Abstract
In the present study, we investigated whether colony‐stimulating factor 1 receptor (CSF1R) is expressed on brain macrophages and microglia in the human and macaque brain and whether it is upregulated and activated after lentivirus infection in vivo and contributes to development of encephalitic lesions. We examined, using multi‐label and semi‐quantitative immunofluorescence microscopy, the protein expression level and cellular localization of CSF1R in brain tissues from uninfected controls and SIV‐infected adult macaques with or without encephalitis and also from uninfected controls, HIV‐infected encephalitic subjects and virally suppressed subjects. In the normal uninfected brain, CSF1R protein was detected only on microglia and brain macrophages but not on neurons, astrocytes or oligodendrocytes. Microglia constitutively expressed CSF1R at low levels, and its expression was largely unchanged in non‐encephalitic and encephalitic animals. Brain macrophages, including perivascular macrophages (PVMs), expressed higher levels of CSF1R compared to microglia. Interestingly, we found significantly increased expression of CSF1R on the infected PVMs and lesional macrophages in the brains of encephalitic macaques. Moreover, the per cell expression of CSF1R determined by its mean pixel intensity (MPI) correlated positively with the MPI of SIV Gag p28 in SIV‐infected PVMs. Using phosphorylated CSF1R at tyrosine residue 723 and phosphorylated signal transducer and activator of transcription 5 at tyrosine reside 694 as markers for CSF1R activation, we found selective activation of CSF1R signaling in infected brain macrophages in encephalitis. We also found colocalization of CSF1R and its ligand CSF1 in PVMs and lesional macrophages in the brains of encephalitic macaques and humans. Notably, elevated brain CSF1R expression was found in virally suppressed subjects. These findings point to opportunities for developing a specific approach targeting infected brain macrophages, with several brain‐penetrant CSF1R inhibitors that are available now, in order to eliminate central nervous system macrophage reservoirs, while not affecting resting uninfected microglia and PVMs that show no CSF1R activation.
Keywords: AIDS, HIV encephalitis, macrophage, microglia, M‐CSF, neuroinflammation
Introduction
Macrophage reservoirs for HIV within the central nervous system (CNS) are thought to contribute to the development and progression of neurological complications known collectively as HIV‐associated neurocognitive disorder (HAND) 4, 43, 46, which is of significant clinical concern due to its continued prevalence in virally suppressed individuals on antiretroviral therapy (ART) 44. Infected perivascular macrophages (PVMs) and microglia mediate HIV neuropathogenesis through the spread of virus and production of soluble factors that can lead to impairment of neuronal and glial cell function. Due to this, understanding how the macrophage viral reservoir is established and maintained is essential for designing therapeutic strategies aimed at elimination of this reservoir and the prevention/treatment of HAND.
Through genetic knockout and pharmacological targeting in mice, colony‐stimulating factor 1 (CSF1)/CSF1 receptor (CSF1R) signaling has been shown to be essential for myeloid cell survival, proliferation and differentiation 11, 12, 14, 15, 24, 38, 41, 45. CSF1 [also known as macrophage colony‐stimulating factor (M‐CSF)] and interleukin‐34 (IL‐34) constitute the ligands for CSF1R 37. Although there exist differences in receptor affinity, signaling strength and expression profiles between CSF1 and IL‐34 6, CSF1R ligation by either CSF1 or IL‐34 leads to phosphorylation of the same tyrosine residues of the intracellular domain of the receptor, suggesting potential shared functions by the two ligands through CSF1R 6, 52. Interestingly, CSF1 signaling has been shown to play important roles in HIV infection of macrophages and HIV‐associated CNS disease 2, 10, 20, 22, 23, 28, 32, 33, 36, 39, 42, 47. It appears that by forming a positive feedback loop with HIV‐1, CSF1 can enhance HIV‐1 pathogenesis and progression driven by macrophages. A recent study using simian immunodeficiency virus (SIV)‐infected rhesus macaques showed that PVMs and macrophages in encephalitic lesions (the so‐called lesional macrophages) produce high levels of CSF1 in vivo 22, 23. Moreover, levels of CSF1 in the cerebrospinal fluid (CSF) were positively correlated with the degree of neurocognitive impairment in HIV‐infected patients on suppressive ART 36.
Largely due to lack, until recently, of anti‐human or macaque CSF1R antibodies that are effective on formalin‐fixed paraffin embedded (FFPE) tissues, little has been known about its expression in humans or nonhuman primates. In the current study, using a newly developed CSF1R antibody that works for immunohistochemical staining in FFPE tissues, we sought to examine its expression in brains of SIV‐infected macaques and HIV‐infected individuals compared to uninfected controls, while characterizing the phenotype of cells expressing CSF1R in the brain in relation to virus infection and efficacy of a potential treatment for eliminating macrophage reservoirs through CSF1R blockade.
Materials and Methods
Animals
Ten adult rhesus macaques listed in Table 1 were used in this study. The animals were housed at Tulane National Primate Research Center (TNPRC), with procedures approved by the Tulane University Institutional Animal Care and Use Committee in accordance with the National Institutes of Health “Guide for the Care and Use of Laboratory Animals” and recommendations of the Weatherall report, “The use of non‐human primates in research.” All possible measures were taken to minimize discomfort for the animals for routine procedures such as physical examination and blood collection, where animals were fully anesthetized with ketamine HCl under the direction of a veterinarian. Animals were humanely euthanized at the TNPRC with respect to its endpoint policy. The criteria for euthanasia included 15% weight loss in 2 weeks, unresponsive opportunistic infection, persistent anorexia, severe intractable diarrhea, progressive neurological signs, significant cardiac and/or pulmonary signs or any other serious illness. In accordance with American Veterinary Medical Association Guidelines, euthanasia was conducted by anesthesia with ketamine HCl (10 mg/kg) followed by a sodium pentobarbital overdose.
Table 1.
Animals used in the study.
| Animal necropsy ID | Age (years) at euthanasia and sex | Infection status | Length of SIV infection (weeks) |
|---|---|---|---|
| 11A014 | 4.77M | Uninfected | n.a. |
| 11A023 | 4.69M | Uninfected | n.a |
| 11A314 | 8.99M | Uninfected | n.a |
| 11A563 | 8.19M | SIVnoE | 10.0 |
| 12A136 | 5.69M | SIVnoE | 41.6 |
| 12A305 | 7.95M | SIVnoE | 50.4 |
| 10A067 | 5.70M | SIVE | 24.0 |
| 10A786 | 12.06M | SIVE (mild) | 6.7 |
| 12A715 | 5.62M | SIVE (severe) | 13.0 |
| 13A049 | 20.88F | SIVE | 9.7 |
Abbreviation: n.a. = not applicable.
