Abstract
Brain−gut neural communications have long been considered limited because of conspicuous numerical mismatches. The vagus, the parasympathetic nerve connecting brain and gut contains thousands of axons, whereas the gastrointestinal (GI) tract contains millions of intrinsic neurons in local plexuses. The numerical paradox was initially recognized in terms of efferent projections, but the number of afferents, which comprise the majority (≈ 80%) of neurites in the vagus, is also relatively small. The present survey of recent morphological observations suggests that vagal terminals, and more generally autonomic and visceral afferent arbors in the stomach as well as throughout the gut, elaborate arbors that are extensive, regionally specialized, polymorphic, polytopic, and polymodal, commonly with multiplicities of receptors and binding sites—smart terminals. The morphological specializations and dynamic tuning of one-to-many efferent projections and many-to-one convergences of contacts onto afferents create a complex architecture capable of extensive peripheral integration in the brain−gut connectome and offset many of the disparities between axon and target numbers. Appreciating this complex architecture can help in the design of therapies for GI disorders.
Keywords: afferent, autonomic, efferent, intestines, parasympathetic, postganglionic, preganglionic, stomach, viscera, visceral afferent, vagus
Graphical abstract
In this short review, we examine and illustrate some of the recent observations that have supported the conclusion that autonomic, and specifically vagal, efferents and visceral afferents from the nodose ganglia establish, respectively, one-to-many and many-to-one connections to create the brain−gut connectome.
Introduction
A century ago, Langley1 highlighted the mismatch between the limited number of vagal preganglionic neurons that project to the GI tract and the extensive network of enteric neurons in the gut wall. He concluded that the limited number of motor axons must contact a circumscribed number of specialized “mother” or “vagal” cells (more recently called “command neurons” by Wood2) in the ENS, and the specialized enteric neurons then coordinated a limited set of effectors. Perhaps because of Langley’s overall influence on physiology and his pivotal role in articulating the features of the ANS, his extrapolation based on the mismatch was codified. His hypothesis was widely, if often implicitly, accepted for much of the last century. Notably, though, in a century of experimentation, command neurons have never been unambiguously identified among gut intrinsic neurons.
None of the earlier observations that shaped the assumptions surrounding the vagal axon:target mismatch attempted to incorporate systematically the prospect that vagal neurites might end in extensive arbors capable of considerable integration locally within the gut wall. The vagal terminals in the gut wall were apparently assumed to be “free nerve endings” and these free nerve endings were assumed to terminate simply. In contrast, however, over the last two or three decades, we and others have reported a variety of experiments that have consistently indicated that both vagal efferents and afferents end in complex, extensive and highly differentiated terminal arbors. These arbor specializations often span different tissue domains, or are “polytopic”. They are sometimes also polymorphic, commonly polymodal, and/or display conspicuous and complex region-specific morphologies. The specialized neurons also regularly express receptors for various signaling molecules.
Contrary to the expectation established by Langley’s early idea, vagal efferent projections to the GI tract develop extensive terminal arbors to establish “one-to-many” networks (without intervening mother cells or command neurons) to create brain-to-gut communication.
Reciprocally, vagal afferent innervation of the GI tract employs complex and highly differentiated terminal arbors in the periphery to establish “many-to-one” dynamic networks to generate gut-to-brain signaling.
In this short review, we examine and illustrate some of the recent observations that have supported the conclusion that autonomic, and specifically vagal, efferents and visceral afferents from the nodose ganglia establish, respectively, one-to-many and many-to-one connections to create the brain−gut connectome. Improvements in neural tracing and labeling have played a large role in characterizing the architecture of the terminals, and we illustrate the arbors using some of these tracing technologies. We also identify recent advances in electrophysiology and other techniques mapping neurochemical phenotypes in the connectome that support the newer perspective on the brain−gut axis.
Vagal efferents
Vagal preganglionic efferents extensively innervate the ganglia of the myenteric plexus in the stomach smooth muscle wall. Such profiles have long been observed,3 but with older, nonspecific staining protocols the source or origin and extent of individual varicose fibers remained controversial. With the introduction of neural tracers, it became possible to (a) establish a source through the use of local injections (e.g., the dorsal motor nucleus of the vagus or dmnX; see Berthoud et al.4) of tracers, (b) anterogradely label terminals with high definition5, 6 (see Fig. 1), and (c) reconstruct arborization patterns and selectively labeled individual fibers throughout an organ (see Figs. 2 and 3).
Figure 1.
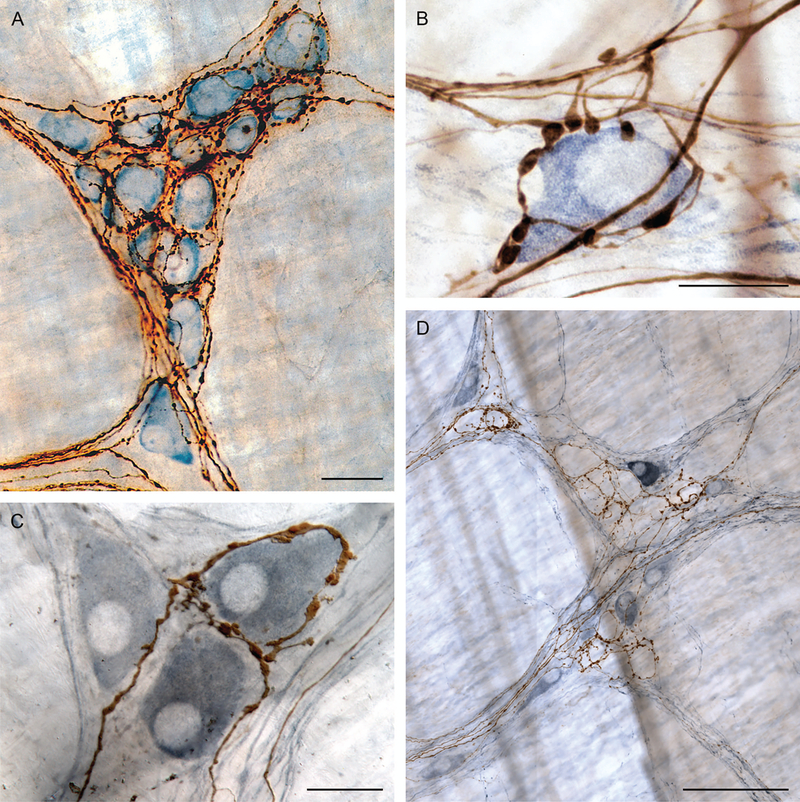
Preganglionic projections to myenteric ganglia. (A) Simultaneous labeling of multiple motor projections (brown fibers: PHA-l labeling): large dorsal motor nucleus of vagus injections label efferent contacts on essentially all cells in the individual myenteric ganglia in the stomach (myenteric postganglionic neurons counterstained with Cuprolinic Blue). (B) Selective labeling of limited numbers of vagal preganglionic efferents with small injections (brown fibers: dextran-biotin labeling) reveal contacts around individual myenteric postganglionic neurons (individual postganglionic stained immunohistochemically for nNOS: steel gray secondary). (C) One of three nNOS-positive cells (steel gray secondary) selectively encircled by two preganglionic branches (brown fibers: dextran-biotin labeling). (D) Myenteric ganglia with immunohistochemistry for nNOS (steel gray positive postganglionic neurons) illustrate that some vagal preganglionic terminals selectively encircle the unstained (nNOS-negative) cells of the ganglion. Other preganglionic fibers (not shown; cf. panels B and C) preferentially contact nNOS-positive neurons within the myenteric plexus. Scale bars in plates = 30 μm (panel A), 16 μm (panel B), 25 μm (panel C), and 100 μm (panel D). Panel A reproduced by permission of John Wiley & Sons from Holst et al.5 Panel B reproduced by permission of Elsevier from Walter et al.6
Figure 2.
