Abstract
With few effective treatments available, the global rise of metabolic diseases, including obesity, type 2 diabetes mellitus, and cardiovascular disease, seems unstoppable. Likely caused by an obesogenic environment interacting with genetic susceptibility, the pathophysiology of obesity and metabolic diseases is highly complex and involves crosstalk between many organs and systems, including the brain. The vagus nerve is in a key position to bidirectionally link several peripheral metabolic organs with the brain and is increasingly targeted for neuromodulation therapy to treat metabolic disease. Here, we review the basics of vagal functional anatomy and its implications for vagal neuromodulation therapies. We find that most existing vagal neuromodulation techniques either ignore or misinterpret the rich functional specificity of both vagal efferents and afferents as demonstrated by a large body of literature. This lack of specificity of manipulating vagal fibers is likely the reason for the relatively poor beneficial long‐term effects of such therapies. For these therapies to become more effective, rigorous validation of all physiological endpoints and optimization of stimulation parameters as well as electrode placements will be necessary. However, given the large number of function‐specific fibers in any vagal branch, genetically guided neuromodulation techniques are more likely to succeed.
Keywords: neuromodulation, electrical stimulation, obesity, diabetes, anti‐inflammatory pathways, gut–brain communication, genetically guided, vagotomy
The vagus nerve bidirectionally links several peripheral metabolic organs with the brain and is increasingly targeted for neuromodulation therapy to treat metabolic disease. The authors of this article review the basics of vagal functional anatomy and its implications for vagal neuromodulation therapies, and they find that these therapies often ignore or misinterpret the rich functional specificity of both vagal efferents and afferents.
Introduction
The prevalence of metabolic diseases is steadily increasing with no real cure in sight. The overpowering environmental, social, cultural, and economic changes in the past century, together with genetic susceptibility, are widely considered to be the main causes of obesity, type 2 diabetes mellitus (T2DM), cardiovascular disease, and many other diseases, including cancer, associated with chronic overnutrition and a positive energy balance. Because these staggering changes cannot be easily reversed or neutralized, our ability to fight metabolic diseases is mainly limited to symptomatic treatment, such as pharmacology, behavioral modification, and surgery. Except for bariatric/metabolic surgery, the effectiveness of such symptomatic treatments has been rather disappointing, likely owing to incomplete understanding of the critical components of physiological regulation of body weight and glucose homeostasis.
The vagus nerve is a bidirectional communication pathway between the metabolic periphery and the brain and as such has the potential to be a major player in body weight and glucose homeostasis1 (Fig. 1); it is now 40 years since subdiaphragmatic vagotomy was first proposed as a treatment for severe obesity.2 Vagal mechanisms are also likely to play important roles in the sustained beneficial effects of gastric bypass and other bariatric surgeries and devices.3 In the last 40 years, however, we have learned a great deal about the anatomy and function of the 10th cranial nerve, which will help develop much more selective and refined techniques to leverage vagal mechanisms for the treatment of metabolic diseases. In this overview, we will summarize some of these advances and discuss the potential of vagal neuromodulation in the fight against metabolic and associated diseases. Because of space limitations, many important contributions of dedicated scientists in this field, particularly as it pertains to vagal sensory functions, will not be discussed in detail, but the interested reader can find synopses of their work here.4, 5, 6, 7, 8, 9
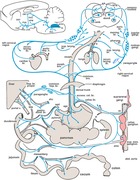
Figure 1.
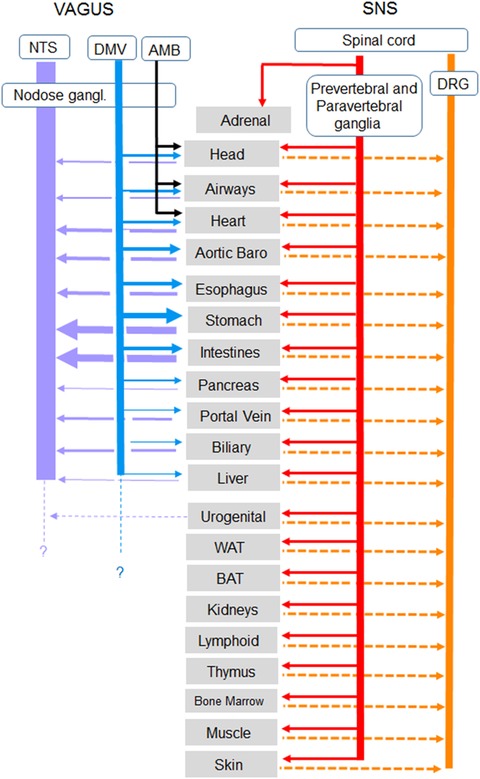
Overview of autonomic nervous system innervation of organs and tissues important for metabolic regulation. For vagal innervation, the line thickness roughly reflects the number of afferent and efferent axons in the respective branches, as mainly observed in rats. There are not sufficient data available for similar analyses in the sympathetic and dorsal root systems. AMB, nucleus ambiguous; DMV, dorsal motor nucleus of the vagus; DRG, dorsal root ganglia; NTS, nucleus tractus solitarius.
Functional anatomy of the vagus nerve
What organs and tissues relevant for metabolic diseases are innervated by the vagus nerve?
Although acetylcholinesterase immunohistochemistry provided the first hint of vagal innervation of specific organs, tissues, and cells,10, 11, 12 systematic study began with the availability of anterograde and retrograde tracers discovered in the late 1970s and early 1980s. In a first wave of research, retrograde tracers, either classical monosynaptic tracers, such as horseradish peroxidase,13 or the trans‐synaptic retrograde tracer pseudorabies virus,14 were injected into the organ of choice and labeled cells were identified in vagal and sympathetic neurons. However, since the peripheral injections were often large and leaky, there soon were a fair number of false positive findings.15, 16 Thus, in a second wave of research, anterograde tracers were injected into the vagal motor nucleus in the brainstem17, 18, 19 or into the nodose ganglia,20, 21 and after allowing for axonal transport, the labeled terminals of the respective efferent or afferent neurons were observed in peripheral tissues.