Macaque tissue samples
Five‐micrometer‐thick FFPE tissue sections of the parietal cortices of the rhesus macaques listed in Table 1 were cut and placed on charged slides for use in immunohistochemistry (IHC) and immunofluorescence (IF) microscopy. In total, tissues from ten macaques, three uninfected controls and seven macaques intravenously infected with SIVmac251 virus (20 ng of SIV p27), were used. Perfusion was not performed at necropsy. Evidence of SIVE in four of the infected macaques was defined by the presence of SIV proteins in the brain and the accumulation of macrophages and multinucleated giant cells.
Human tissue samples
FFPE tissue sections of basal ganglia were obtained from the Manhattan HIV Brain Bank, through the National NeuroAIDS Tissue Consortium. A total of four HIVE cases, four HIV‐1‐infected virally suppressed cases and four seronegative controls that had been previously described elsewhere were examined (Table 2) 30.
Table 2.
Subjects involved in the study.
| Project ID | Age (years) at death and sex | Infection status at death | Length of time on suppressive ART (years) |
|---|---|---|---|
| 01640 | 63M | Uninfected | n.a. |
| 01819 | 65M | Uninfected | n.a. |
| 01821 | 26F | Uninfected | n.a. |
| 01923 | 61M | Uninfected | n.a. |
| 01555 | 47M | HIVE | n.a. |
| 01580 | 50M | HIVE | n.a. |
| 01598 | 45F | HIVE | n.a. |
| 02390 | 57F | HIVE | n.a. |
| 00658 | 52M | ART | >7 |
| 00719 | 44M | ART | ~2 |
| 01878 | 63M | ART | >7 |
| 02087 | 51M | ART | >24 |
Abbreviation: n.a. = not applicable.
Immunohistochemistry
IHC was performed using the antibodies listed in Table 3, as previously described 17. Deparaffinization and rehydration was performed after incubation for 16 h at 58°C–62°C. Sections were then pretreated for antigen retrieval with a citrate or Tris‐based Antigen Unmasking Solution (Vector Laboratories, Burlingame, CA) in a microwave (1000 W) for 20 minutes. After cooling for 20 minutes, sections were washed twice with Tris‐buffered saline (TBS) containing 0.05% Tween‐20 for 5 minutes, followed by incubation with a peroxidase blocking solution (Bloxall, Vector Laboratories) for 5 minutes. After washing again, sections were incubated with either 5% normal horse or goat serum in TBS for 30 minutes, and then incubated with primary antibodies for 1 h at room temperature or overnight at 4°C. After washing, sections were incubated with a biotinylated or horseradish peroxidase micropolymer‐conjugated secondary antibody (Vector Laboratories) for 30 minutes. Dako Antibody Diluent (Dako, Carpinteria, CA) was used for both primary and secondary antibody dilutions. Following another wash, the sections incubated with biotinylated secondary antibody were incubated for 30 minutes with an avidin–biotin peroxidase complex (Vectastain ABC Elite kit, Vector Laboratories). Sections were then developed with diaminobenzidine (DAB; Dako) with Mayer's Hematoxlyin (Dako) used as a nuclear counterstain. Sections were dehydrated and mounted using VectaMount (Vector Laboratories). Sections were visualized using a Nikon Coolscope digital microscope.
Table 3.
Antibodies used in the study.
| Antigen | Clone | Isotype | Manufacturer | IHC Conc. | IF Conc. |
|---|---|---|---|---|---|
| CD68 | KP1 | Mouse IgG1 | Thermo Scientific | 1:200 | 1:40 |
| CD163 | EDHu‐1 | Mouse IgG1 | BioRad | 1:250 | 1:50 |
| CSF1 | 2D10 | Mouse IgG1 | Millipore | 1:1000 | 1:200 |
| CSF1R | SP211 | Rabbit IgG | Spring Bioscience | 1:150 | 1:50 |
| Glut1 | SPM498 | Mouse IgG2a | Invitrogen | 1:2000 | 1:200 |
| PCNA | PC10 | Mouse IgG2a | Santa Cruz | 1:1000 | 1:200 |
| p‐CSF1R | Rabbit IgG | LSBio | 1:50 | ||
| p‐CSF1R | F.540.2 | Rabbit IgG | Invitrogen | 1:300 | |
| p‐Stat5 | E208 | Rabbit Mono | Abcam | 1:8000 | 1:1000 |
| p‐Stat5a/b | AX1 | Mouse IgG2b | Advantex Bio | 1:500 | 1:100 |
| SIVp28 | 3F7 | Mouse IgG1 | Fitzgerald | 1:1000 | 1:200 |
Immunofluorescence microscopy
To determine the phenotype of CSF1R expressing cells within the brain, double‐ or triple‐label immunofluorescence using cell type‐specific markers with markers for proliferation and SIV infection was performed. As described above, the sections were deparaffinized and rehydrated followed by antigen retrieval. This and all subsequent steps were followed by two 5‐minute phosphate buffer saline (PBS) washes containing 0.2% fish skin gelatin (FSG; Sigma Aldrich) unless otherwise stated. Sections were permeabilized using PBS/FSG solution containing 0.1% Triton X‐100 for 1 h. Sections were then incubated with either 5% normal goat or horse serum in PBS for 30 minutes, and were immediately followed by primary antibody diluted in PBS/FSG either for 1 h at room temperature or overnight at 4°C. After primary antibody incubation, the sections were incubated with Alexa Fluor 350‐, 488‐ or 594‐conjugated secondary antibodies (Molecular Probes; diluted at 1:500 or 1:1000 in PBS/FSG) at room temperature for 1 h. Subsequent primary antibody incubations for double‐ or triple‐label immunofluorescence followed the same procedure above. After immunofluorescence staining was complete, the sections were then treated with 10 mM CuSO4 solution in 50mM ammonium acetate buffer for 45 minutes to quench autofluorescence. The sections were immediately rinsed with distilled water and then mounted with Aqua‐Mount aqueous mounting medium (Thermo Scientific, Waltham, MA).
Immunohistochemistry image analysis and data collection
A Nikon Coolscope digital microscope was used to image immunohistochemically stained slides. Ten white matter (WM) and 10 gray matter (GM) images were captured at 20× using the same settings for all macaque and human tissues examined. The images were then processed in ImageJ/FIJI. The images were converted to grayscale before using thresholding to exclude background. The %area of the positive staining was then obtained using the “Measure” function. Values were transferred to Microsoft Excel and GraphPad Prism 7.2 where mean %area was calculated, graphically represented and statistical analysis was performed.