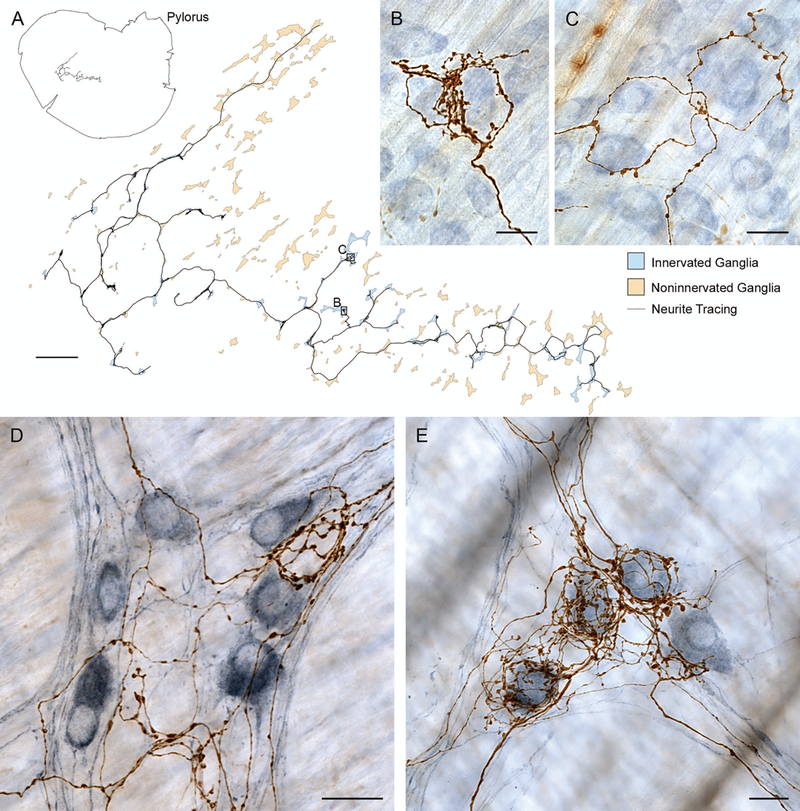
Digitization of individual vagal efferents establishes that single preganglionics link multiple neighboring myenteric ganglia and selectively innervate different phenotypes of myenteric postganglionic neurons. (A) Neurolucida® (MBF Bioscience, Williston, VT) reconstruction of whole mount and fiber location (upper left) and preganglionic arbor contacting multiple myenteric neurons in multiple ganglia (blue ganglia) within a larger field of ganglia un-innervated by the fiber (pale orange ganglia). Insets (B) and (C) illustrate the digitized vagal preganglionic fiber (dextran-biotin labeled) and its selectivity for certain myenteric neurons within the ganglia (counterstained with Cuprolinic blue). (D and E) In other specimens, immunohistochemistry used to selectively label nNOS neurons (steel gray chromogen) illustrates that some vagal preganglionic efferents (brown dextran-biotin labeled fibers) preferentially or selectively ring nNOS-negative or unstained (i.e., presumably cholinergic) profiles (e.g., panel D) whereas other efferents preferentially encircle nNOS-positive (presumably nitrergic) postganglionic neurons (e.g., panel E). Scale bars in plates = 1000 μm (panel A) and 25 μm (panels B−E).
Figure 3.
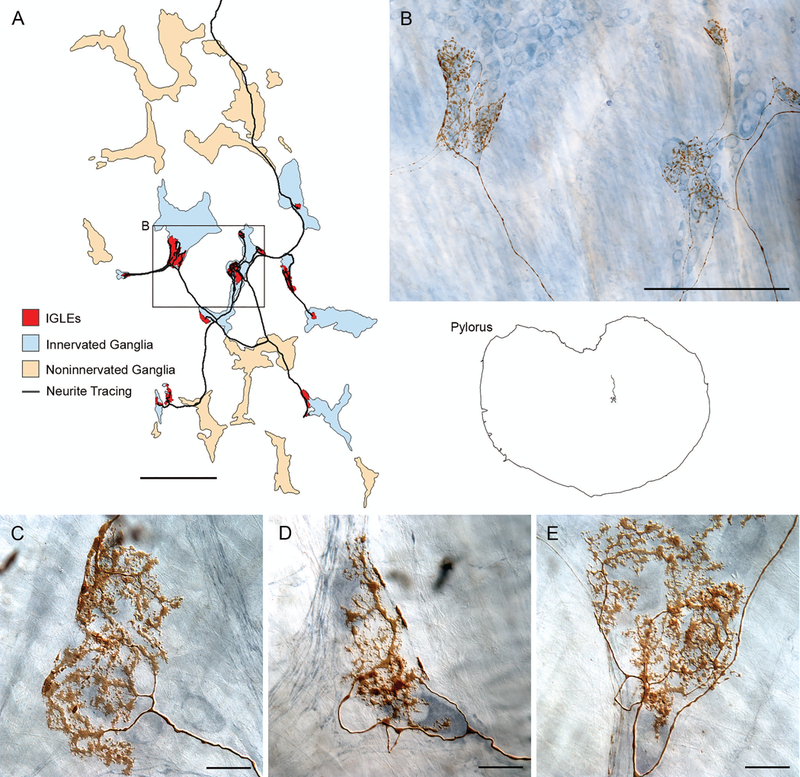
Individual intraganglionic laminar endings (IGLEs) issued by a single afferent innervate multiple neighboring myenteric ganglia (red regions), apparently generating large receptive fields, but (unlike vagal efferents) IGLEs do not obviously discriminate or selectively innervate either nNOS-positive or -negative neurons within their receptive fields. (A) A Neurolucida reconstruction of a gastric whole mount and an IGLE afferent within it (right side of image) as well as an enlargement of the IGLE afferent (left part of figure). (B) A photomicrograph of a region of the IGLE afferent illustrated in (A), which reproduces several of the IGLE plates issued by the afferent as well as the myenteric ganglia (stained with a polyneuronal Cuprolinic blue protocol) that the afferent contacts. ( C, D, and E) Examples of IGLE plates from other specimens that illustrate the terminal patterns of IGLE afferents and display how they appear to contact local ensembles of adjacent neurons, whether they are nNOS-positive or -negative (panels C−E are counterstained by nNOS immunohistochemistry). Scale bars in plates = 500 μm (panel A), 250 μm (panel B), and 25 μm (panels C−E).
At the level of the myenteric plexus, individual tracer-labeled vagal preganglionic projections commonly enter ganglia and form rings of contacts surrounding many of the neurons within the plexus (Fig. 1A and 1C). The rings routinely make conspicuous, highly varicose presumptive contacts on the postganglionic neurons. Ultrastructural observations7 substantiate that such varicosities are synaptic contacts with myenteric ganglionic neurons and commonly contain round and translucent, presumably cholinergic, vesicles massed in the region of the presynaptic site of the cell membrane. In addition to cholinergic vesicles, some presynaptic varicosities of vagal efferents also contain (presumptively) dopaminergic vesicles. The vast majority, possibly all, of the myenteric ganglion neurons in the plexus of the stomach wall appear to receive such terminal projections of vagal preganglionics (see Fig. 1A, 1B, and 1C).
When the terminal arbors of vagal efferents are traced and digitally reconstructed, they display a consistent pattern. Individual efferents entering their terminal projection fields form arbors that ramify so as to contact myenteric ganglion cells of multiple neighboring ganglia in a particular regional field within the myenteric plexus. Figure 2A illustrates a digitized and flattened 3D tracing of a representative vagal arbor. As Figure 2A also illustrates, the terminal arbors issued by an individual preganglionic vagal motor axon innervate a collection or string of essentially all neighboring ganglia within a circumscribed field and limit their terminal contacts to those ganglia. Preganglionic arbors do not regularly issue meandering branches that “stray” far beyond the circumscribed and coherent terminal field. The pattern suggests that adjacent terminal fields supplied by neighboring preganglionics composed of “plates of neighboring ganglia,” collectively girdle the stomach with an extensive, potentially complete, tessellated network of projections.
Further, the local or regional networks formed by individual preganglionics are apparently cross-coupled in a pattern that may produce dimensionally larger ensembles organized for more extensive regional coordination. Individual preganglionics or “motor tiles”, such as the one in Figure 2A, apparently overlap insofar as multiple preganglionics seem to converge on individual ganglia. The pattern is conspicuous when one compares the extrinsic projections to a single ganglion in animals with multiple (or large) injections into the dmnX (e.g., Fig. 1A) versus the efferent projection to a ganglion when one uses single (or smaller) injections into the motor nucleus (e.g., Figs. 1B, 1C, 2B, and 2C). In the case of larger injections, virtually all of the postganglionic cells appear innervated, whereas in the case of the smaller injections, a single preganglionic fiber appears to innervate only a fraction of all of the neurons in a ganglion.
Additional features suggest that the vagal preganglionics generate a terminal architecture to support complex motor programming of the stomach. In brief, one feature stems from considering the pattern of peristaltic waves that moves chyme through the stomach and intestines; a second feature is the cholinergic and nitrergic ganglionic organization in the myenteric plexus that generates peristalsis.