On the basis of such studies in rats with various anterograde tracers, it became clear that the alimentary canal, and specifically the stomach and gastroduodenal junction, is by far the most densely vagally innervated abdominal organ.19, 22 According to classical parasympathetic anatomy, vagal preganglionic fibers originating in the dorsal motor nucleus innervate postganglionic neurons of mainly the myenteric and, to a lesser extent, the submucosal plexus,18 where they are in a position to powerfully affect gastro‐duodenal motor and secretory functions (Fig. 2). Similarly, vagal afferents traced from the nodose ganglia innervate the external muscle layer, myenteric plexus, and the mucosa, where they are in a position to mediate both mechano‐ and chemosensory information to the brain.20, 22 Both efferent and afferent vagal axons also innervate the entire length of the small and large intestines, although with decreasing density in the colon.19, 23, 24 The extent of vagal afferent innervation to the small intestinal mucosa is particularly impressive and suggests comprehensive direct monitoring of mucosal chemistry by the brain, as the next synapse of these bipolar sensory neurons is located in the nucleus tractus solitarius (NTS) in the brainstem.25, 26, 27 Gastrointestinal vagal afferents are channeled via the NTS to various forebrain structures, for example, the hypothalamus and limbic areas, partly in a lateralized manner. This was exemplified by a recent study demonstrating gut‐induced reward via a right‐sided nodose ganglion‐NTS‐parabrachial nuclei pathway to midbrain dopaminergic neurons and the striatum.28
Figure 2.
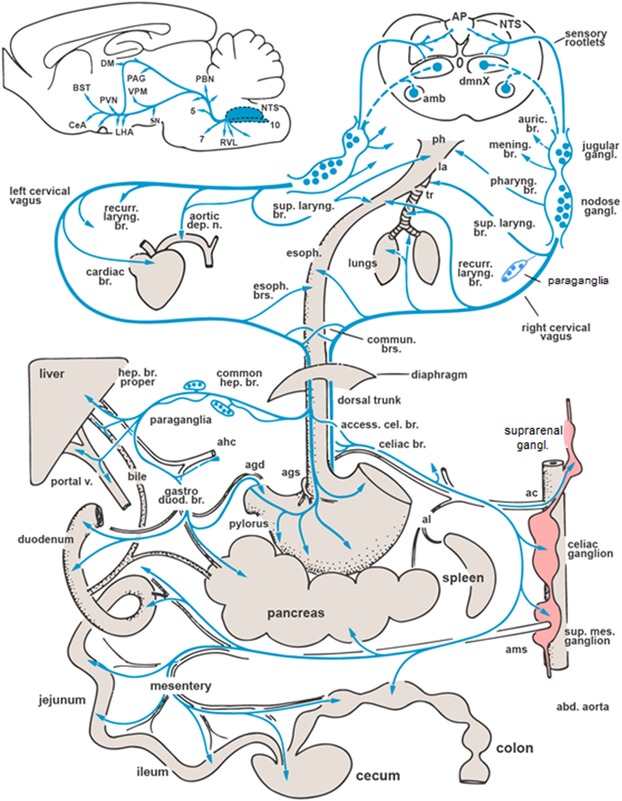
Major vagal nerve branches and central projections from the nucleus tractus solitarius (NTS) in the rat, based on anterograde and retrograde tracing experiments. Note that the arrows only indicate the innervated organs and tissues and do not distinguish afferents and efferents. Also note that the spleen is not innervated by the vagus. amb, nucleus ambiguous; AP, area postrema; BST, bed nucleus of the stria terminalis; CeA, central nucleus amygdala; DM, dorsal nucleus hypothalamus; dmnX, dorsal motor nucleus of the vagus; LHA, lateral hypothalamic area; PAG, periaqueductal gray; PBN, parabrachial nucleus; PVN, paraventricular nucleus hypothalamus; RVL, rostral ventrolateral medulla; SN, substantia nigra; agd, gastroduodenal artery; ags, left gastric artery; ahc, hepatic artery proper; al, splenic artery; ams, superior mesenteric artery. (Figure modified after Ref. 16.)
In addition to the gastrointestinal tract, the rat pancreas is also innervated by both vagal efferents and afferents (Fig. 3). As in the gastrointestinal tract, labeled preganglionic vagal fibers innervate primarily cholinergic or nitrergic neurons located in small intrapancreatic ganglia,29, 30 in a position to affect both endocrine and exocrine functions. Intrapancreatic ganglia are also found in mouse31, 32 and human pancreas33, 34 (Fig. 4). Intriguingly, vagal afferents focus on pancreatic islets, suggesting a detector function for high local concentrations of pancreatic hormones, in particular insulin.35 This idea was supported by a more recent study, which provided functional evidence that pancreas‐innervating nodose ganglion neurons were activated by insulin.36 Vagal afferents from pancreatic islets may be relayed via the NTS to hypothalamic nuclei orchestrating feeding behavior, thus supplementing the role of blood‐borne insulin.
Figure 3.
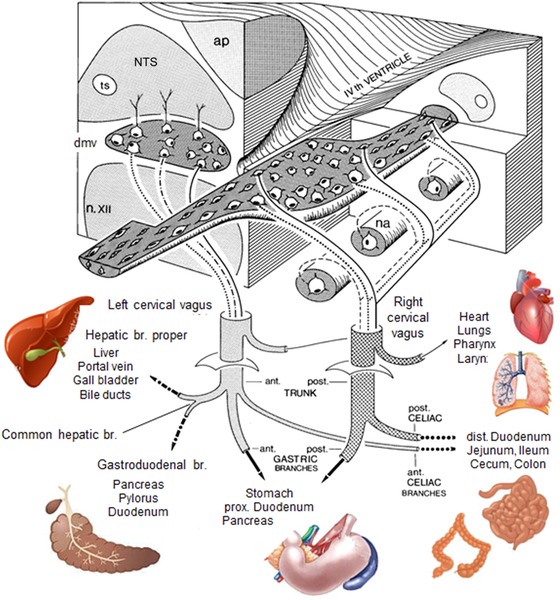
Overview of the central representation of functional vagal efferent outflow, based on experiments combining retrograde tracing, electrical stimulation, and specific vagal branch cuts in rats. Note the organotopic longitudinal columnar organization within the dorsal motor nucleus of the vagus, as indicated by solid, dashed, and punctuated lines, respectively. ap, area postrema; dmv, dorsal motor nucleus vagus; na, nucleus ambiguous; n. XII, hypoglossal nucleus; NTS, nucleus tractus solitarius.
Figure 4.
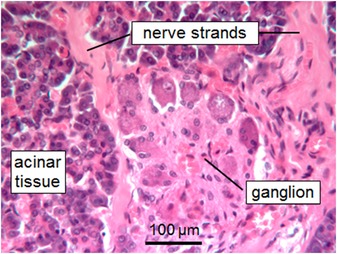
Intrapancreatic ganglion in the head of the human pancreas stained with hematoxylin/eosin. Postmortem processed tissue from a body donated to the Institute of Anatomy and Cell Biology, University of Erlangen.