Immunofluorescence image analysis and data collection
A Zeiss Axio Observer.Z1 fluorescence microscope was used to analyze and image the fluorescently labeled sections. The images were captured and merged by Zeiss AxioVision Release 4.8.2. Exposure times were adjusted to reduce background on all values and kept constant on the color channel or channels being quantified across all the photos included in each data set. The images were then converted to JPEG files and processed in ImageJ/FIJI. First, the images were color balanced to constant values across the images used for quantification. Then, the data analysis differed depending on the size of the data set. In smaller data sets (<100 cells), each cell was chosen by visual morphology and outlined carefully in order to exclude external pixels and to prevent the exclusion of real cellular pixels. Parameters such as area and mean pixel value (in relation to the cell) (0–255, where 0 is no fluorescence and 255 is full fluorescent capacity) were measured and the data were transferred to Microsoft Excel. For much larger data sets (>100 cells) with high variation in background, cells were selected and analyzed via variable thresholding where a minimum pixel value was set as the threshold of where real staining can be distinguished from background staining and autofluorescence. This difference can be seen by a sharp increase in pixel values from surrounding pixels. Cells then had their boundaries determined automatically via the conversion to a binary image and using the “Analyze Particles” function. Size and circularity ranges of the particles were determined based on the cell targets. After all of the particles were gathered, they were examined for the appropriate properties of the target cell to identify and remove artifacts and other cells from the data collection. Values were transferred to Excel and GraphPad Prism 7.2 and then had corrections done on the values image by image depending on the threshold and subtracting that value out since the minimum value at that point is the threshold value.
Statistical analysis
GraphPad Prism 7.2 was used for the graphing and analysis of data for significance. Newman–Keuls test in conjunction with two‐way or one‐way ANOVA or two‐tailed, unpaired t‐test with Welch's correction were used to determine the significance of CSF1R %area and CSF1R mean pixel intensity (MPI) of PVMs, microglia and uninfected vs. infected cells. The Spearman's correlation coefficient was calculated to determine the relationship between CSF1R MPI and SIVp28 MPI of infected cells. A two‐tailed p‐value was calculated.
Results
Expression of CSF1R in normal brain
Little is known about CSF1R protein expression and function in human and macaque brain. This has been due to the paucity, until recently, of antibodies against human CSF1R that work for staining of FFPE tissues. We recently identified a new rabbit anti‐human CSF1R monoclonal antibody that is effective in FFPE tissues and cross‐reacts with rhesus macaque CSF1R, so we examined CSF1R protein expression by IHC in normal human and macaque brain tissues (Figure 1). Little staining was observed within the white matter (WM) of both human and macaque brain tissue (Figure 1B and E) except around vessels (Figure 1C and F). Gray matter (GM) showed higher expression than white matter (Figure 1A and D), although not significant (Supplementary Figure S1). Some weak staining was observed on what morphologically appeared to be microglia in the GM, which may account for the higher CSF1R expression levels in GM. Using multi‐label immunofluorescent staining on human and rhesus macaque brain tissues, we found that these CSF1R+ cells are Iba1+ microglia (Figure 1H and K) and PVMs (Figure 1G and J), with microglia appearing to express lower levels of CSF1R than PVMs (Figure 1I and L). These observations suggest that humans and rhesus macaques show similar CSF1R expression in normal brain, and that cells expressing CSF1R in the brain are only PVMs and microglia. We found no CSF1R expression on other cells within the brain.
Figure 1.
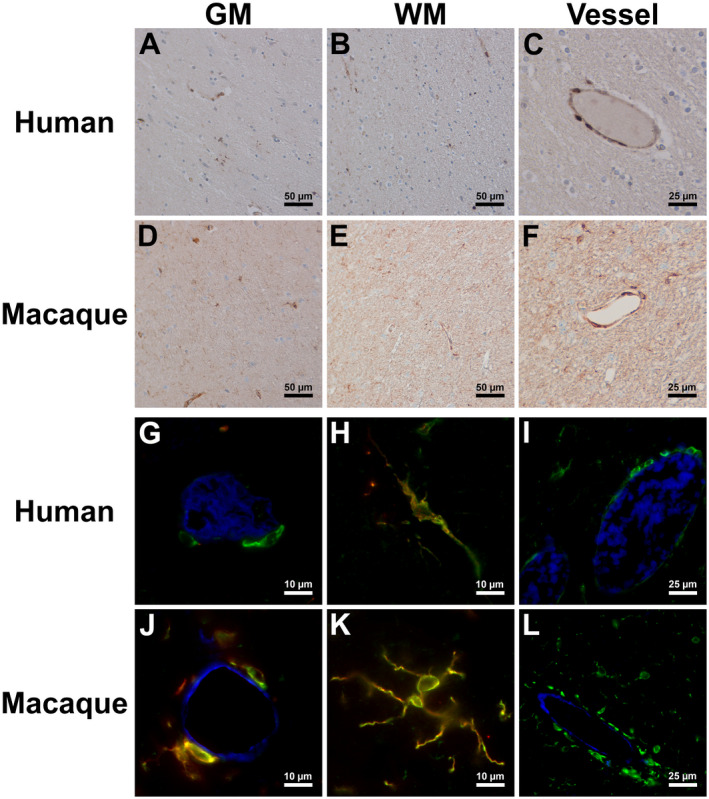
Expression of CSF1R in uninfected human and macaque brain. Single‐label immunohistochemistry for CSF1R counterstained with hematoxylin showed expression in human and macaque GM (A and D), WM (B and E) and around vessels (C and F), respectively. Triple‐label immunofluorescence for CSF1R (green), Iba1 (red) and Glut1 (blue) showed CSF1R expression in humans and macaques on PVMs around vessels (G and J), microglia in the parenchyma (H and K) and that PVMs appeared to express higher levels of CSF1R than microglia based on observations of CSF1R immunofluorescent intensity (I and L).
Upregulation of CSF1R expression in HIV/SIV‐infected brain
Since CSF1R protein was expressed exclusively on brain myeloid cells that are targets of virus and sources of virus persistence in the brain with HIV/SIV infection, next we investigated by IHC if the expression of this receptor changed with infection (Figure 2). Expression of CSF1R was highly upregulated in the brains of SIV‐infected macaques compared to uninfected macaques. CSF1R expression in GM was comparable between encephalitic and non‐encephalitic animals (Supplementary Figure S1). In addition, encephalitic animals showed significantly higher expression in WM compared to non‐encephalitic macaques. This upregulation was due mainly to the presence of SIVE lesions that are characterized by accumulation of CSF1R+ macrophages (Figure 2C and F). There were no observable differences between non‐encephalitic and encephalitic macaque brain tissues when lesions were excluded. The GM of infected humans with encephalitis and macaques with or without encephalitis showed more CSF1R+ cells than that of normal human and macaque brains (Figure 2A and D, Supplementary Figure S1). CSF1R expression was also increased in the WM of infected humans and macaques when compared to the normal uninfected WM (Figure 2B and E, Supplementary Figure S1). To examine whether the expression of this receptor was upregulated on a per cell basis during HIV/SIV infection, we performed quantitative immunofluorescence microscopy on macaque brain tissue. Measuring the mean intensity per pixel of each cell expressing CSF1R as a measure of cellular protein expression, we found no significant differences in expression of CSF1R on PVMs (Figure 2G) or microglia (Figure 2H) among three macaque groups, namely uninfected, SIV‐infected, non‐encephalitic (SIVnoE) and SIV‐infected, encephalitic (SIVE). When PVM MPI data from all three groups was compared to microglia MPI data from all three groups, a statistically significant difference was observed between these two cell types (Figure 2I), with CSF1R expression being significantly higher on PVMs than microglia. When comparing activated vs. ramified microglia in the SIVnoE and SIVE groups using morphology to distinguish between the two, a statistically significant increase in CSF1R expression was found on the activated microglia compared to ramified microglia (Figure 2J), suggesting that activation of microglia during infection leads to an increase in CSF1R expression.