Peristaltic waves that move food more distally in the GI tract, including the stomach, consist of patterns of leading smooth muscle relaxation reducing intraluminal pressure immediately aboral to a bolus of food or chyme timed to coordinate with a muscular contraction immediately oral to the bolus. This choreography or coordination of reciprocating relaxation and contraction must travel along the GI tract in the appropriate phase relationships to move nutrient material along the alimentary canal. Though in its most elemental pattern, peristalsis can be generated by the enteric nervous system without extrinsic input,8, 9 optimally timed and most efficient wave patterns are coordinated with extrinsic nervous system modulation. The enteric nervous system, of course, has a rich and varied collection of neurochemical phenotypes;10 however, nearly all myenteric neurons in the stomach wall are also either nitrergic, producing and using nitric oxide as a transmitter, or cholinergic, expressing and using acetylcholine as a transmitter. The nitrergic neurons typically inhibit smooth muscle contractions, whereas the cholinergic neurons excite smooth muscle activity. In terms of peristaltic activity, the relaxing distal leading edge of the peristaltic wave seems to reflect nitrergic activity, whereas the contracting and more proximal trailing edge of the wave reflects cholinergic potentiation of contraction.
A recent and ongoing analysis of vagal efferent projections suggests one integrative function of the vagal efferent arbors. Jaffey and coworkers (unpublished) have evaluated whether individual vagal preganglionic arbors selectively contact nitrergic (or NOS-positive) ganglion cells, selectively contact cholinergic (NOS-negative) ganglion cells, or both. Throughout the stomach, two distinct preganglionic phenotypes occur. One phenotype of efferent preferentially contacts NOS-positive, presumably “inhibitory,” ganglion cells in multiple neighboring myenteric ganglia (Fig. 2E). The other phenotype selectively contacts NOS-negative (Fig. 2D), presumably cholinergic myenteric neurons that are “excitatory” and cause contractions, in multiple neighboring myenteric ganglia. (A third phenotype is also observed and appears to crosslink NOS-positive and -negative postganglionic neurons.) The common dichotomized or “bipolar” architectural pattern of preganglionics suggests that one population of efferents crosslinks NOS-positive neurons throughout its respective projection fields to coordinate relaxation, whereas a second population crosslinks NOS-negative cholinergic neurons through its target fields to coordinate contraction. If so, cross coupling of the two phenotypes could then program and phase peristaltic activity. Thus, many vagal preganglionics apparently are organized in a push-pull architectural pattern that might well be responsible for pacing and coordinating aspects of traveling peristaltic waves generated in the stomach.
Vagal afferents
Three broad phenotypic classes of vagal afferents (intraganglionic laminar endings, intramuscular arrays, and mucosal arbors—see below) innervate the stomach wall. The distinctive, defining architecture of each of the classes suggests that these afferent projections also support complex and extensive integrative capacities in the brain−gut connectome. Moreover, the topography of afferents suggests that much pre-processing, or peripheral integration, in the stomach wall offsets the limited number of “transmission lines” for afferent neurite relays in the vagus nerve.
Further, though there is some tendency in the experimental literature to assume that the different individual afferent inputs to the central nervous system (CNS) operate separately and need to be considered separately (perhaps because they commonly need to be dissected individually in most experimental protocols), if one thinks of the visceral afferent stream to the CNS as representing a cross-fiber pattern representation, then the combinatorial possibilities become enormous. The different phenotypes, regional locations, intensities of responses, patterns of responses, and “hormonal/paracrine tone,” all may well be factored into continuous cross-fiber real-time representations of GI conditions.
Intraganglionic laminar endings (IGLEs)
Of the three general phenotypes of gastric afferents, intraganglionic laminar endings or IGLEs were the first to be recognized morphologically,11 established as vagal by vagotomy,12 and named.13 IGLEs are found throughout the GI tract including the esophagus,14 stomach,15 small intestine,16, 17 cecum, and colon.15
IGLEs are associated with the ganglia of the myenteric plexus. Conventionally, an IGLE consists of a plate of lamelliform terminal puncta apparently in contact with a ganglion. The plate is typically situated superficial to the ganglion, effectively lying in association with laminae between the ganglion and either the longitudinal muscle above the ganglion or the circular muscle below the ganglion. When an IGLE afferent fiber reaches its target field in the stomach wall, it arborizes extensively, producing IGLE plates in association with a number of neighboring ganglia (Fig. 3).1
Individual IGLE plates (Figs. 3B, 3C, 3D, 3E and 4A’) commonly produce a web of flattened puncta that tend to variously over- or under-lay the ganglion (though the former pattern predominates) and appear to establish close approximations or contacts with many of the ganglion cells. The puncta commonly appear lamelliform in appearance, and these lamellar puncta often issue secondary and tertiary puncta emerging from an initial punctum, not the parent neurite itself. The lamelliform puncta of the IGLE plates, when well stained, often appear to issue spiny protrusions or fingers seemingly analogous to dendritic spines seen in the cerebral cortex (Figs. 3C, 3D, 3E, and 4A’). IGLE plates also often produce a neurite “bar” along the surface of the ganglion or its immediate connectives; such “bars” often appear to be coalesced by or formed from the lamelliform puncta, rather than directly from the neurite branch itself (Fig. 3B, 3C, and 3D). The IGLE plates have been the subject of only limited ultrastructural work, but available EM observations on IGLEs in the esophagus and gastric cardia18 and in the forestomach19 indicate that IGLEs, perhaps via their “fingers” or spines and similar protrusions which appear to extend into the parenchyma around ganglion cells, do make contacts with the ganglion cells and do contain translucent vesicles and limited numbers of opaque vesicles.
Figure 4.
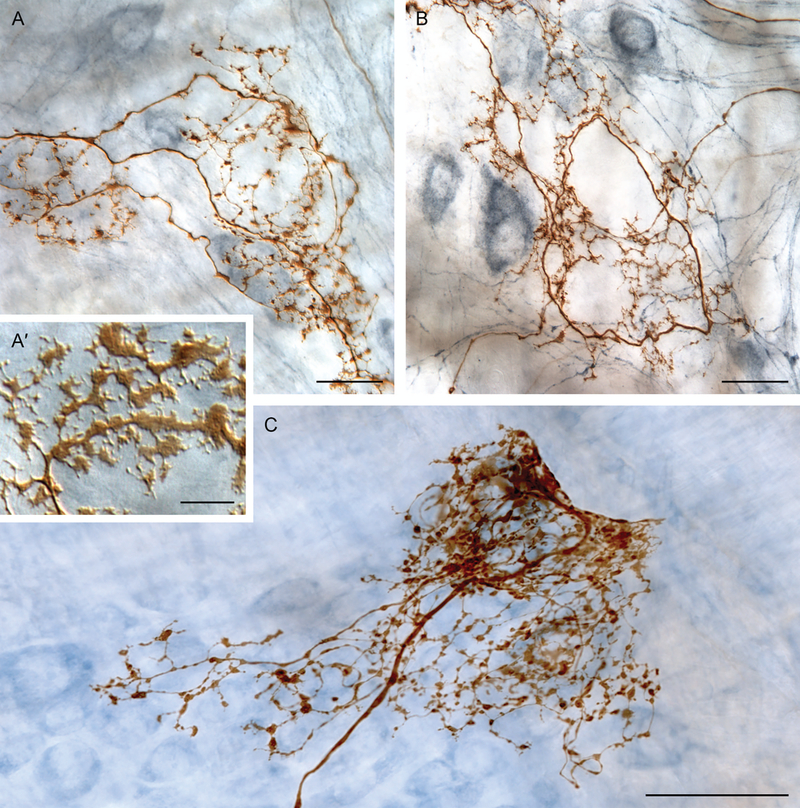
The conventional IGLE phenotype exhibits regional specializations. IGLE branches, particularly in the distal stomach, often have more varicose, less lamelliform specializations of puncta. (A’) Inset illustrates common IGLE puncta morphology of flattened lamelliform puncta, frequently displaying spinous extensions, routinely observed in the proximal stomach. (A, B, and C) In the distal stomach, IGLE afferents often issue some—or even a majority of—branches that terminate in rounded or beaded varicosities, rather than flattened and lamelliform puncta. These IGLE specializations can consist of branches with a mixture of varicosities and smaller lamelliform puncta (e.g., panel A), branches issuing almost exclusively simpler varicosities (e.g., panel B), or branches terminating in dense aggregations of simple and varicose puncta (e.g., panel C). Scale bars = 10 μm (panel A’), 25 μm (panels A and B), and 50 μm (panel C).
