Broad spectrum and personalized gRNAs for CRISPR/Cas9 HIV-1 therapeutics. AIDS Res Hum Retrovirus 2018. August 27. doi: 10.1089/AID.2017.0274. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schooley RT, Spritzler J, Wang H, Lederman MM, Havlir D, Kuritzkes DR, et al. AIDS clinical trials group 5197: a placebo-controlled trial of immunization of HIV-1-infected persons with a replication-deficient adenovirus type 5 vaccine expressing the HIV-1 core protein. J Infect Dis. 2010;202:705–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Munson P, Liu Y, Bratt D, Fuller JT, Hu X, Pavlakis GN, et al. Therapeutic conserved elements (CE) DNA vaccine induces strong T-cell responses against highly conserved viral sequences during simian-human immunodeficiency virus infection. Hum Vaccin Immunother. 2018;14:1820–1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jacobson JM, Bucy RP, Spritzler J, Saag MS, Eron JJ Jr, Coombs RW, et al. Evidence that intermittent structured treatment interruption, but not immunization with ALVAC-HIV vCP 1452, promotes host control of HIV replication: the results of AIDS Clinical Trials Group 5068. J Infect Dis. 2006;194:623–32. [DOI] [PubMed] [Google Scholar]
- 47.García F, Climent N, Guardo AC, Gil C, León A, Autran B, et al. A dendritic cell-based vaccine elicits T cell responses associated with control of HIV-1 replication. Sci Transl Med. 2013;5:166ra2. [DOI] [PubMed] [Google Scholar]
- 48.Jacobson JM, Routy JP, Welles S, DeBenedette M, Tcherepanova I, Angel JB, et al. Dendritic cell immunotherapy for HIV-1 infection using autologous HIV-1 RNA: a randomized, double-blind, placebo-controlled clinical trial. J Acquir Immune Defic Syndr. 2016; 72:31–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Macatangay BJ, Riddler SA, Wheeler ND, Spindler J, Lawani M, Hong F, et al. Therapeutic vaccination with dendritic cells loaded with autologous HIV type 1-infected apoptotic cells. J Infect Dis. 2016;213:1400–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bar KJ, Sneller MC, Harrison LJ, Justement JS, Overton ET, Petrone ME, et al. Effect of HIV Antibody VRC01 on Viral Rebound after Treatment Interruption. N Engl J Med. 2016;375:2037–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Scheid JF, Horwitz JA, Bar-On Y, Kreider EF, Lu CL, Lorenzi JC, et al. HIV-1 antibody 3BNC117 suppresses viral rebound in humans during treatment interruption. Nature. 2016;535:556–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gunn BM, Alter G. Modulating antibody functionality in infectious disease and vaccination. Trends Mol Med. 2016;22:969–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ferrari G, Haynes BF, Koenig S, Nordstrom JL, Margolis DM, Tomaras GD. Envelope-specific antibodies and antibody-derived molecules for treating and curing HIV infection. Nat Rev Drug Discov. 2016. December;15(12):823–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Leibman RS, Richardson MW, Ellebrecht CT, Maldini CR, Glover JA, Secreto AJ, et al. Supraphysiologic control over HIV-1 replication mediated by CD8 T cells expressing a re-engineered CD4-based chimeric antigen receptor. PLoS Pathog. 2017;13:e1006613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Byrareddy SN, Arthos J, Cicala C, Villinger F, Ortiz KT, Little D, et al. Sustained virologic control in SIV+ macaques after antiretroviral and α4β7 antibody therapy. Science. 2016;354:197–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.DiMascio M, Lifson JD, Srinivasula S, DeGrange P, Keele B, Wang Y, et al. Evaluation of an antibody to alpha4beta7 in the control of SIV infection. 22nd International AIDS Conference. Amsterdam, Netherlands, July 23–27 2018. 22nd International AIDS Conference Amsterdam, Netherlands, July 23–27 2018. [Google Scholar]
- 57.Fauci AS. Durable control of HIV infection in the absence of antiretroviral therapy: opportunities and obstacles. [DOI] [PubMed]
- 58.Evans VA, van der Sluis RM, Solomon A, Dantanarayana A, McNeil C, Garsia R, et al. Programmed cell death-1 contributes to the establishment and maintenance of HIV-1 latency. AIDS. 2018;32:1491–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gay CL, Bosch RJ, Ritz J, Hataye JM, Aga E, Tressler RL, et al. Clinical Trial of the Anti-PD-L1 Antibody BMS-936559 in HIV-1 Infected Participants on Suppressive Antiretroviral Therapy. J Infect Dis. 2017;215:1725–1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lange CG, Lederman MM, Medvik K, Asaad R, Wild M, Kalayjian R, Valdez H. Nadir CD4+ T-cell count and numbers of CD28+ CD4+ T-cells predict functional responses to immunizations in chronic HIV-1 infection. AIDS. 2003;17:2015–23. [DOI] [PubMed] [Google Scholar]
- 61.Zeng M, Southern PJ, Reilly CS, Beilman GJ, Chipman JG, Schacker TW, Haase AT. Lymphoid tissue damage in HIV-1 infection depletes naïve T cells and limits T cell reconstitution after antiretroviral therapy. PLoS Pathog. 2012. January;8:e1002437. [DOI] [PMC free article] [PubMed] [Google Scholar]