Figure 2.
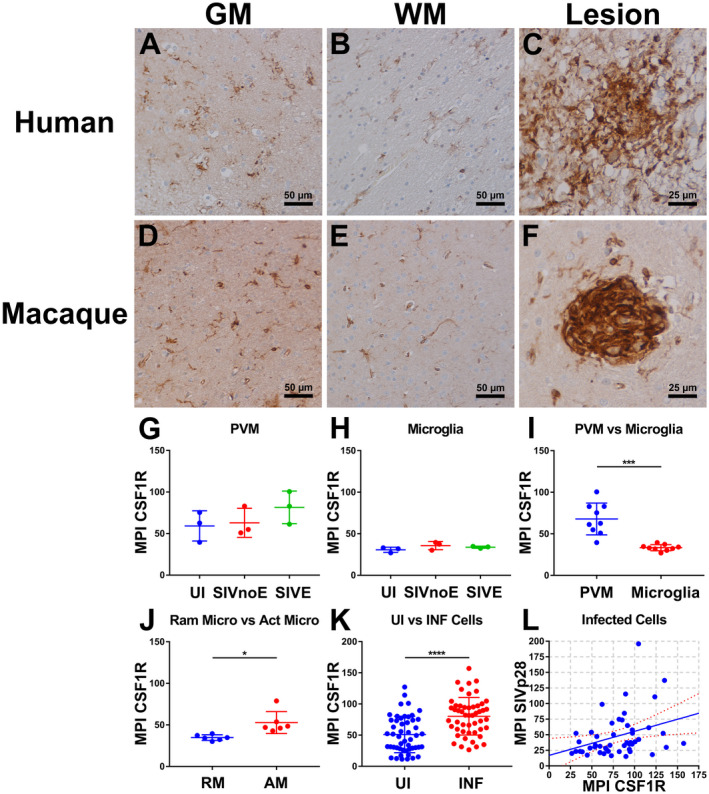
Expression of CSF1R in HIV/SIV infected human and macaque brain with expression depending on cell type and infection. Single‐label immunohistochemistry for CSF1R counterstained with hematoxylin showed expression of CSF1R with HIV/SIV infection within human and macaque GM (A and D), WM (B and E) and within lesions (C and F), respectively. The column scatter plots show PVM (G) and microglial expression (H) between the uninfected, SIVnoE and SIVE groups, with no significance seen. PVMs expressed significantly higher levels of CSF1R than microglia (I) when the values of all groups were used for comparison (P = 0.0005; unpaired two‐tailed t‐test; n = 9 per group). There was also a significant increase in expression of CSF1R on activated microglia vs. ramified microglia (P = 0.0188; unpaired two‐tailed t‐test; n = 6 per group) (J). SIV‐infected cells showed a significant increase in CSF1R expression compared to uninfected cells (P < 0.0001; unpaired two‐tailed t‐test; n = 50 per group) (K). Quantification of infected cells showed a weak but significant positive correlation between CSF1R expression and SIVp28 expression (r = 0.3332, P = 0.0181) (L). For A–J, three SIVE cases, for K and L, four SIVE cases were used.
Although there was no significant increase in CSF1R expression on cells within the brain with SIV infection, an upward trend in CSF1R expression was observed as the degree of infection increased (Figure 2G and H). We, therefore, hypothesized that this may be due to a selective upregulation of CSF1R on SIV‐infected macrophages, and that the expression levels between groups not being significantly different was due to the majority of CSF1R+ macrophages/microglia included in the previous analysis not being infected by SIV. After separating CSF1R+ cells based on expression of SIV p28, SIVp28+ cells showed a significantly higher level of expression of CSF1R compared to SIVp28– cells in SIVE brain (Figure 2K). To evaluate the correlation between expression levels of CSF1R and SIV p28, the MPIs of CSF1R and SIV p28 that were simultaneously obtained from the same cells were plotted. Interestingly, there was a weak but significant positive correlation between CSF1R and SIV p28 MPIs (Figure 2L). This confirms that SIV infection of PVMs and microglia within the brain leads to an increase in their expression of CSF1R.
CSF1R activation in SIVE/HIVE brain
HIV infection has been shown to induce production of CSF1 by macrophages and elicit resistance to apoptosis through activation/phosphorylation of CSF1R on infected macrophages 10, 47. Previous research shows upregulation of CSF1 in the brains of SIV‐infected macaques 23, but the activation of the CSF1R tyrosine kinase or its downstream effectors likely by CSF1 binding has not been shown in vivo. We, therefore, sought to examine at the cellular level the activation of macrophage/microglial CSF1R in the brain either with SIV infection or by local high levels of CSF1. CSF1R+ macrophages in SIVE and HIVE lesions (Figure 3C and K) coexpressed a phosphorylated (activated) form of CSF1R (Figure 3D and L). Little CSF1 expression was seen in uninfected brain (Figure 3A and I), but in macaques with SIVE and humans with HIVE, CSF1 was observed at high levels within lesions and was colocalized with CSF1R+ cells (Figure 3B and J), strongly suggesting a positive feedback loop between viral infection and CSF1/CSF1R signaling in macrophages. Signal transducer and activator of transcription 5 (Stat5) is a known downstream effector of the CSF1R pathway 1, 6. STAT5a and STAT5b are highly phosphorylated in the CSF1Rhigh population compared to the CSF1Rlow population. With a recently developed antibody against phospho‐STAT5a/b (pSTAT5a/b) that cross‐reacts with rhesus macaque, little‐to‐no pStat5a/b was observed in the brains of uninfected macaques and humans (Figure 3E and M), while increased expression was observed in SIVE and HIVE lesions (Figure 3F and N). pSTAT5a/b was observed most frequently in SIVp28 and CSF1R double‐positive cells, suggesting that SIV infection can cause stronger activation of the CSF1R pathway in macrophages, while CSF1 induces a widespread activation of CSF1R in SIVE brain (Figure 3D).
Figure 3.