IGLE plates appear to function (though perhaps not exclusively) as tension receptors in the smooth muscle wall. In an early assessment of the structural features of IGLE plates, Neuhuber and Clerc20 suggested that the IGLE plates would be distorted by shearing when tension created shearing forces and distorted the laminar interface—including the myenteric plexus tissue--between the longitudinal and circular muscle layers. Based on several lines of indirect evidence, including morphology and distributions, Phillips and Powley21 compared IGLEs and intramuscular arrays (IMAs, described below) and concluded that IGLEs might indeed serve as tension receptors in the GI tract, whereas IMAs might function as stretch or length detectors in the wall of the GI tract (and though the analogy still seems apt, it is, of course, imperfect—see Timmermans and Adriaensen22). In our analysis, we also emphasized that “tension” and “stretch” were regularly confounded and confused in early electrophysiological experiments. More recently, Zagorodnyuk, Brookes et al.23, 24 were able to record from neurites associated with tracer-identified vagal afferent terminals and concluded that IGLEs were tension receptors.
Individual vagal IGLE afferents elaborate these individual ganglionic plates in an extensively branching terminal arbor (cf. Fig. 3A). Typically, the afferent neurite travels some distance in the myenteric plexus and then, on reaching the specific region of gastric wall that it innervates, the neurite arborizes into a complex of branches ending in IGLE plates on a field of adjacent ganglia. Much like the local concentration of ganglia innervated by an individual efferent (see above), IGLE afferents arborize in local concentrations of ganglia, each receiving a plate of puncta from the afferent in question. Also much like the local concentration of ganglia innervated by an individual efferent, the pattern of a concentrated field of IGLE plates issued by a single common IGLE afferent suggests that the IGLE plates may be in some sense integrating the activity or information distributed within a terminal “receptive field.”
Other observations also suggest that the architecture of the IGLE afferent arbors invests the fibers with local integrative potential: Individual afferent plates and entire individual afferent arbors commonly seem to establish lamelliform contacts with both inhibitory (NOS-positive) and presumptive excitatory (or NOS-negative/cholinergic) postganglionic neurons within individual ganglia (Figs. 3C, 3D, 3E, and 4A; Jaffey et al., unpublished). Potentially, IGLEs as tension receptors are well positioned to, and may track waves of inhibitory and excitatory responses associated with peristalsis and provide feedback about motility as extrinsic efferent inflows pace or recalibrate and coordinate forces of tension with motor scores.
Vagal afferents, and specifically vagal IGLE afferents, also appear to be polymodal and to integrate additional information and presumably modify afferent traffic (and vagovagal reflex activity) used by the CNS to generate both physiology and behavior involved in nutrient handling and energy balance. Substantial evidence establishes that vagal afferents innervating the GI tract have receptors, and are affected by both endocrine and paracrine release, for myriad GI hormones secreted by enteroendocrine cells (EEC) and specialized glandular tissues,25, 26 also, see below).
The evidence for CCK, for example, is particularly strong. Binding studies, in situ hybridization experiments, electrophysiological effects, deployment of blockers, and denervation experiments all point to the fact that vagal afferents to the GI tract are sensitive to, and tuned by, fluxes of CCK and other hormones of metabolism. Since some of these CCK-responsive vagal afferents are located in regions innervated almost exclusively by IGLE afferents (e.g., the “tension receptors” reported by Blackshaw and Grundy,27 see also Okano-Matsumoto et al.28), it appears a reasonable inference that vagal IGLE afferents do indeed comprise part of the pool of vagal afferents that dynamically integrate neural signals with local peripheral and circulating levels of CCK.
Another group of observations suggests that vagal IGLE afferents may have “smart” and adaptive arbors that supply substantial integrative potential out of all proportion to the number of neurites found within the nerve trunk. Other afferents (e.g., somatosensory afferents innervating the skin) sometimes support “axon reflexes” in which a membrane potential in a terminal branch invades other branches of the same afferent terminal, causing the release of transmitters or neuromodulators that produce local motor-like responses or tissue effects in the periphery and independent of any central relay. Such axon-reflex capability has been posited for vagal afferents as well,20, 29 and two features of IGLEs give the conclusion feasibility. Firstly, in a regional adaptation of the general IGLE morphology, some of the IGLEs located in the more distal corpus and antrum have not only many branches terminating in the characteristic plates of flattened, lamellar puncta, they also have other branches or collaterals that enter the more central region of the myenteric ganglia, display rounded, varicose contacts in proximity to postganglionic neurons (see Fig. 4A, 4B, and 4C). Secondly, as mentioned above, ultrastructural observations have reported presumptive synaptic vesicles in IGLE processes, suggesting that the afferent terminal arbors may release neuropeptides or other transmitter molecules in the target tissues.
Finally, and most speculatively in terms of IGLEs’ structured specializations shaping function, one might expect that IGLEs situated between the longitudinal muscle and the myenteric plexus might be more sensitive to longitudinal muscle stress whereas IGLEs located between the circular muscle and the plexus would be more affected by circular muscle stress.
Intramuscular arrays (IMAs)
IMAs were the second phenotype of vagal afferent terminals in the stomach to be recognized and well characterized morphologically. In 1992, our laboratory group29 labeled vagal afferent terminals with the anterograde tracer DiI, simultaneously characterizing the morphology of the endings and establishing, by limited nodose injections, that the terminals were vagal. Shortly thereafter, using HRP as an anterograde tracer, Wang and Powley30 further began to survey the endings and introduced the terminology of “intramuscular arrays.” As the name suggests and as we have described,31, 32 and in contrast with both vagal efferents and IGLE afferents both of which innervate the myenteric plexus situated between the two smooth muscle layers of the muscularis externa projecting to the stomach, intramuscular arrays or IMAs specifically innervate gastric smooth muscle proper. Individual IMAs distribute region-specific arbors either in the longitudinal smooth muscle layer superficial to the myenteric plexus or the circular smooth muscle layer deep to the myenteric plexus (and only rarely both muscle layers32).
Within the targeted smooth muscle layer, vagal IMAs arborize, branching so as to produce a series of short elements, bridging perpendicular to the smooth muscle fibers (Fig. 5B, 5C, and 5E), that bifurcate into long neurites which run parallel to and with bundles of smooth muscle fibers (Fig. 5A, 5B, 5C, and 5E) and the associated interstitial cells of Cajal (ICC) networks between muscle fibers (Fig. 5D). These long terminal branches express varicosities and flattened elements that appear to associate with and contact ICCs, forming an array of long parallel neurites. Immunohistochemical surveys of the IMAs indicate that the processes of the IMA-ICC complexes often intermingle with different neurochemically distinct afferent and efferent neurites.33 Ultrastructural observations of the IMAs corroborate the contacts with smooth muscles and ICCs, document the presence of vesicles, and illustrate that the IMA neurites and terminal branches often course in small fascicles of a few neurites.19
Figure 5.