Vagal innervation of the liver is a subject of great ambiguity. Although there is extant literature on cholinergic effects on liver function, there is no clear demonstration of vagal efferent or afferent innervation of the liver parenchyma in rodents and humans. This is in stark contrast to the quite abundant innervation by tyrosine hydroxylase–positive sympathetic efferent fibers. In the rat, a few cholinergic vagal efferent fibers terminate in the hepatic hilus and in the wall of bile ducts and the portal vein.37, 38, 39 In the absence of neurons within the hepatic parenchyma and hilus, it is not clear where the classical postganglionic neurons for the potential vagal liver innervation are located. In the rat, there are a handful of neurons within paraganglia associated with the common hepatic branch and in a few small ganglia embedded in the periarterial plexus of the celiac and hepatic arteries,40 but their projections to the hepatic parenchyma have not been demonstrated. Vagal afferent fibers are distributed to the portal vein and extra‐ and intrahepatic biliary ducts but not to the liver lobules in the rat.39
Despite one report to the contrary,41 abdominal and subcutaneous white adipose tissues do not seem to be innervated by vagal efferent and afferent axons in the rat. Anterograde tracing from both the motor nucleus and nodose ganglia has never revealed labeled axon terminals in any of the major adipose tissue pads and there is a paucity of markers for vagal efferent innervation, such as VAChT, VIP, and NOS.42 The most likely explanation for the positive report is tracer leakage from adipose tissue to the gastrointestinal tract.15, 43 There is also no evidence for vagal innervation of brown adipose tissue, as well as the spleen and kidneys.44, 45
The pitfalls of functional mapping with electrical stimulation and selective vagotomies
Electrical stimulation of the vagus nerve has been an important tool to establish its functional anatomy, but because of its innervation of multiple organs and the temporal characteristics of evoked metabolic responses, it is easy to make erroneous conclusions. Early studies have devised elaborate setups in order to prove direct vagally mediated effects on an organ. To prove that the vagus is able to stimulate insulin secretion by direct innervation of the pancreas, Bergman and Miller used an experimental dog with a vascularly perfused pancreas and preserved vagal innervation connected to a support dog.46 Electrical stimulation of the vagus nerve of the experimental dog rapidly stimulated insulin secretion, ruling out mediation by an indirect humoral factor. Unfortunately, many studies since then did not use vascularly perfused organs when studying the effects of electrical vagal stimulation. This may not be a problem if the vagal responses of interest are very fast, such as heart rate, blood pressure, and gastric motility or if the vagus can be selectively stimulated very close to the organ of interest. However, if the vagus cannot be cleanly stimulated near the organ or the responses are slow, such as biochemical changes in the liver,47 then vascular perfusion is mandatory, as humoral substances released upstream by vagal stimulation (gastrointestinal tract and pancreas) can by themselves be responsible for the observed effects (Fig. 5).
Figure 5.
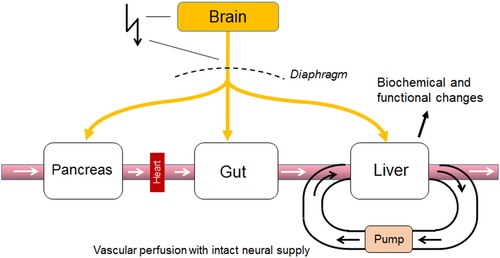
Vascular perfusion with intact neural supply to disconnect a given organ from the systemic circulation as the gold standard for identification of vagal functional anatomy. Electrical stimulation of the vagal trunks (or chemical stimulation in the brain) can release humoral substances upstream (e.g., the pancreas and gut) and, if the organ was not vascularly disconnected, can thus be potentially responsible for the observed functional effects in the observed organ (e.g., the liver), particularly when the responses are slow, such as biochemical changes.
Selective vagotomies are another useful tool for studying the functional anatomy of the vagus nerve, but it is only as good as the specificity in relation to innervating a specific organ or tissue. It should be kept in mind that none of the five abdominal vagal branches (the anterior (ventral) and posterior (dorsal) gastric, the ventral (accessory) and dorsal celiac, and the common hepatic branch) selectively innervate a specific abdominal organ or tissue (Figs. 2 and 3), so the effects of selective abdominal branch vagotomies have to be interpreted with caution. In particular, the common hepatic branch does not only innervate the liver proper, but also innervates bile ducts and the portal vein, as well as parts of the gastric antrum, the pyloric sphincter, the proximal duodenum, and the pancreas through the gastroduodenal branch that splits off the common hepatic branch near the bifurcation of the hepatic and gastroduodenal arteries.39, 48 Therefore, transection of the common hepatic branch affects not only the liver area, but also the gastrointestinal tract and pancreas.19, 49, 50 Thus, the conclusions drawn in a great many studies using common hepatic branch vagotomy51, 52, 53, 54, 55, 56, 57 are highly questionable and should be re‐evaluated with proper methodology. If specific inferences are to be made regarding vagal efferents or afferents in a given abdominal branch, the burden of proof is even higher, since in none of these studies were the two separated.
In summary, incomplete knowledge of detailed vagal innervation patterns and the pitfalls inherent to both neuronal tracing and electrical stimulation methods have led to erroneous interpretation of data in physiological and pathophysiological metabolic studies. Thus, far‐reaching misconceptions should be corrected by careful re‐examination.
Vagal stimulation and blockade for the treatment of obesity and neural diseases
Cervical and transcutaneous auricular branch vagal stimulation
Left cervical vagal electrical stimulation has been approved and used for the treatment of refractory epilepsy since 1997 and treatment‐resistant depression since 2005. It has also been reported to cause weight loss,58, 59 increase energy expenditure and BAT thermogenesis,60 and reduce food craving in adults with depression.61 Transcutaneous electrical stimulation of the auricular branch of the vagus nerve (also known as Arnold's nerve), a pure cutaneous nerve innervating parts of the external ear, obtained European approval for the treatment of refractory epilepsy and pain more recently (for an extensive recent review, see Ref. 62). In addition, a 12‐week randomized pilot clinical trial in patients with impaired glucose tolerance revealed beneficial effects of transcutaneous auricular branch stimulation on fasting glucose, oral glucose tolerance, HbA1c, and systolic blood pressure.63 Furthermore, direct electrical auricular vagal branch stimulation in diet‐induced obese rats for 6 weeks significantly reduced body weight and adiposity without reducing food intake, possibly by increasing BAT thermogenesis.64
The auricular branch contains almost exclusively afferent fibers,65 most of which do not directly project to the NTS, but indirectly through the lateral trigeminal islands.66, 67 The stimulation parameters, which are typically in the range of 10–20 Hz, 0.25–2 mA intensity, and 0.1–0.5 ms pulse width, are optimized to activate mainly vagal afferent fibers. Effective activation of vagal afferents (in the auricular branch) is supported by the measurement of brain activity with fMRI, showing the activation of brain structures previously shown to receive vagal afferent input,68 such as the left locus coeruleus, thalamus, insula, prefrontal cortex, posterior cingulate gyrus, and the bilateral postcentral gyrus, and deactivation in the right nucleus accumbens and cerebellar hemisphere.69, 70 Thus, left cervical vagal and auricular branch stimulation most likely activate vagal afferents and act on various brain areas to affect behavior as well as reflex autonomic outflow.