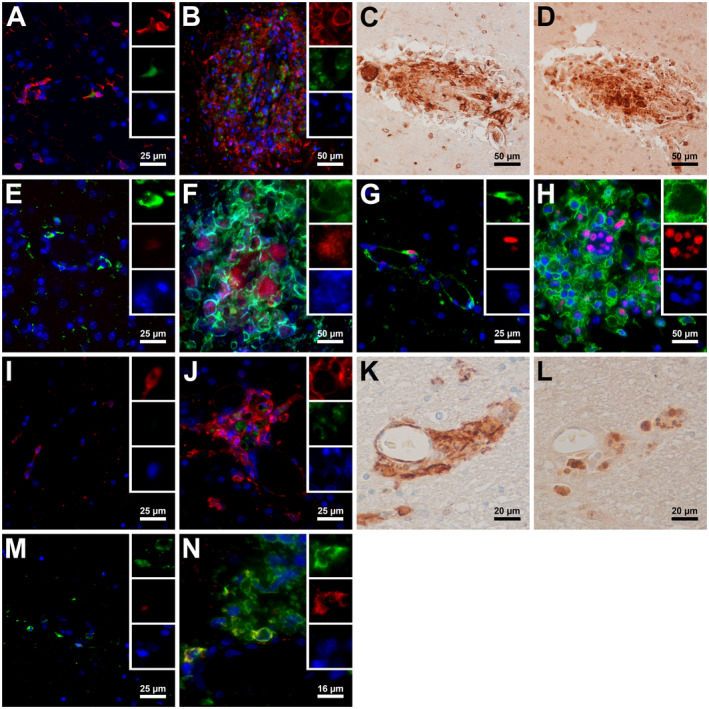
CSF1R and downstream pathway activation within the brain due to SIV and HIV infection. Triple‐label immunofluorescence for CSF1R (red), CSF1 (green) and DAPI (blue) in macaques and humans showed that little to no CSF1 expression was observed in uninfected brains (A and I), while encephalitic lesions of SIV‐infected macaques and HIV‐infected humans showed high levels of CSF1 present (B and J). Single label IHC for CSF1R (C and K) and phosphorylated CSF1R (D and L) counterstained with hematoxylin showed that activated/phosphorylated CSF1R was present in CSF1R+ lesions on serial sections of macaque and human brains. Triple‐label immunofluorescence for CSF1R (green), phosphorylated Stat5a/b (red) and DAPI (blue) (E, M, and N) or SIVp28 (blue) (F) shows high levels of p‐Stat5a/b present in macaque and human lesions (F and N) with rare and very low expression of p‐Stat5a/b being found in uninfected macaque and human brains (E and M). Triple‐label immunofluorescence for CSF1R (green), PCNA (red) and DAPI (blue) found rare occurrences of PCNA in uninfected macaque brain (G) typically only in PVMs with high levels and upregulation being present in SIV lesions (H).
We recently demonstrated that proliferating (PCNA+/Ki‐67+) macrophages become more prevalent in SIVE and correlate with severity of SIVE lesions 17. Since macrophage proliferation can be induced by activation of CSF1R by CSF1, we examined PCNA expression in relation to CSF1R. Uninfected macaques showed little to no PCNA expression within the brain (Figure 3G), while SIVE macaques showed higher PCNA expression in CSF1Rhigh brain macrophages, especially within lesions (Figure 3H). This suggests that SIV‐induced CSF1 production and/or activation of CSF1R and its pathways promote proliferation of CSF1R+ brain macrophages.
Elevated expression of CSF1R in virally suppressed patients
Previously, Lentz et al. demonstrated that levels of CSF1 in the CSF of HIV patients receiving combination ART correlate with the degree of cognitive impairment 36. With the persistence of HAND despite suppressive ART, it stands to reason that the expression and activation of CSF1R are altered in the brain during chronic infection and long‐term suppressive ART. We, therefore, sought to determine if this was the case in the brains of HIV‐1‐infected aviremic individuals after suppressive ART (“virally suppressed” patients) (Table 2 and Figure 4). By IHC, we found heightened levels of expression of CSF1R in the brains of virally suppressed patients (Figure 4A–C), compared to uninfected individuals (Figure 1A–C, Supplementary Figure S1). Of note, CSF1R expression appeared the most elevated in the GM of the virally suppressed patients (Figure 4A, Supplementary Figure S1). This appears to be due to the increased numbers of CSF1R+ microglia in particular within the GM. Collectively, these data present in vivo evidence that HIV/SIV infection promotes the upregulation and activation of CSF1R in macrophages/microglia by inducing CSF1 production by infected cells in the brain.
Figure 4.
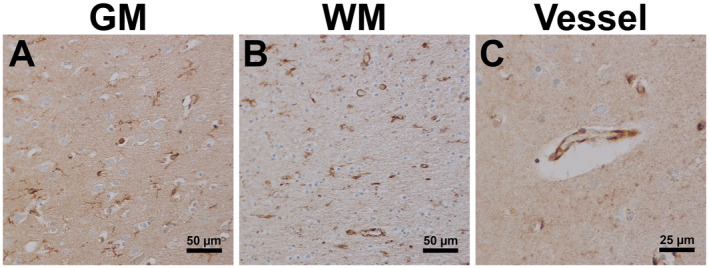
Persistent expression of CSF1R in HIV suppressed ART treated individuals. Single‐label immunohistochemistry for CSF1R counterstained with hematoxylin showed that HIV infected individuals on suppressive ART treatment had increased expression of CSF1R within GM (A), WM (B) and around vessels (C) compared to uninfected individuals (Fig. 1).
Discussion
The present study demonstrates that CSF1R is upregulated and activated in PVMs and lesional macrophages and contributes to development of lentiviral encephalitis possibly by promoting macrophage proliferation and survival in the perivascular space and within encephalitic lesions. We show that CSF1, pSTAT5a/b and PCNA are coexpressed with CSF1R in infected PVMs and lesional macrophages in the brains of encephalitic animals, further suggesting that autocrine CSF1/CSF1R signaling is operative. We also found that CSF1R was persistently expressed at high levels on microglia and PVMs in the brains of HIV‐1‐infected aviremic individuals on suppressive ART.
While we were preparing our manuscript, Knight et al. (2018) reported for the first time that CSF1 and CSF1R were upregulated in the brains of SIV‐infected pigtailed macaques 29. Moreover, the authors were able to show that CSF1R is persistently expressed in the brains of virally suppressed macaques after ART, consistent with our findings in virally suppressed patients. Elevated expression of CSF1R in the brain of virally suppressed subjects may be maintained by the continuous presence of its ligand CSF1. This suggests that CSF1‐mediated inflammation persists in the brain without active viral replication.