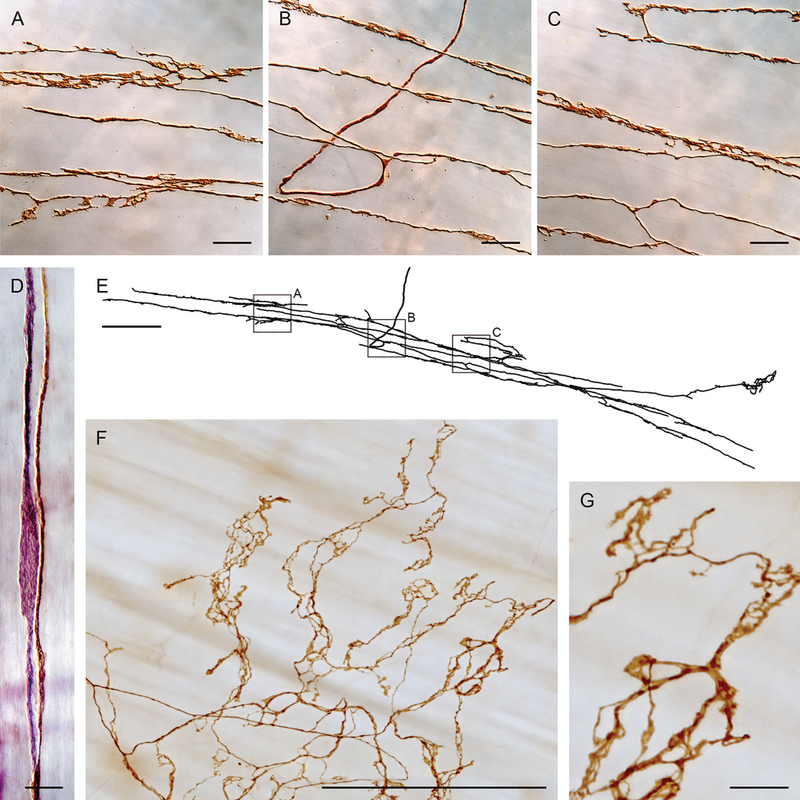
Intramuscular arrays (IMAs), in contrast to vagal preganglionic efferents and IGLE afferents, directly innervate the muscle layers and form arrays of branches that typically run with the ICC network found in the smooth muscle layers. Panels A, B, and C are photomicrographs of branches of the IMA digitized with Neurolucida in panel E. Panel D illustrates how IMA branches (dextran-biotin labeled brown fiber) course with ICCs (elongated purple cell—Vector® VIP labeled for cKit antibody) in the muscle layers. Panel F illustrates the specialized “web ending” variant of IMAs seen near the distal antral/pyloric insertion of sling muscle fibers. Panel G is a higher power image of the IMA apparatus and its contacts or varicosities illustrated in panel F. Scale bars = 20 μm (panels A−C), 10 μm (panel D), 250 μm (panels E and F), and 25 μm (panel G). Panels A–E reproduced by permission of Elsevier from Powley and Phillips.33
Systematic tracings and digital reconstructions indicate that IMA afferents most commonly consist of a “parent” neurite coursing to the innervation site and then ramifying into a single, often extensive, array of long, parallel terminal branches. Occasionally, a parent arbor will issue one or more secondary arbors.
Functionally, the operation(s) of IMAs remain an open empirical question. Based on the features of the afferents and several tentative inferences, we have provisionally suggested that vagal afferent IMAs have the architecture and contacts of stretch or length detectors (whereas IGLE afferents discussed above have the architecture and contacts of tension receptors). Our stretch-receptor suggestion21, 33 seems to fit the available evidence and to have heuristic value. It is, however, still provisional and certainly an approximation.22 Unfortunately, most electrophysiological analyses of vagal afferents that could potentially have distinguished between stretch or tension receptors have confounded the two types of force and thus do not permit any definitive conclusion. It is worth noting that in our initial description of IMAs,29 we too conflated tension and stretch reception and discussed IMAs in terms of “tension.” Consistent with a more particular and explicit distinction that IMAs may record stretch or length whereas IGLEs may record tension, Zagorodnyuk, Brookes, and coworkers23, 24 in their recent analyses of tracer-labeled IGLEs considered IGLEs to be tension receptors.
Vagal IMA afferents display particularly clearly the point that the complex vagal terminals in the stomach express predictable regional specializations in their morphology, which may generate different functional capacities. To date, the regional surveys of IMA specializations have been more comprehensive than the observations on gastric IGLEs or other afferents, and in the case of IMAs in particular, the regional specializations also seem consistent with the hypothesized role of stretch receptors.
At any rate, though, IMAs do display a multiplicity of adaptations to the region they innervate. IMAs in the forestomach are particularly densely distributed in both longitudinal (closer to greater curvature) and circular (closer to the lesser curvature) muscle layers, and the endings are extensive and elongated, features consistent with the reservoir function of the forestomach.30, 32 IMAs in the corpus and proximal antrum are prominent, particularly in circular muscle, consistent with mixing and pumping functions associated with the regions.32 In the antrum, near the pyloric attachment of sling muscles, IMAs also organize into a highly specialized web apparatus34 (Fig. 5F and 5G). The long sling muscles running superficially from the pyloric lesser curvature to the LES, are innervated by exceptionally elongated IMAs.34 And both the clasp muscles and sling muscles where they encircle the LES, are densely innervated by IMA afferents.35 The thick sphincter circular muscle ring of the pylorus is heavily innervated by relatively short IMAs, though with a densely arborized branching pattern.36 In the case of the pyloric IMAs, circumstantial evidence again suggests that IMA function may be modulated by CCK release: the pyloric IMAs appear colocalized with the distribution of CCK receptors in pyloric circular muscle,37 and the receptors appear to be densely distributed on the ICCs38 of the IMA-ICC complexes in that circular muscle.
And most generally, the stretch receptor hypothesis of IMAs is reinforced by the fact that the stomach, which because of a sphincter at either end operates as a reservoir of nutrients that distends, contracts and stretches with every meal, is extensively innervated with vagal IMA afferent terminals, whereas the intestines, which essentially constitute long “open-ended” tubes without sphincters (between the pylorus and ileocecal junction) have a minimum of IMAs.
Gastric mucosal arbors
While vagal IGLE afferents and IMA afferents comprise the general categories of vagal afferents that innervate the muscularis externa, the mucosa and submucosa of the stomach wall are innervated by a different class of complex vagal terminal. Of the three broad phenotypes of gastric afferent terminals, these are the last to be characterized morphologically with tracer injections. We recently described their architecture, established (by nodose injections of the tracer) that they are vagal, and designated them “gastric antral mucosal arbors” because of their architecture and location in the gastric glandular mucosa.31 The architecture of the mucosal endings in the corpus or most of the stomach have yet to be fully examined.
Nonetheless, though only recently and partially described morphologically and mapped, the broad outline of their architecture has come into focus. The pattern again suggests that the gastric mucosal arbors have topographies consistent with extensive, broadly tuned structures capable of integrating considerable information on stomach function. As illustrated in Figure 6, individual vagal afferents course through the submucosa to begin arborizing at the base of the gastric glandular mucosa. The parent neurite divides repeatedly, forming a number of higher order branches that create dense networks of afferent terminals in the deeper layers of the mucosa and a subset of terminals that course along the inner epithelial wall of the gastric glands to continue to terminal endings immediately below the lumenal mucosal lining (Fig. 6A). These gastric mucosal arbors consist of varicose branches, many of which run in close proximity to EEC (Fig. 6B), potentially affected by the paracrine release of those EEC,26, 39 as well as by the secretory products elaborated by the local gastric glands and mucosa. Presumably, those higher order terminal branches coursing up to the lumenal epithelium of the gastric mucosa would also respond directly or indirectly to the contents and composition of the gastric chyme.40
Figure 6.
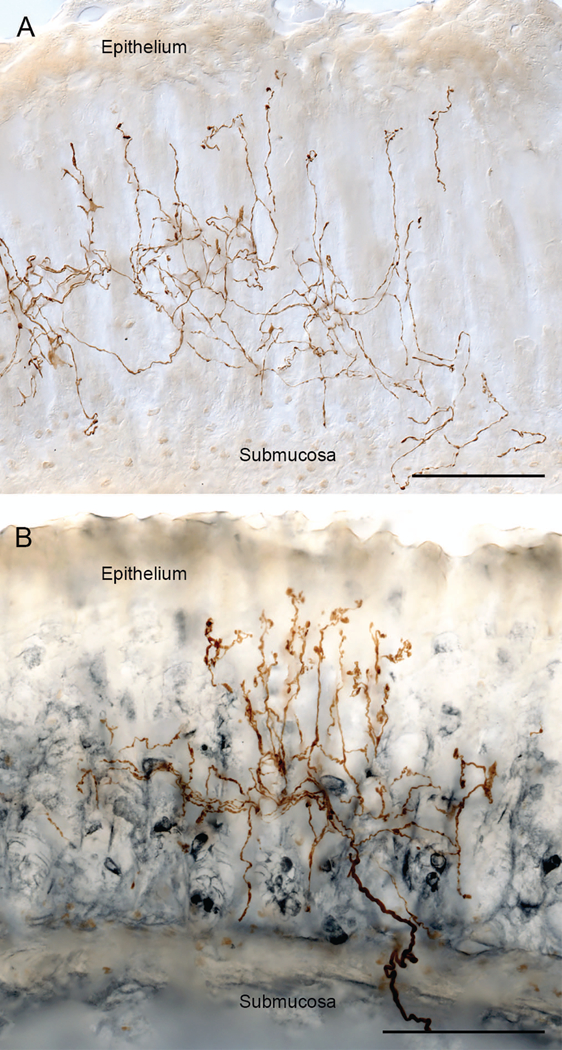
Vagal mucosal arbors are afferents that arborize deep in the mucosal layer and send a subset of their branches paralleling the gastric glands and reaching the basal side of the epithelial wall in direct contact with the contents of the stomach. Panel A illustrates a dextranbiotin filled mucosal arbor (brown branches) in the mucosal layer. Panel B illustrates how the mucosal fibers branch and ramify in the deeper mucosal layers in the zone heavily populated with EEC (in the specimen in panel B, the brown dextran-biotin labeled afferent articulates with EEC immunohistochemically stained for gastrin (steel gray secondary)). Scale bars = 100 μm.