Vagal blockade for the treatment of obesity and metabolic disease
As mentioned in the introduction, it is now 40 years since subdiaphragmatic vagotomy was first proposed as a treatment for severe obesity.2 Another way that has been claimed to block all vagal signaling from and to the abdomen is through stimulation of the subdiaphragmatic vagal trunks with high‐frequency (5 kHz) electrical stimulation. VBloc® Therapy has been shown to lower body weight beyond sham stimulation in subjects with obesity. In a randomized, double‐blind, sham‐controlled multicenter clinical trial with 239 participants with a body mass index of 40–45 (or 35–40 with one or more obesity‐related conditions), the intent‐to‐treat analysis showed that vagal nerve block therapy for 12 months resulted in significantly greater (9.2%) total weight loss compared with the sham group (6.0%).71, 72 Furthermore, patients who continued on open‐label vBloc therapy for another 12 months regained very little body weight, so that after 2 years they had 8% weight loss and significant improvements in low‐density lipoprotein, HbA1c, and blood pressure.73
While these initial data are somewhat encouraging, the underlying mechanisms of vBloc therapy are not well understood. Given the large body of literature demonstrating the capacity of vagal afferents to signal satiety to the brain, it is counterintuitive that blockade of these vagal afferent signals should result in decreased food intake and body weight loss. Modeling has shown that complete conduction block in the abdominal vagus nerves is unlikely with current stimulation parameters,74 suggesting that some vagal afferents may be excited rather than blocked. Additional modeling with different stimulation parameters and electrode configurations will be necessary to determine how it works. It also remains to be seen whether chronic cuff electrodes continue to work and do not cause nerve damage. Studies in a rat model suggest that chronic cuffing of the cervical vagus inhibits efferent fiber integrity.75
Stimulation of gastric vagal nerve branches (gastric pacing)
Several electrical stimulation devices that target gastric vagal branches in order to reduce appetite, body weight, and improve glucose handling have recently been put on the market (see Ref. 76 for a recent review). The Transcend® implantable gastric stimulator, placed near the lesser curvature, although showing some promise in early investigations in European centers, was unable to produce significant weight loss in a 12‐month randomized controlled clinical trial (SHAPE) conducted in the United States.77 Smart devices, using interactive sensor technology and programmable stimulators, are also currently being evaluated, but without adequate controls for electrical stimulation of vagal branches, their efficacy is unclear.78, 79 Although studies in rats using similar electrical stimulation of gastric vagal branches have identified potential mediating mechanisms in the hypothalamus,80, 81 there are no mechanistic studies aimed to understand the role of function‐specific vagal afferents and efferents in the effects on appetite, body weight, and glycemic control. Given the mixed nature of nerve fascicles on the gastric surface, involvement of sympathetic and dorsal root afferents in the observed effects cannot be excluded.
Currently, the rationale for both vagal stimulation and blocking strategies rests on incomplete acknowledgment of vagal efferent and afferent anatomy and its intertwining with sympathetic and spinal afferent neurons at peripheral levels. This may also explain contradictory results of clinical studies.
The vagus nerve, inflammation, and cancer
The cholinergic, vagal anti‐inflammatory pathway
Interactions between the nervous and immune systems have long been studied, but their potential involvement in metabolic disease progression is relatively novel. It has been proposed that an ancient paraventricular neural system enabling protection and defense through somatic reflexes in lower vertebrates evolved into a much more elaborate system in modern vertebrates, engaging both arms of the autonomic nervous system and the neuroendocrine axis, and providing protection not only from external but also internal insults to homeostasis.82 A sensory arm of this system informs the brain about the state of peripheral immune cells, such as macrophages, mast cells, T cells, and Kupffer cells in the spleen, liver, gut, lungs, and pancreas, through either the circulation or primary afferents of dorsal root ganglia (DRG) and vagal origin (Fig. 6). Close anatomical appositions between mast cells and vagal afferent terminals in the intestinal mucosa83 and similar structures in the airways84 suggest vagal pathways by which immune‐relevant signals from the environment are transmitted to the brain.
Figure 6.
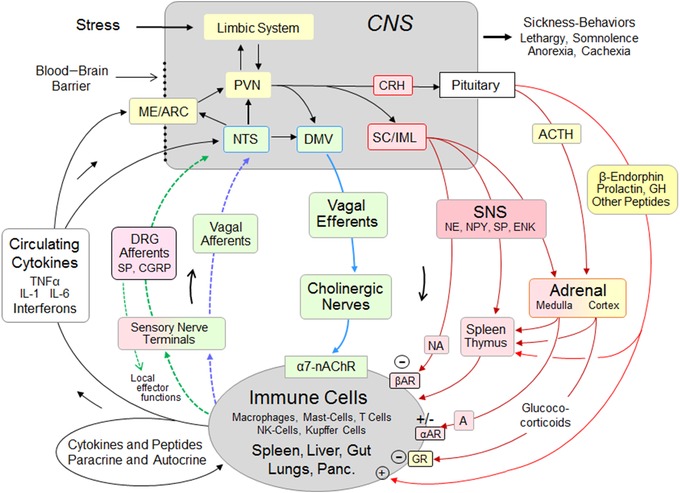
Role of the vagal and sympathetic systems in reciprocal immune‐to‐brain communication and neuroimmunomodulation. Besides their role as afferents to the central nervous system, peptidergic dorsal root ganglia (DRG) neurons can influence immune cells via their so‐called local effector function. DMV, dorsal motor nucleus vagus; PVN, paraventricular nucleus hypothalamus; ME/ARC, median eminence/arcuate nucleus; SC/IML, spinal cord/intermediolateral column; A, adrenaline; NA, noradrenaline; αAR, alpha‐adrenergic receptor; βAR, beta‐adrenergic receptor; ACTH, adrenocorticotrophic hormone; CRH, corticotrophin‐releasing hormone; α7‐nAChR, alpha‐7 nicotinic acetylcholine receptor; ENK, encephalin; CGRP, calcitonin gene‐related peptide; NPY, neuropeptide Y; SP, substance P; GR, glucocorticoid receptor.