In fact, there is ample evidence that CSF1 may play a critical role in HIV infection of macrophages 2, 10, 22, 23, 28, 32, 33, 39, 42, 47. It appears that by forming a positive feedback loop with HIV‐1, CSF1 can enhance HIV‐1 pathogenesis and progression driven by macrophages. CSF1 treatment in vitro makes monocyte‐derived macrophages susceptible to HIV infection, possibly by upregulating surface expression of known viral receptors 2, 28. It is a direct effect of CSF1 since anti‐CSF1 antibody prevents the infection 32. It has also been shown that HIV infection of macrophages promotes CSF1 secretion, which can then, in an autocrine manner, activate CSF1R signaling 20, 25, 33, 47. The dependence of brain‐resident macrophages on CSF1 for survival further underscores the relevance of this cytokine to HIV‐1‐associated CNS disease, as long‐lived brain macrophages represent a significant HIV reservoir in the brain. Interestingly, levels of CSF1 in the CSF were positively correlated with the degree of neurocognitive impairment in HIV‐infected patients on suppressive ART 36. A recent study using SIV‐infected rhesus macaques showed that PVMs and lesional macrophages produce high levels of CSF1 in vivo 23. Our current study indicates that PVMs express much higher levels of CSF1R than resting ramified microglia, and provides the first in vivo evidence that HIV/SIV infection of the CNS induces further upregulation and activation (measured by phosphorylation of CSF1R and STAT5) of CSF1R in PVM and activated microglia but not in resting microglia. Previously, we found increased proliferation of brain macrophages in HIV and SIV encephalitis, in line with these new findings regarding CSF1R signaling in the brain, correlating with the severity of SIV encephalitis 17.
Recent studies in mice have revealed that CSF1/CSF1R signaling directly controls the maintenance (survival and proliferation) of adult microglia and regulates microglia‐mediated neuroinflammation in several mouse models of neurodegenerative diseases including AD 6, 37, 52. Using newly developed brain‐penetrant inhibitors of CSF1R kinase (many of which are currently in clinical trials for the treatment of cancers including glioblastoma), these studies demonstrate that brain macrophages, including microglia, are dependent on CSF1R signaling for survival in adult, normally aged and AD brains, and that activated microglia/macrophages in the brain are the main contributors that are directly responsible for persistent neuroinflammation seen in these models. Levels of CSF1 in the cerebrospinal fluid and serum are elevated in patients with Alzheimer's disease 13, 26, 31, 35, 40. Very recently, a new RNA‐seq study of human aged and AD brain tissues have demonstrated upregulation of CSF1 and CSF1R mRNA in the AD brain 51. Interestingly, it was shown that CSF1 augments Aβ42‐induced microglial production of proinflammatory cytokines and that Aβ42 enhances microglial proliferation in response to CSF1 50. Taken together, these findings suggest that M‐CSF is a player in chronic neuroinflammation.
Since women have an increased risk of developing AD compared to men 16, it would be interesting and important to examine brain and CSF levels of CSF1 in age‐matched adult male and female macaques. Unfortunately, we only have brain tissue from one female macaque, which makes it impossible to address sex‐specific differences in CSF1 levels and lesion susceptibility.
While combination ART has dramatically improved the longevity and quality of life for HIV‐infected individuals, it is not a cure. Long‐lived cells such as resting memory CD4+ T cells and tissue‐resident macrophages can be latently infected and serve as reservoirs for the virus due to their resistance to viral cytopathic effects 7, 8, 9, 18, 21, 27, 34. Within the CNS, PVMs and to a lesser extent microglia are the cells found to harbor latent and productive HIV/SIV, with PVMs being the primary reservoir 3, 19, 49, 53. Even in the absence of virus production in the brain or CNS disease, HIV DNA is consistently found in PVMs 48. With the brain being implicated as a site for viral persistence in both human and macaque models, and HAND continuing to become a more severe problem within the HIV population regardless of current viral suppressive treatments, finding a way to eliminate this reservoir or prevent the development of HAND remain top priorities.
With current cancer research finding the targeting of the CSF1R pathway as a possible cure or treatment for cancer through the elimination of the tumor‐promoting macrophages/microglia by CSF1R inhibition 5, such a strategy may prove useful for eradicating or drastically reducing the viral reservoir within the brains of HIV‐infected individuals by targeting infected cells for elimination. Our findings suggest that this strategy may truly be an effective way of targeting infected persistent macrophages within the brain while limiting the death of the brain's uninfected PVM and microglia populations. While further research and experiments will be needed to look into this and other possible strategies before any real conclusions can be made, our data provide important insights into the workings and characterization of HIV/SIV within the brain that will assist in the global effort to cure or truly control HIV.
Conflict of Interest
The authors do not have a conflict of interest to declare.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Supporting information
Figure S1. CSF1R expression in macaque and human brain. Single‐label immunohistochemistry for CSF1R showed differing levels of expression in macaque (A) and human brain (B) depending on brain area (GM or WM) and infection status. (n = 3 per group for macaque groups, n = 4 per group for human groups) (UI GM vs SIVnoE GM, p = 0.0146; UI WM vs SIVE WM, p < 0.0001; SIVnoE GM vs SIVnoE WM, p = 0.0018; SIVnoE WM vs SIVE WM, p = 0.0002) (A) (UI GM vs HIVE GM, p < 0.0001; UI GM vs ART GM, p = 0.0022; UI WM vs HIVE WM, p = 0.0001; UI WM vs ART WM, p = 0.0247; HIVE GM vs HIVE WM, p = 0.0336; HIVE GM vs ART GM, p = 0.0005; HIVE WM vs ART WM, p < 0.0001) (B) *, p < 0.05; **, p < 0.01; ***, p < 0.001; ****, p < 0.0001.
Acknowledgments
This work was supported by NIH Grants R01MH107333 and R21MH108458 (W.‐K.K.); also supported in part by R01AI097059 and R33AI110163 (M.J.K). This work was also supported by U24MH100931 (Manhattan HIV Brain Bank) and U24MH100925 (NNTC Data Coordinating Center).