It should also be noted that the arbors of the antral mucosal afferents are “bushy” or extensive in z-, as well as in x- and y-, up to about 500 µm in x- and z-, and about 200 Δm in y- (within the thickness of the mucosa). One significance of this “bushy” pattern of arborization, if the afferent had mechanoreceptive properties, would be that the arbor might not have major orientation sensitivity, but rather might respond more to net pressure or deformation, regardless of orientation. Such functional patterns have been described electrophysiologically for vagal afferents in the mucosa,41, 42 and bushy 3D arbors sensitive to deformation or pressure in any orientation might well provide the functionality for the stomach to monitor the pressure associated with contractions, peristaltic waves, grinding of chyme, or maceration of food material.
Considerable evidence suggests that these arbors have been partially characterized electrophysiologically (and, if so, they are apparently polymodal arbors, and perhaps with multiple receptive fields43), but they, as discussed, have only recently—and partially—been morphologically characterized.
Esophagus and intestines: sympathetics and spinal visceral afferents
A primary focus of the present review is the vagal innervation specifically of the stomach. This focus was adopted in the interest of space, but also because the gastric innervation, compared to that in the rest of the GI tract, has been the most thoroughly inventoried. The conclusion that the autonomic innervation of the GI tract is complex and extensive, based on a variety of distinctive phenotypes specialized to different regions, however, appears to be general, possibly ubiquitous. Certainly, the pattern of extensive, complex, and specialized smart terminals is not limited to the stomach, to the vagus, or to parasympathetic projections.
Rather than reflecting a specific stomach feature, complex and specialized vagal terminals are found throughout the GI tract. Vagal preganglionic motor fibers are found from the esophagus, through the stomach, as well as through the intestines to at least the proximal colon.44 Similarly, vagal IGLE afferents are found from the esophagus to the proximal colon.14, 16, 17 Vagal IMAs are apparently found in the esophagus, the stomach and its sphincters, and within the intestines (low density; Phillips and Powley, unpublished data). Furthermore, as we have recently described in a survey of the proximal small intestines, the vagus elaborates two additional and unique phenotypes of afferent terminals in the postgastric GI tract. One of these afferents produces terminal branches that coil around intestinal crypts or glands (see Fig. 7B), and the second intestinal phenotype forms arbors in the villi of the intestine (Fig. 7A).
Figure 7.
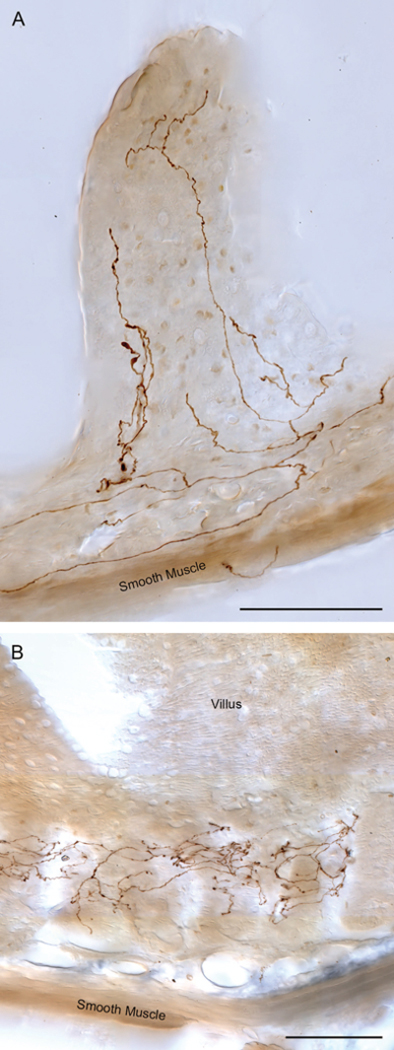
Vagal afferents innervate villi and crypts (or intestinal glands) throughout the small intestines. Panel A illustrates a villus ending (brown neurites labeled with dextran-biotin) in the distal jejunum. Vagal villus afferents throughout the small intestine issue multiple branches that course along the basal side of the epithelial wall and run apically to the villus tips. Several adjacent villi can be innervated by one afferent arbor. Along the small intestine, the vagal villus afferents exhibit local specializations with, generally, more numerous branches in individual villi in the proximal intestines and less numerous branches in the distal intestines. Panel B illustrates the arbor of a villus crypt—or gland—afferent (brown neurites labeled with dextran-biotin) encircling multiple neighboring glands immediately below intestinal villi. This afferent, located in the distal duodenum, characteristically links several neighboring glands into a presumptive receptive field. Scale bars = 100 μm.
Moreover, extensive and specialized endings are not restricted to the vagus. For example, postganglionic sympathetic axons (originating in the celiac and superior mesenteric ganglia) innervating both the stomach and intestines form complex and polytopic projections and have complex distributions45 (Fig. 8). Similarly, the special vagal efferent projections (which are issued by the neurons of the compact or “retrofacial” subnucleus of the nucleus ambiguus) terminate in esophageal striated muscle and end in complex, extensive and polytopic patterns consistent with the smart terminal inference.35 In addition, visceral afferents issued by the “sacral” component of the “craniosacral” parasympathetic division of the autonomic nervous system supply complex afferents to the distal GI tract. Brookes and colleagues23, 24, 46, 47 have applied tracers to the distal stump of fiber bundles (including dorsal root afferents) to the distal colon and rectum and observed a rectal IGLE-like profile (rIGLEs) in the rectum. Spencer and coworkers47, 48 have injected dextran conjugates into the lumbosacral DRGs and reported a multiplicity of types and variants of afferent arbors in the distal GI tract.
Figure 8.

Sympathetic efferent fibers innervating the gut exhibit complex terminal arbors. The labeled fiber in this photomicrograph (brown neurite labeled with dextran biotin) courses through and contacts myenteric ganglion cells (entering at lower right) and then turns and continues to extensively arborize in the smooth muscle wall, where it produces branches coursing both longitudinally (top to bottom of figure) and circularly (left to right of figure). Scale bar = 31 μm. Reproduced by permission of John Wiley & Sons from Walter et al.45
Receptors, hormones, and paracrine integration
Though, in the initial sections of this review we have concentrated on a few examples of receptor molecules where there is good evidence for a coincidence of the maps for individual vagal efferent or afferent arbor phenotypes and the maps for distributions of identified receptor or binding site types, a considerable amount of additional evidence indicates more generally— with less clear (or unexamined) coincidence of a single phenotype and particular receptor type—that vagal projections to the stomach are rich with hormone, paracrine factor, and another molecular binding site expression. For example, vagal afferents (precise target and architecture unspecified) examined in terms of nodose ganglia neuronal somata expression (mRNA, immunohistochemistry, etc.) produce receptors for CCK, serotonin, lipid amides, Y2, CART, MCH-1, T1R2/T1R3 heterodimers, GPR120, etc.49, 50, 25 The case is compelling that vagal afferents including those that innervate the stomach are effectively influenced by the local tissues, the chemistry and the environment of the circulation, and the contents of the GI tract. Given the high percentages of afferent somata in the nodose ganglion that express the various separate types of paracrine/hormone/etc. binding sites, it must follow that some or all individual nodosal afferents express binding sites for multiplicities of different hormonal and humoral signals.