In the brain, a key neural network, including the brainstem, hypothalamus, and limbic system, acts in concert with local microglia and astrocytes to organize appropriate behavioral, autonomic, and neuroendocrine actions.85, 86 The effector arm of the system is complex, with checks and balances involving the sympathetic nervous system, vagus nerve, and neuroendocrine systems. The vagal cholinergic anti‐inflammatory component of the effector system was first demonstrated in a rat sepsis model, when direct bilateral electrical stimulation of the peripheral end of the cervical vagi inhibited hepatic TNF‐α synthesis and attenuated peak serum TNF‐α levels.87 The α7 nicotinic acetylcholine receptor on peripheral immune cells was subsequently identified as an essential modulator of inflammation,88 and the splenic nerve as the mediator of the cholinergic vagal anti‐inflammatory reflex.89, 90, 91 However, because the vagus nerve does not directly innervate the spleen, it was later suggested that vagal input to the celiac ganglia92, 93 may drive adrenergic sympathetic postganglionic fibers projecting from the celiac ganglia to the spleen that stimulate acetylcholine release from splenocytes. However, a re‐examination was unable to support these hypotheses.94, 95 In parallel studies on gastrointestinal tract inflammation, vagal stimulation was also shown to ameliorate surgery‐induced inflammation and postoperative ileus via the α7 nAChR and Jak2‐STAT3 activation of resident intestinal muscularis macrophages,96 independent of the spleen.97 The extent to which cholinergic vagal efferent nerve terminals in the gastrointestinal tract and pancreas directly contact macrophages or indirectly via cholinergic enteric (postganglionic) neurons remains to be more carefully investigated. Besides vagal outflow, the sympathetic nervous system is also critically involved in modulating peripheral inflammatory processes.85, 95, 98, 99 C1 neurons in the medullary ventrolateral medulla appear to be a hub relaying information about various stressors to both vagal and, even more importantly, sympathetic preganglionic neurons.85
The concept of a vagal anti‐inflammatory pathway has also encouraged trials with vagal stimulation in inflammatory diseases of organs far away from vagal innervation, and vagus nerve stimulation was successfully used in patients with rheumatoid arthritis.100 This may seem surprising, as joints typically affected by arthritis are definitely not vagally innervated. It is important to realize that the left cervical stimulator used in this study likely stimulates vagal afferents, as mentioned above, and modulates sympathetic and neuroendocrine reflex activity. To this end, experiments performed in the late 1990s with bradykinin‐induced plasma extravasation in the rat knee joint as an arthritis model provided a hypothetical framework for these therapeutic effects. It was shown that subdiaphragmatic vagal afferents from the intestine were part of a complex neuroendocrine network involving the adrenal medulla that inhibited synovial plasma extravasation (for a review, see Ref. 101). These studies highlight the complex relationship between the vagus and inflammation.
The vagus nerve and cancer progression
A role for the vagus nerve in cancer progression has also been suggested. The old notion of a variety of cancers spreading by invasion of peripheral nerves, which heralds poor outcome, has increasingly stimulated research activity in recent years.102 Basic to this phenomenon are interactions between malignant cells and nerve fibers and their sheaths, involving various neurotrophins and their receptors.102, 103 In particular, the role of the vagus in modulating malignant disease was studied in gastric and pancreatic carcinomas, both in mouse models and patients. Subdiaphragmatic vagotomy was protective in gastric carcinoma,104, 105 but tumor promoting in pancreatic106, 107 carcinoma. In an experimental tour de force, these authors aimed at deciphering the molecular signaling mechanisms. It turned out that cholinergic signals in the stomach, supposedly elicited by vagal activation of enteric cholinergic neurons, acted via m3 muscarinic receptors through the Wnt/Hippo pathway, favoring tumor induction and growth.105 In the pancreas, vagally induced acetylcholine release activated the EGFR/MAPK pathway through m1 muscarinic receptors, suppressing tumorigenesis.107 Thus, vagotomy is thought to release a brake, thus allowing for sympathetic adrenergic signaling, which is tumor promoting in this organ.107, 108 Perhaps equally important, vagotomy abrogates the vagal anti‐inflammatory effect, resulting in increased TNF‐α levels that favor inflammation and tumor growth in the pancreas.106
However, things are probably not that simple, given the complexity of gastric and pancreatic innervation by vagal efferents and afferents that are simultaneously cut by vagotomy, in addition to sympathetic efferents and spinal DRG afferents, the latter playing a proinflammatory and tumor‐promoting role in the pancreas.109 Furthermore, important neuron populations, for example, VIPergic and nitrergic, were not yet considered in these paradigms. They account for roughly one‐third of the gastric myenteric plexus in the guinea pig,110 are prominent also in pancreatic ganglia of rat and humans,30, 33, 111 and are targeted by vagal preganglionics in rats.30, 112, 113
Although a number of preclinical and clinical studies have hinted at significant vagal modulation of inflammation and gastrointestinal cancers, the mechanisms are far from clear, as are the relative contribution of vagal and sympathetic efferents and afferents innervating specific organs and tissues. Again, incomplete knowledge and even ignorance of vagal innervation patterns have resulted in misconceptions.