References
- 1. Aikawa Y, Katsumoto T, Zhang P, Shima H, Shino M, Terui K et al (2010) PU.1‐mediated upregulation of CSF1R is crucial for leukemia stem cell potential induced by MOZ‐TIF2. Nat Med 16:580–585, 1p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bergamini A, Perno CF, Dini L, Capozzi M, Pesce CD, Ventura L et al (1994) Macrophage colony‐stimulating factor enhances the susceptibility of macrophages to infection by human immunodeficiency virus and reduces the activity of compounds that inhibit virus binding. Blood 84:3405–3412. [PubMed] [Google Scholar]
- 3. Bissel SJ, Wiley CA (2004) Human immunodeficiency virus infection of the brain: pitfalls in evaluating infected/affected cell populations. Brain Pathol 14:97–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Burdo TH, Lackner A, Williams KC (2013) Monocyte/macrophages and their role in HIV neuropathogenesis. Immunol Rev 254:102–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cannarile MA, Weisser M, Jacob W, Jegg AM, Ries CH, Ruttinger D (2017) Colony‐stimulating factor 1 receptor (CSF1R) inhibitors in cancer therapy. J Immunother Cancer 5:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chihara T, Suzu S, Hassan R, Chutiwitoonchai N, Hiyoshi M, Motoyoshi K et al (2010) IL‐34 and M‐CSF share the receptor Fms but are not identical in biological activity and signal activation. Cell Death Differ 17:1917–1927. [DOI] [PubMed] [Google Scholar]
- 7. Chun TW, Davey RT Jr, Ostrowski M, Shawn JJ, Engel D, Mullins JI et al (2000) Relationship between pre‐existing viral reservoirs and the re‐emergence of plasma viremia after discontinuation of highly active anti‐retroviral therapy. Nat Med 6:757–761. [DOI] [PubMed] [Google Scholar]
- 8. Chun TW, Stuyver L, Mizell SB, Ehler LA, Mican JA, Baseler M et al (1997) Presence of an inducible HIV‐1 latent reservoir during highly active antiretroviral therapy. Proc Natl Acad Sci U S A 94:13193–13197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cribbs SK, Lennox J, Caliendo AM, Brown LA, Guidot DM (2015) Healthy HIV‐1‐infected individuals on highly active antiretroviral therapy harbor HIV‐1 in their alveolar macrophages. AIDS Res Hum Retroviruses 31:64–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cunyat F, Rainho JN, West B, Swainson L, McCune JM, Stevenson M (2016) Colony‐stimulating factor 1 receptor antagonists sensitize human immunodeficiency virus type 1‐infected macrophages to TRAIL‐mediated killing. J Virol 90:6255–6262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dai XM, Ryan GR, Hapel AJ, Dominguez MG, Russell RG, Kapp S et al (2002) Targeted disruption of the mouse colony‐stimulating factor 1 receptor gene results in osteopetrosis, mononuclear phagocyte deficiency, increased primitive progenitor cell frequencies, and reproductive defects. Blood 99:111–120. [DOI] [PubMed] [Google Scholar]
- 12. De I, Nikodemova M, Steffen MD, Sokn E, Maklakova VI, Watters JJ et al (2014) CSF1 overexpression has pleiotropic effects on microglia in vivo . Glia 62:1955–1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Du YS, Zhu H, Fu J, Yan SF, Roher A, Tourtellotte WW et al (1997) Amyloid‐beta peptide‐receptor for advanced glycation endproduct interaction elicits neuronal expression of macrophage‐colony stimulating factor: a proinflammatory pathway in Alzheimer disease. Proc Natl Acad Sci U S A 94:5296–5301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Elmore MR, Najafi AR, Koike MA, Dagher NN, Spangenberg EE, Rice RA et al (2014) Colony‐stimulating factor 1 receptor signaling is necessary for microglia viability, unmasking a microglia progenitor cell in the adult brain. Neuron 82:380–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Erblich B, Zhu L, Etgen AM, Dobrenis K, Pollard JW (2011) Absence of colony stimulation factor‐1 receptor results in loss of microglia, disrupted brain development and olfactory deficits. PLoS One 6:e26317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ferretti MT, Iulita MF, Cavedo E, Chiesa PA, Schumacher DA, Santuccione CA et al (2018) Sex differences in Alzheimer disease—the gateway to precision medicine. Nat Rev Neurol 14:457–469. [DOI] [PubMed] [Google Scholar]
- 17. Filipowicz AR, McGary CM, Holder GE, Lindgren AA, Johnson EM, Sugimoto C et al (2016) Proliferation of perivascular macrophages contributes to the development of encephalitic lesions in HIV‐infected humans and in SIV‐infected macaques. Sci Rep 6:32900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Finzi D, Hermankova M, Pierson T, Carruth LM, Buck C, Chaisson RE et al (1997) Identification of a reservoir for HIV‐1 in patients on highly active antiretroviral therapy. Science 278:1295–1300. [DOI] [PubMed] [Google Scholar]
- 19. Fischer‐Smith T, Croul S, Sverstiuk AE, Capini C, L'Heureux D, Regulier EG et al (2001) CNS invasion by CD14+/CD16+ peripheral blood‐derived monocytes in HIV dementia: perivascular accumulation and reservoir of HIV infection. J Neurovirol 7:528–541. [DOI] [PubMed] [Google Scholar]
- 20. Gallo P, De Rossi A, Sivieri S, Chieco‐Bianchi L, Tavolato B (1994) M‐CSF production by HIV‐1‐infected monocytes and its intrathecal synthesis. Implications for neurological HIV‐1‐related disease. J Neuroimmunol 51:193–198. [DOI] [PubMed] [Google Scholar]
- 21. Gama L, Abreu CM, Shirk EN, Price SL, Li M, Laird GM et al (2017) Reactivation of simian immunodeficiency virus reservoirs in the brain of virally suppressed macaques. AIDS 31:5–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gerngross L, Fischer T (2015) Evidence for cFMS signaling in HIV production by brain macrophages and microglia. J Neurovirol 21:249–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gerngross L, Lehmicke G, Belkadi A, Fischer T (2015) Role for cFMS in maintaining alternative macrophage polarization in SIV infection: implications for HIV neuropathogenesis. J Neuroinflammation 12:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gomez‐Nicola D, Fransen NL, Suzzi S, Perry VH (2013) Regulation of microglial proliferation during chronic neurodegeneration. J Neurosci 33:2481–2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gruber MF, Weih KA, Boone EJ, Smith PD, Clouse KA (1995) Endogenous macrophage CSF production is associated with viral replication in HIV‐1‐infected human monocyte‐derived macrophages. J Immunol 154:5528–5535. [PubMed] [Google Scholar]
- 26. Hasegawa Y, Sawada M, Ozaki N, Inagaki T, Suzumura A (2000) Increased soluble tumor necrosis factor receptor levels in the serum of elderly people. Gerontology 46:185–188. [DOI] [PubMed] [Google Scholar]
- 27. Honeycutt JB, Thayer WO, Baker CE, Ribeiro RM, Lada SM, Cao Y et al (2017) HIV persistence in tissue macrophages of humanized myeloid‐only mice during antiretroviral therapy. Nat Med 23:638–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kalter DC, Nakamura M, Turpin JA, Baca LM, Hoover DL, Dieffenbach C et al (1991) Enhanced HIV replication in macrophage colony‐stimulating factor‐treated monocytes. J Immunol 146:298–306. [PubMed] [Google Scholar]