Our focus in this review is on how the crosslinking, coordination and integration of terminals morphologically “hardwired” in the vagal−gut architecture may generate processing in the periphery. In addition to this hardwiring of processing syntheses, however, it is also important to recognize that such inherent integrative capacity is further amplified by sensitivity to endocrine/paracrine factors and dynamic humoral adjustments of the innervation. Considerable evidence now indicates that vagal efferents and afferents (typically not specified in terms of their phenotypes and targets and in many cases only characterized as “vagal”—insofar as the associated response(s) can be blocked by vagotomy—rather than even specifically motor or sensory) are influenced by, i.e., respond with various up- or downregulations to, the conditions of the gut. These labile gut factors include diet, microbiome, disease, and insult. For example, manipulations of the microbiome of the gut certainly affect the brain−gut connectome51 and can reflect dynamic changes of the afferents that monitor the status of the internal milieu. Furthermore, the cascade of inflammatory responses and associated cytokines that can impact the GI tract can produce major changes in the vagal pathways comprising the brain−gut connectome.52,53
Therapeutic manipulations of the brain−gut connectome
Given the myriad GI disorders that are poorly treated pharmacologically (e.g., gastroparesis, reflex disorder, eating disorders, obesity, IBS, celiac disease; etc.), the need for prescriptions for selective manipulations of the vagus and/or optimally locating of GES electrodes is apparent. The fact that vagal projections to the upper GI tract are smart, or morphologically “wired” for coordination or integration, has significant promise for therapeutic interventions. On the other hand, assessments of the vagus nerve, some of its efferents and afferent phenotypes, and some of its terminal concentrations are too incomplete to yet formulate comprehensive prescriptions. Too little is yet known about the precise locations of the different neurons (i.e., their neurites) in the vagus nerve or their terminal distribution patterns in the GI tract to specify a prescription for selective activation of particular vagal effectors, nonetheless a review of the smart vagal projections to the stomach highlights several principles: (1) Successful vagal nerve stimulation (VNS) will likely have to be engineered to selectively affect specific phenotypes of vagal efferent and/or afferent neurites. (2) For a given disorder, a decision will have to be made as to whether to directly or indirectly (by engaging vagovagal reflex circuitry) affect GI function. (3) Successful gastric electrical stimulation (GES) will likely have to be engineered to selectively affect specific sites with concentrations of particular phenotypes efferents or afferents. (4) Again, in the case of GES for a particular disorder (as in the case of VNS), a choice of directly or indirectly engaging motor outflows will have to be made. (5) Successful VNS or GES will likely have to be tuned or engineered to selectively affect one phenotype of efferent or afferent intermingled with other projections of other phenotypes of similar caliber or similar target sites.
Summary
In general, observations of the architecture of the different autonomic projections to the gastrointestinal tract revealed by neural tracers underscore two conclusions: (a) the autonomic innervation of the GI tract, or the degree of communication between the brain and the gut is underestimated and cannot be adequately understood from simple counts of cell bodies or axons responsible for the coordination and (b) the autonomic projections to the gut are characterized by extensive and smart terminal arbors that may perform substantial integration in the periphery, even before the signals are communicated to the CNS.
Acknowledgments
The authors thank C.N. Billingsley, M.-C. Holst, J.B. Kelly, F.N. Martin, G.C. Walter, and F.-B. Wang for their expert help in the use and refinement of the several different tracer protocols as well as their technical assistance employed in tissue preparation. The morphological analyses of the authors were supported by NIH grants OT2OD023847 and DK-027627 to TLP and RJP.
Footnotes
Some sources treat the plate as an “IGLE”; whereas other sources refer to the entire afferent issuing the complete set of the one plate and all others issued by a common neurite as an “IGLE”. For consistency and to avoid ambiguities, here, we distinguish the plate associated with a particular ganglion as an “IGLE” and we refer to the entire afferent as an “IGLE afferent.”
Competing interests
The authors declare no competing interests, and the National Institutes of Health provided all monetary support.
References
- 1.Langley JN 1922. Connexions of the enteric nerve cells. J. Physiol. (Lond.). 56:xxxix [Google Scholar]
- 2.Wood JD 1987. Physiology of the Enteric Nervous System In Physiology of the Gastrointestinal Tract. Johnson LR, Ed.: 2nd ed. New York, Raven Press. [Google Scholar]
- 3.Hill CJ 1927. A contribution to our knowledge of the enteric plexuses. Philos. Trans. R. Soc. Lond. B Biol. Sci. 215:355–387. [Google Scholar]
- 4.Berthoud H-R, Jedrzejewska A, & Powley TL. 1990. Simultaneous labeling of vagal innervation of the gut and afferent projections from the visceral forebrain with Dil injected into the dorsal vagal complex in the rat. J. Comp. Neurol. 301(1):65–79. [DOI] [PubMed] [Google Scholar]
- 5.Holst M-C, Kelly JB & Powley TL. 1997. Vagal preganglionic projections to the enteric nervous system characterized with PHA-L. J. Comp. Neurol. 381:81–100. [DOI] [PubMed] [Google Scholar]
- 6.Walter GC, Phillips RJ, Baronowsky EA & Powley TL. 2009. Versatile, high-resolution anterograde labeling of vagal efferent projections with dextran amines. J. Neurosci. Methods. 178:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hayakawa T, Kuwahara S, Maeda S, Tanaka K & Seki M. 2006. Direct synaptic contacts on the myenteric ganglia of the rat stomach from the dorsal motor nucleus of the vagus. J. Comp. Neurol. 498:352–362. [DOI] [PubMed] [Google Scholar]
- 8.Cannon WB 1911. The Mechanical Factors of Digestion. New York: Longmans, Green & Co. [Google Scholar]
- 9.Davenport HW 1989. Gastrointestinal physiology, 1895–1975: motility In Schultz SG, Ed. Handbook of Physiology: Section 6: The Gastrointestinal System, v. 1 pt. 1. Bethesda: The American Physiological Society [Google Scholar]
- 10.Furness JB 2006. The Enteric Nervous System. Oxford: Wiley-Blackwell. [Google Scholar]
- 11.Lawrentjew BI 1929. Experimentell-morphologische Studien uber den feineren Bau des Autonomen Nervensystems. II. Uber den Aufbau der ganglien der Speiserohre nebst einigen Bemerkungen uber das Vorkommen und die Verteilung zweier Arten von Nervenzellen in dem autonomen Nervensystem. Z. Mikrosk. Anat. Forsch. 18:233–267. [Google Scholar]
- 12.Rodrigo J, de Felipe J, Robles-Chillida EM, Perez Anton JA, Mayo I & Gomez A. 1982. Sensory vagal nature and anatomical access paths to esophagus laminar nerve endings in myenteric ganglia. Determination by surgical degeneration methods. Acta. Anat. (Basel).112:47–57. [DOI] [PubMed] [Google Scholar]
- 13.Rodrigo J, Hernandez J, Vidal MA & Pedrosa JA. 1975. Vegetative innervation of the esophagus. II. Intraganglionic laminar endings. Acta. Anat. (Basel). 92:79–100. [DOI] [PubMed] [Google Scholar]
- 14.Neuhuber WL, Raab M, Berthoud H-R & Worl J. 2006. Innervation of the mammalian esophagus. Adv. Anat. Embryol. Cell. Biol. 185:1–73. [PubMed] [Google Scholar]
- 15.Berthoud H-R, Patterson LM, Neumann F & Neuhuber WL. 1997. Distribution and structure of vagal afferent intraganglionic laminar endings (IGLEs) in the rat gastrointestinal tract. Anat. Embryol. (Berl.). 195:183–191. [DOI] [PubMed] [Google Scholar]
- 16.Wang F-B & Powley TL. 2007. Vagal innervation of intestines: Afferent pathways mapped with new en bloc horseradish peroxidase adaptation. Cell Tissue Res. 329: 221–230. [DOI] [PubMed] [Google Scholar]
- 17.Wang F-B, Young YK & Kao C-K. 2012. Abdominal vagal afferent pathways and their distributions of intraganglionic laminar endings in the rat duodenum. J. Comp. Neurol. 520:1098–1113. [DOI] [PubMed] [Google Scholar]