Genetically guided functional mapping and selective vagal manipulation
As discussed in the preceding sections, one of the biggest obstacles for developing effective vagal (as well as sympathetic and other peripheral nerves) neuromodulation therapies is the inability to selectively target function‐specific fibers. This leads inevitably to costimulation (or silencing) of fibers with other functions and, thus, unwanted side effects. Designing finer and better electrodes and smarter stimulation algorithms may still yield improved specificity in the future. A promising alternative approach, however, is genetically guided manipulation of a select pool of nerve fibers. Most of these studies have been carried out in the mouse, given the ease of its genetic manipulation. For example, since the melanocortin receptor‐4 (MC4R) is expressed in vagal efferents and afferents,114 a mouse was generated that expresses green fluorescent protein (GFP) under the control of the Mc4r promoter.115 Without any histochemical processing, GFP‐labeled vagal fibers could then be identified in abdominal organs. Another study used pancreatic injections of a custom retrograde tracer, pseudorabies virus, capable of Cre‐conditional replication (PRV‐Ba2001‐GFP) in mice with nuclear Cre‐expression specifically restricted to pancreatic β cells when induced by tamoxifen (MIP‐CreERT mice).116 This allowed identification of the vagal and sympathetic efferent outflow selectively to pancreatic β cells, potentially enabling their selective stimulation or silencing. In a third study, specific populations of vagal afferents were targeted by injecting a Cre‐inducible adeno‐associated virus expressing the strong red fluorophore tdTomato (AAV‐flex‐tdTomato) into the nodose ganglia of mice with Cre expressed under the promoter of either the glucagon‐like peptide‐1 receptor (Glp1r‐ires‐Cre mice) or the G protein–coupled receptor 65 (Gpr65‐ires‐Cre mice).117 This selectively highlighted specific vagal afferents either innervating exclusively the stomach tension receptors (IGLEs) or proximal intestinal mucosal receptors, likely sensing the luminal environment.117 Importantly, shining blue light on the vagal trunks in mice with inducible channel rhodopsin expression (Gpr65‐ires‐Cre; lox‐ChR2 mice) resulted in the rapid and complete suppression of gastric motility, suggesting that a sensory‐motor vagal loop with a relay in the brainstem was activated that normally suppresses gastric motility upon arrival of nutrients in the proximal duodenum. Collectively, these studies provide proof‐of‐concept for genetics‐based selective manipulation of pools of function‐specific vagal efferents or afferents. Besides light‐sensitive channel rhodopsin, channels sensitive to designer drugs (DREADDs), heat, magnetism, or radio waves have also been successfully engineered into cells, with the promise of remotely controlling specific cell functions in the fight against metabolic diseases.118, 119
In summary, recent studies in genetically engineered mice have advanced our understanding of vagal, and in particular afferent, functional anatomy. This will hopefully contribute to designing more specific stimulation strategies aimed at treating metabolic disorders.
Conclusions
Given its distribution pattern and functionality, the vagus nerve is in a strong position to influence physiological processes responsible for the control of food intake and regulation of body weight. This has spurred the development of numerous neuromodulation techniques and devices to manipulate vagal nerve function as therapies to treat obesity and its comorbid conditions, such as T2DM, cardiovascular disease, hepatosteatosis, inflammation, and cancer. However, there is a clear disconnect between the mechanistic rationale for such devices and the known functional anatomy of the vagus nerve, which leaves their underlying mechanisms completely unanswered. The specificity of what exactly is manipulated and is, in fact, responsible for specific beneficial effects should be the focus of future preclinical and clinical studies for the field to move ahead.
Competing interests
The authors declare no competing interests.
Acknowledgments
This work was partially funded by the National Institutes of Health, Grants DK 047348 and OT2OD023864 (H.R.B.), and by the Deutsche Forschungsgemeinschaft NE534/3‐1 (W.L.N.).
References
- 1. Berthoud, H.R. 2008. The vagus nerve, food intake and obesity. Regul. Pept. 149: 15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kral, J.G. 1978. Vagotomy for treatment of severe obesity. Lancet 1: 307–308. [DOI] [PubMed] [Google Scholar]
- 3. Berthoud, H.R. , Shin A.C. & Zheng H.. 2011. Obesity surgery and gut–brain communication. Physiol. Behav. 105: 106–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mei, N. 1985. Intestinal chemosensitivity. Physiol. Rev. 65: 211–237. [DOI] [PubMed] [Google Scholar]
- 5. Niijima, A. 1989. Nervous regulation of metabolism. Prog. Neurobiol. 33: 135–147. [DOI] [PubMed] [Google Scholar]
- 6. Page, A.J. et al 2012. Peripheral neural targets in obesity. Br. J. Pharmacol. 166: 1537–1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Blackshaw, L.A. et al 2007. Sensory transmission in the gastrointestinal tract. Neurogastroenterol. Motil. 19: 1–19. [DOI] [PubMed] [Google Scholar]
- 8. Grundy, D. 2006. Signalling the state of the digestive tract. Auton. Neurosci. 125: 76–80. [DOI] [PubMed] [Google Scholar]
- 9. Jungermann, K. & Stumpel F.. 1999. Role of hepatic, intrahepatic and hepatoenteral nerves in the regulation of carbohydrate metabolism and hemodynamics of the liver and intestine. Hepatogastroenterology 46(Suppl. 2): 1414–1417. [PubMed] [Google Scholar]
- 10. Gurtner, T. , Kreutzberg G.W. & Holle F.. 1967. [The vagus nerve and cholinergic system in the stomach of humans. II. Cholinergic supply of the human stomach]. Munch. Med. Wochenschr. 109: 1763–1769. [PubMed] [Google Scholar]
- 11. Naik, N.T. & Cauna N.. 1971. Histochemical observations on cholinesterase activity in the autonomic ganglia of the human sympathetic trunk and vagus nerve. Histochem. J. 3: 47–53. [DOI] [PubMed] [Google Scholar]
- 12. Kyosola, K. , Veijola L. & Rechardt L.. 1975. Cholinergic innervation of the gastric wall of the cat. Histochemistry 44: 23–30. [DOI] [PubMed] [Google Scholar]
- 13. Ellison, J.P. & Clark M.G.. 1975. Retrograde axonal transport of horseradish peroxidase in peripheral autonomic nerves. J. Comp. Neurol. 161: 103–113. [DOI] [PubMed] [Google Scholar]