- 29. Knight AC, Brill SA, Queen SE, Tarwater PM, Mankowski JL (2018) Increased microglial CSF1R expression in the SIV/macaque model of HIV CNS disease. J Neuropathol Exp Neurol 77:199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ko A, Kang G, Hattler JB, Galadima HI, Zhang J, Li Q et al (2018) Macrophages but not astrocytes harbor HIV DNA in the brains of HIV‐1‐infected aviremic individuals on suppressive antiretroviral therapy. J Neuroimmune Pharmacol 14:110–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kong QL, Zhang JM, Zhang ZX, Ge PJ, Xu YJ, Mi RS et al (2002) Serum levels of macrophage colony stimulating factor in the patients with Alzheimer's disease. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 24:298–301. [PubMed] [Google Scholar]
- 32. Kutza J, Crim L, Feldman S, Hayes MP, Gruber M, Beeler J et al (2000) Macrophage colony‐stimulating factor antagonists inhibit replication of HIV‐1 in human macrophages. J Immunol 164:4955–4960. [DOI] [PubMed] [Google Scholar]
- 33. Kutza J, Fields K, Grimm TA, Clouse KA (2002) Inhibition of HIV replication and macrophage colony‐stimulating factor production in human macrophages by antiretroviral agents. AIDS Res Hum Retroviruses 18:619–625. [DOI] [PubMed] [Google Scholar]
- 34. Lamers SL, Rose R, Maidji E, Agsalda‐Garcia M, Nolan DJ, Fogel GB et al (2016) HIV DNA is frequently present within pathologic tissues evaluated at autopsy from combined antiretroviral therapy‐treated patients with undetectable viral loads. J Virol 90:8968–8983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Laske C, Stransky E, Hoffmann N, Maetzler W, Straten G, Eschweiler GW et al (2010) Macrophage colony‐stimulating factor (M‐CSF) in plasma and CSF of patients with mild cognitive impairment and Alzheimer's disease. Curr Alzheimer Res 7:409–414. [DOI] [PubMed] [Google Scholar]
- 36. Lentz MR, Degaonkar M, Mohamed MA, Kim H, Conant K, Halpern EF et al (2010) Exploring the relationship of macrophage colony‐stimulating factor levels on neuroaxonal metabolism and cognition during chronic human immunodeficiency virus infection. J Neurovirol 16:368–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lin H, Lee E, Hestir K, Leo C, Huang M, Bosch E et al (2008) Discovery of a cytokine and its receptor by functional screening of the extracellular proteome. Science 320:807–811. [DOI] [PubMed] [Google Scholar]
- 38. MacDonald KP, Palmer JS, Cronau S, Seppanen E, Olver S, Raffelt NC et al (2010) An antibody against the colony‐stimulating factor 1 receptor depletes the resident subset of monocytes and tissue‐ and tumor‐associated macrophages but does not inhibit inflammation. Blood 116:3955–3963. [DOI] [PubMed] [Google Scholar]
- 39. Mlcochova P, Sutherland KA, Watters SA, Bertoli C, de Bruin RA, Rehwinkel J et al (2017) A G1‐like state allows HIV‐1 to bypass SAMHD1 restriction in macrophages. EMBO J 36:604–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Murphy GM Jr, Yang L, Cordell B (1998) Macrophage colony‐stimulating factor augments beta‐amyloid‐induced interleukin‐1, interleukin‐6, and nitric oxide production by microglial cells. J Biol Chem 273:20967–20971. [DOI] [PubMed] [Google Scholar]
- 41. Olmos‐Alonso A, Schetters ST, Sri S, Askew K, Mancuso R, Vargas‐Caballero M et al (2016) Pharmacological targeting of CSF1R inhibits microglial proliferation and prevents the progression of Alzheimer's‐like pathology. Brain 139:891–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pauls E, Ruiz A, Badia R, Permanyer M, Gubern A, Riveira‐Munoz E et al (2014) Cell cycle control and HIV‐1 susceptibility are linked by CDK6‐dependent CDK2 phosphorylation of SAMHD1 in myeloid and lymphoid cells. J Immunol 193:1988–1997. [DOI] [PubMed] [Google Scholar]
- 43. Rappaport J, Volsky DJ (2015) Role of the macrophage in HIV‐associated neurocognitive disorders and other comorbidities in patients on effective antiretroviral treatment. J Neurovirol 21:235–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Saylor D, Dickens AM, Sacktor N, Haughey N, Slusher B, Pletnikov M et al (2016) HIV‐associated neurocognitive disorder—pathogenesis and prospects for treatment. Nat Rev Neurol 12:234–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Spangenberg EE, Lee RJ, Najafi AR, Rice RA, Elmore MR, Blurton‐Jones M et al (2016) Eliminating microglia in Alzheimer's mice prevents neuronal loss without modulating amyloid‐beta pathology. Brain 139:1265–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Spudich S, Gonzalez‐Scarano F (2012) HIV‐1‐related central nervous system disease: current issues in pathogenesis, diagnosis, and treatment. Cold Spring Harb Perspect Med 2:a007120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Swingler S, Mann AM, Zhou J, Swingler C, Stevenson M (2007) Apoptotic killing of HIV‐1‐infected macrophages is subverted by the viral envelope glycoprotein. PLoS Pathog 3:1281–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Thompson KA, Cherry CL, Bell JE, McLean CA (2011) Brain cell reservoirs of latent virus in presymptomatic HIV‐infected individuals. Am J Pathol 179:1623–1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Thompson KA, Varrone JJ, Jankovic‐Karasoulos T, Wesselingh SL, McLean CA (2009) Cell‐specific temporal infection of the brain in a simian immunodeficiency virus model of human immunodeficiency virus encephalitis. J Neurovirol 15:300–311. [DOI] [PubMed] [Google Scholar]
- 50. Vincent VA, Selwood SP, Murphy GM Jr (2002) Proinflammatory effects of M‐CSF and A beta in hippocampal organotypic cultures. Neurobiol Aging 23:349–362. [DOI] [PubMed] [Google Scholar]
- 51. Walker DG, Tang TM, Lue LF (2017) Studies on colony stimulating factor receptor‐1 and ligands colony stimulating factor‐1 and interleukin‐34 in Alzheimer's disease brains and human microglia. Front Aging Neurosci 9:244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wei S, Nandi S, Chitu V, Yeung YG, Yu W, Huang M et al (2010) Functional overlap but differential expression of CSF‐1 and IL‐34 in their CSF‐1 receptor‐mediated regulation of myeloid cells. J Leukoc Biol 88:495–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Williams KC, Corey S, Westmoreland SV, Pauley D, Knight H, deBakker C et al (2001) Perivascular macrophages are the primary cell type productively infected by simian immunodeficiency virus in the brains of macaques: implications for the neuropathogenesis of AIDS. J Exp Med 193:905–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. CSF1R expression in macaque and human brain. Single‐label immunohistochemistry for CSF1R showed differing levels of expression in macaque (A) and human brain (B) depending on brain area (GM or WM) and infection status. (n = 3 per group for macaque groups, n = 4 per group for human groups) (UI GM vs SIVnoE GM, p = 0.0146; UI WM vs SIVE WM, p < 0.0001; SIVnoE GM vs SIVnoE WM, p = 0.0018; SIVnoE WM vs SIVE WM, p = 0.0002) (A) (UI GM vs HIVE GM, p < 0.0001; UI GM vs ART GM, p = 0.0022; UI WM vs HIVE WM, p = 0.0001; UI WM vs ART WM, p = 0.0247; HIVE GM vs HIVE WM, p = 0.0336; HIVE GM vs ART GM, p = 0.0005; HIVE WM vs ART WM, p < 0.0001) (B) *, p < 0.05; **, p < 0.01; ***, p < 0.001; ****, p < 0.0001.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