- 18.Neuhuber W 1987. Sensory vagal innervation of the rat esophagus and cardia: a light and electron microscopic anterograde tracing study. J. Auton. Nerv. Syst. 20:243–255. [DOI] [PubMed] [Google Scholar]
- 19.Powley TL, Wang X-Y, Fox EA, Phillips RJ, Liu LWC & Huizinga JD. 2008. Ultrastructural evidence for communication between intramuscular vagal mechanoreceptors and interstitial cells of Cajal in the rat fundus. Neurogastroenterol. Motil. 20(1):69–79. [DOI] [PubMed] [Google Scholar]
- 20.Neuhuber WL & Clerc N. 1990. Afferent innervation of the esophagus in cat and rat In The Primary Afferent Neuron. Zenker W & Neuhuber WL, Eds.: New York: Plenum. [Google Scholar]
- 21.Phillips RJ & Powley TL. 2000. Tension and stretch receptors in gastrointestinal smooth muscle: reevaluating vagal mechanoreceptor electrophysiology. Brain Res. Brain Res. Rev. 34(1–2):1–26. [DOI] [PubMed] [Google Scholar]
- 22.Timmermans J-P & Adriaensen D. 2008. Gastrointestinal mechanosensors: analysis of multiple stimuli may require complex sensors. Neurogastroenterol. Motil. 20(1):4–7. [DOI] [PubMed] [Google Scholar]
- 23.Zagorodnyuk VP, Chen BN & Brookes SJ. 2001. Intraganglionic laminar endings are mechanotransduction sites of vagal tension receptors in the guinea pig stomach. J. Physiol. 534:255–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zagorodnyuk VP, Chen BN, Costa M & Brookes SJ. 2003. Mechanotransduction by intraganglionic laminar endings of vagal tension receptors in the guinea pig oesophagus. J. Physiol. 553:575–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Steinert RE & Beglinger C. 2011. Nutrient sensing in the gut: interactions between chemosensory cells, visceral afferents and the secretion of satiation peptides. Physiol. Behav. 105:62–70. [DOI] [PubMed] [Google Scholar]
- 26.Furness JB, Rivera LR, Cho H-J, Bravo DM & Callaghan B. 2013. The gut as a sensory organ. Nat. Rev. Gastroenterol. Hepatol. 10:729–740 [DOI] [PubMed] [Google Scholar]
- 27.Blackshaw LA & Grundy D. 1990. Effect of cholecystokinin (CCK-8) on two classes of gastroduodenal vagal afferent fibre. J. Auton. Nerv. Syst. 31:191–201. [DOI] [PubMed] [Google Scholar]
- 28.Okano-Matsumoto S, McRoberts JA, Tache Y & Adelson DW. 2011. Electrophysiological evidence for distinct vagal pathways mediating CCK-evoked motor effects in the proximal versus distal stomach. J. Physiol. 589(2):371–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berthoud H-R & Powley TL. 1992. Vagal afferent innervation of the rat fundic stomach: morphological characterization of the gastric tension receptor. J. Comp. Neurol. 319:261–276. [DOI] [PubMed] [Google Scholar]
- 30.Wang F-B & Powley TL. 2000. Topographic inventories of vagal afferents in gastrointestinal muscle. J. Comp. Neurol. 421:302–324. [PubMed] [Google Scholar]
- 31.Powley TL, Spaulding RA & Haglof SA. 2011. Vagal afferent innervations of the proximal gastrointestinal tract mucosa: chemoreceptor and mechanoreceptor architecture. J. Comp. Neurol. 519:644–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Powley TL, Hudson CN, McAdams JL, Baronowsky EA & Phillips RJ. 2016. Vagal intramuscular arrays: The specialized mechanoreceptor arbors that innervate the smooth muscle layers of the stomach examined in the rat. J. Comp. Neurol. 524:713–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Powley TL & Phillips RJ. 2011. Vagal intramuscular array afferents form complexes with interstitial cells of Cajal in gastrointestinal smooth muscle: analogues of spindle organs? Neuroscience. 186:188–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Powley TL, Gilbert JM, Baronowsky EA, Billingsley CN, Martin FN & Phillips RJ. 2012. Vagal sensory innervation of gastric sling muscle and antral wall: implications for gastroesophageal reflux disease? Neurogastroenterol. Motil. 24:e526–e537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Powley TL, Mittal RK, Baronowsky EA, Hudson CN, Martin FN, McAdams JL, Mason JK & Phillips RJ. 2013. Architecture of vagal motor units controlling striated muscle of esophagus: peripheral elements patterning peristalsis? Auton. Neurosci. 179:90–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Powley TL, Hudson CN, McAdams JL, Baronowsky EA, Martin FN, Mason JK & Phillips RJ. 2014. Organization of vagal afferents in pylorus: Mechanoreceptors arrayed for high sensitivity and fine spatial resolution? Auton. Neurosci. 183:36–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smith GP, Moran TH, Coyle JT, Kuhar MJ, O’Donahue TL, & McHugh PR. 1984. Anatomic localization of cholecystokinin receptors to the pyloric sphincter. Am. J. Physiol. 246(1):R127–130. [DOI] [PubMed] [Google Scholar]
- 38.Patterson LM, Zheng H, Ward SM & Berthoud H-R. 2001. Immunohistochemical identification of cholecystokinin A receptors on interstitial cells of Cajal, smooth muscle, and enteric neurons in rat pylorus. Cell Tissue Res. 305:11–23. [DOI] [PubMed] [Google Scholar]
- 39.Diwakarla S, Fothergill LJ, Callaghan B & Furness JB. 2017. Heterogeneity of enterochromaffin cells within the gastrointestinal tract. Neurogastroenterol. Motil. 29: e13101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mei N 1985. Intestinal Chemosensitivity. Physiol. Rev. 65(2):211–237. [DOI] [PubMed] [Google Scholar]
- 41.Iggo A 1957. Gastric mucosal chemoreceptors with vagal afferent fibres in the cat. Q. J. Exp. Physiol. Cogn. Med. Sci. 42(4):398–409. [DOI] [PubMed] [Google Scholar]
- 42.Grundy D 1988. Speculations on the structure/function relationship for vagal and splanchnic afferent endings supplying the gastrointestinal tract. J. Auton. Nerv. Syst. 22(3):175–180 [DOI] [PubMed] [Google Scholar]
- 43.Berthoud H-R, Lynn PA & Blackshaw LA. 2001. Vagal and spinal mechanosensors in the rat stomach and colon have multiple receptive fields. Am. J. Physiol. Regul. Integr. Comp. Physiol. 280(5):R1371–1381. [DOI] [PubMed] [Google Scholar]
- 44.Berthoud H-R, Carlson NR & Powley TL. 1991. Topography of efferent vagal innervation of the rat gastrointestinal tract. Am. J. Physiol. 260:R200–207. [DOI] [PubMed] [Google Scholar]
- 45.Walter GC, Phillips RJ, McAdams JL & Powley TL. 2016. Individual sympathetic postganglionic neurons coinnervate myenteric ganglia and smooth muscle layers in the gastrointestinal tract of the rat. J. Comp. Neurol. 524:2577–2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zagorodnyuk VP & Brookes SJ. 2000. Transduction sites of vagal mechanoreceptors in the guinea pig esophagus. J. Neurosci. 20(16):6249–6255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Spencer NJ, Kyloh M, Beckett EA, Brookes S & Hibberd T. 2016. Different types of spinal afferent nerve endings in stomach and esophagus identified by anterograde tracing from dorsal root ganglia. J. Comp. Neurol. 524:3064–3083. [DOI] [PubMed] [Google Scholar]
- 48.Kyloh M, & Spencer NJ. 2014. A novel anterograde neuronal tracing technique to selectively label spinal afferent nerve endings that encode noxious and innocuous stimuli in visceral organs. Neurogastroenterol. Motil. 26:440–444 [DOI] [PubMed] [Google Scholar]
- 49.Dockray GJ 2009. The versatility of the vagus. Physiol. Behav. 97:531–536. [DOI] [PubMed] [Google Scholar]
- 50.Dockray GJ & Burdyga G. 2011. Review: Plasticity in vagal afferent neurons during feeding and fasting: mechanisms and significance. Acta. Physiol. (Oxf.). 201:313–321. [DOI] [PubMed] [Google Scholar]
- 51.Sgritta M, Dooling WW, Buffington SA, Momin EN, Francis MB, Britton RA & Costa-Mattioli M. 2018. Mechanisms underlying microbial-mediated changes in social behavior in mouse models of autism spectrum disorder. Neuron. 101(2):246–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Breit S, Kupferberg A, Rogler G & Hasler G. 2018. Vagus nerve as modulator of the brain-gut axis in psychiatric and inflammatory disorders. Front. Psychiatry. 9:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Steinberg BE, Silverman HA, Robbiati S, Gunasekaran MK, Tsaava T, Battinelli E, Stiegler A, Bouton CE, Chavan SS, Tracey KJ & Huerta PT. 2016. Cytokine-specific neurograms in the sensory vagus nerve. Bioelectron. Med. 3:7–17. [PMC free article] [PubMed] [Google Scholar]