- 14. Jansen, A.S. & Loewy A.D.. 1994. Viral tracing of innervation. Science 265: 121–122. [DOI] [PubMed] [Google Scholar]
- 15. Fox, E.A. & Powley T.L.. 1989. False‐positive artifacts of tracer strategies distort autonomic connectivity maps. Brain Res. Brain Res. Rev. 14: 53–77. [DOI] [PubMed] [Google Scholar]
- 16. Fox, E.A. & Powley T.L.. 1986. Tracer diffusion has exaggerated CNS maps of direct preganglionic innervation of pancreas. J. Auton. Nerv. Syst. 15: 55–69. [DOI] [PubMed] [Google Scholar]
- 17. Connors, N.A. , Sullivan J.M. & Kubb K.S.. 1983. An autoradiographic study of the distribution of fibers from the dorsal motor nucleus of the vagus to the digestive tube of the rat. Acta Anat. (Basel) 115: 266–271. [DOI] [PubMed] [Google Scholar]
- 18. Berthoud, H.R. , Jedrzejewska A. & Powley T.L.. 1990. Simultaneous labeling of vagal innervation of the gut and afferent projections from the visceral forebrain with dil injected into the dorsal vagal complex in the rat. J. Comp. Neurol. 301: 65–79. [DOI] [PubMed] [Google Scholar]
- 19. Berthoud, H.R. , Carlson N.R. & Powley T.L.. 1991. Topography of efferent vagal innervation of the rat gastrointestinal tract. Am. J. Physiol. 260: R200–R207. [DOI] [PubMed] [Google Scholar]
- 20. Neuhuber, W.L. 1987. Sensory vagal innervation of the rat esophagus and cardia: a light and electron microscopic anterograde tracing study. J. Auton. Nerv. Syst. 20: 243–255. [DOI] [PubMed] [Google Scholar]
- 21. Berthoud, H.R. & Powley T.L.. 1992. Vagal afferent innervation of the rat fundic stomach: morphological characterization of the gastric tension receptor. J. Comp. Neurol. 319: 261–276. [DOI] [PubMed] [Google Scholar]
- 22. Berthoud, H.R. & Neuhuber W.L.. 2000. Functional and chemical anatomy of the afferent vagal system. Auton. Neurosci. 85: 1–17. [DOI] [PubMed] [Google Scholar]
- 23. Berthoud, H.R. et al 1997. Distribution and structure of vagal afferent intraganglionic laminar endings (IGLEs) in the rat gastrointestinal tract. Anat. Embryol. (Berl.) 195: 183–191. [DOI] [PubMed] [Google Scholar]
- 24. Wang, F.B. & Powley T.L.. 2000. Topographic inventories of vagal afferents in gastrointestinal muscle. J. Comp. Neurol. 421: 302–324. [PubMed] [Google Scholar]
- 25. Berthoud, H.R. et al 1995. Vagal sensors in the rat duodenal mucosa: distribution and structure as revealed by in vivo DiI‐tracing. Anat. Embryol. (Berl.) 191: 203–212. [DOI] [PubMed] [Google Scholar]
- 26. Kaelberer, M.M. et al 2018. A gut–brain neural circuit for nutrient sensory transduction. Science 361 10.1126/science.aat5236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Powley, T.L. , Spaulding R.A. & Haglof S.A.. 2011. Vagal afferent innervation of the proximal gastrointestinal tract mucosa: chemoreceptor and mechanoreceptor architecture. J. Comp. Neurol. 519: 644–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Han, W. et al 2018. A neural circuit for gut‐induced reward. Cell 175: 665–678.e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Berthoud, H.R. & Powley T.L.. 1991. Morphology and distribution of efferent vagal innervation of rat pancreas as revealed with anterograde transport of Dil. Brain Res. 553: 336–341. [DOI] [PubMed] [Google Scholar]
- 30. Wang, J. , Zheng H. & Berthoud H.R.. 1999. Functional vagal input to chemically identified neurons in pancreatic ganglia as revealed by Fos expression. Am. J. Physiol. 277: E958–E964. [DOI] [PubMed] [Google Scholar]
- 31. Tang, S.C. et al 2018. Pancreatic neuro‐insular network in young mice revealed by 3D panoramic histology. Diabetologia 61: 158–167. [DOI] [PubMed] [Google Scholar]
- 32. Tsumori, T. et al 2013. Intrapancreatic ganglia neurons receive projection fibers from melanocortin‐4 receptor‐expressing neurons in the dorsal motor nucleus of the vagus nerve of the mouse. Brain Res. 1537: 132–142. [DOI] [PubMed] [Google Scholar]
- 33. Bishop, A.E. et al 1980. The location of VIP in the pancreas of man and rat. Diabetologia 18: 73–78. [DOI] [PubMed] [Google Scholar]
- 34. Krivova, Y. et al 2016. Structure of neuro‐endocrine and neuro‐epithelial interactions in human foetal pancreas. Tissue Cell 48: 567–576. [DOI] [PubMed] [Google Scholar]
- 35. Neuhuber, W.L. 1989. Vagal afferent fibers almost exclusively innervate islets in the rat pancreas as demonstrated by anterograde tracing. J. Auton. Nerv. Syst. 29: 13–18. [DOI] [PubMed] [Google Scholar]
- 36. Iwasaki, Y. et al 2013. Insulin activates vagal afferent neurons including those innervating pancreas via insulin cascade and Ca(2+) influx: its dysfunction in IRS2‐KO mice with hyperphagic obesity. PLoS One 8: e67198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Berthoud, H.R. & Neuhuber W.L.. 1996. An anatomical analysis of vagal and spinal afferent innervation of rat liver and associated organs In Liver Innervation and the Neural Control of Hepatic Function. Shimazu T., Ed.: 31–42. London: John Libbey. [Google Scholar]
- 38. Berthoud, H.R. 2004. Anatomy and function of sensory hepatic nerves. Anat. Rec. 280A: 827–835. [DOI] [PubMed] [Google Scholar]
- 39. Berthoud, H.R. , Kressel M. & Neuhuber W.L.. 1992. An anterograde tracing study of the vagal innervation of rat liver, portal vein and biliary system. Anat. Embryol. (Berl.) 186: 431–442. [DOI] [PubMed] [Google Scholar]
- 40. Berthoud, H.R. & Patterson L.M.. 1996. Innervation of rat abdominal paraganglia by calretinin‐like immunoreactive nerve fibers. Neurosci. Lett. 210: 115–118. [DOI] [PubMed] [Google Scholar]
- 41. Kreier, F. et al 2002. Selective parasympathetic innervation of subcutaneous and intra‐abdominal fat–functional implications. J. Clin. Invest. 110: 1243–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Giordano, A. et al 2006. White adipose tissue lacks significant vagal innervation and immunohistochemical evidence of parasympathetic innervation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 291: R1243–R1255. [DOI] [PubMed] [Google Scholar]
- 43. Berthoud, H.R. , Fox E.A. & Neuhuber W.L.. 2006. Vagaries of adipose tissue innervation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 291: R1240–R1242. [DOI] [PubMed] [Google Scholar]
- 44. Cano, G. , Card J.P. & Sved A.F.. 2004. Dual viral transneuronal tracing of central autonomic circuits involved in the innervation of the two kidneys in rat. J. Comp. Neurol. 471: 462–481. [DOI] [PubMed] [Google Scholar]
- 45. Cano, G. et al 2001. Characterization of the central nervous system innervation of the rat spleen using viral transneuronal tracing. J. Comp. Neurol. 439: 1–18. [DOI] [PubMed] [Google Scholar]
- 46. Bergman, R.N. & Miller R.E.. 1973. Direct enhancement of insulin secretion by vagal stimulation of the isolated pancreas. Am. J. Physiol. 225: 481–486. [DOI] [PubMed] [Google Scholar]
- 47. Shimazu, T. & Ogasawara S.. 1975. Effects of hypothalamic stimulation on gluconeogenesis and glycolysis in rat liver. Am. J. Physiol. 228: 1787–1793. [DOI] [PubMed] [Google Scholar]
- 48. Eisen, S. et al 2005. Inhibitory effects on intake of cholecystokinin‐8 and cholecystokinin‐33 in rats with hepatic proper or common hepatic branch vagal innervation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 289: R456–R462. [DOI] [PubMed] [Google Scholar]
- 49. Berthoud, H.R. et al 1983.. Evidence for a role of the gastric, coeliac and hepatic branches in vagally stimulated insulin secretion in the rat. J. Auton. Nerv. Syst. 7: 97–110. [DOI] [PubMed] [Google Scholar]
- 50. Berthoud, H.R. , Fox E.A. & Powley T.L.. 1990. Localization of vagal preganglionics that stimulate insulin and glucagon secretion. Am. J. Physiol. 258: R160–R168. [DOI] [PubMed] [Google Scholar]
- 51. German, J. et al 2009. Hypothalamic leptin signaling regulates hepatic insulin sensitivity via a neurocircuit involving the vagus nerve. Endocrinology 150: 4502–4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Filippi, B.M. et al 2014. Insulin signals through the dorsal vagal complex to regulate energy balance. Diabetes 63: 892–899. [DOI] [PubMed] [Google Scholar]
- 53. Gao, X. et al 2015. Vagus nerve contributes to the development of steatohepatitis and obesity in phosphatidylethanolamine N‐methyltransferase deficient mice. J. Hepatol. 62: 913–920. [DOI] [PubMed] [Google Scholar]
- 54. Knight, C.M. et al 2011. Mediobasal hypothalamic SIRT1 is essential for resveratrol's effects on insulin action in rats. Diabetes 60: 2691–2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Lam, T.K. et al 2005. Hypothalamic sensing of circulating fatty acids is required for glucose homeostasis. Nat. Med. 11: 320–327. [DOI] [PubMed] [Google Scholar]
- 56. Uno, K. et al 2012. Hepatic peroxisome proliferator‐activated receptor‐γ‐fat‐specific protein 27 pathway contributes to obesity‐related hypertension via afferent vagal signals. Eur. Heart J. 33: 1279–1289. [DOI] [PubMed] [Google Scholar]
- 57. Kimura, K. et al 2016. Central insulin action activates Kupffer cells by suppressing hepatic vagal activation via the nicotinic alpha 7 acetylcholine receptor. Cell Rep. 14: 2362–2374. [DOI] [PubMed] [Google Scholar]
- 58. Pardo, J.V. et al 2007. Weight loss during chronic, cervical vagus nerve stimulation in depressed patients with obesity: an observation. Int. J. Obes. (Lond.) 31: 1756–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Burneo, J.G. et al 2002. Weight loss associated with vagus nerve stimulation. Neurology 59: 463–464. [DOI] [PubMed] [Google Scholar]
- 60. Vijgen, G.H. et al 2013. Vagus nerve stimulation increases energy expenditure: relation to brown adipose tissue activity. PLoS One 8: e77221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Bodenlos, J.S. et al 2007. Vagus nerve stimulation acutely alters food craving in adults with depression. Appetite 48: 145–153. [DOI] [PubMed] [Google Scholar]
- 62. Howland, R.H. 2014. Vagus nerve stimulation. Curr. Behav. Neurosci. Rep. 1: 64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Huang, F. et al 2014. Effect of transcutaneous auricular vagus nerve stimulation on impaired glucose tolerance: a pilot randomized study. BMC Complement. Altern. Med. 14: 203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Li, H. et al 2015. Effects and mechanisms of auricular vagus nerve stimulation on high‐fat‐diet–induced obese rats. Nutrition 31: 1416–1422. [DOI] [PubMed] [Google Scholar]
- 65. Safi, S. , Ellrich J. & Neuhuber W.. 2016. Myelinated axons in the auricular branch of the human vagus nerve. Anat. Rec. (Hoboken) 299: 1184–1191. [DOI] [PubMed] [Google Scholar]
- 66. Saxon, D.W. & Hopkins D.A.. 1998. Efferent and collateral organization of paratrigeminal nucleus projections: an anterograde and retrograde fluorescent tracer study in the rat. J. Comp. Neurol. 402: 93–110. [PubMed] [Google Scholar]
- 67. Nomura, S. & Mizuno N.. 1984. Central distribution of primary afferent fibers in the Arnold's nerve (the auricular branch of the vagus nerve): a transganglionic HRP study in the cat. Brain Res. 292: 199–205. [DOI] [PubMed] [Google Scholar]
- 68. Conway, C.R. et al 2013. Association of cerebral metabolic activity changes with vagus nerve stimulation antidepressant response in treatment‐resistant depression. Brain Stimul. 6: 788–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kraus, T. et al 2007. BOLD fMRI deactivation of limbic and temporal brain structures and mood enhancing effect by transcutaneous vagus nerve stimulation. J. Neural Transm. (Vienna) 114: 1485–1493. [DOI] [PubMed] [Google Scholar]
- 70. Dietrich, S. et al 2008. [A novel transcutaneous vagus nerve stimulation leads to brainstem and cerebral activations measured by functional MRI]. Biomed. Tech. (Berl.) 53: 104–111. [DOI] [PubMed] [Google Scholar]
- 71. Ikramuddin, S. et al 2014. Effect of reversible intermittent intra‐abdominal vagal nerve blockade on morbid obesity: the ReCharge randomized clinical trial. JAMA 312: 915–922. [DOI] [PubMed] [Google Scholar]
- 72. Morton, J.M. et al 2016. Effect of vagal nerve blockade on moderate obesity with an obesity‐related comorbid condition: the ReCharge study. Obes. Surg. 26: 983–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Apovian, C.M. et al 2017. Two‐year outcomes of vagal nerve blocking (vBloc) for the treatment of obesity in the ReCharge trial. Obes. Surg. 27: 169–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Pelot, N.A. , Behrend C.E. & Grill W.M.. 2017. Modeling the response of small myelinated axons in a compound nerve to kilohertz frequency signals. J. Neural Eng. 14: 046022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Somann, J.P. et al 2018. Chronic cuffing of cervical vagus nerve inhibits efferent fiber integrity in rat model. J. Neural Eng. 15: 036018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Lebovitz, H.E. 2016. Interventional treatment of obesity and diabetes: an interim report on gastric electrical stimulation. Rev. Endocr. Metab. Disord. 17: 73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Islam, S. et al 2016. Long‐term outcomes of gastric electrical stimulation in children with gastroparesis. J. Pediatr. Surg. 51: 67–71. [DOI] [PubMed] [Google Scholar]
- 78. Miras, M. et al 2015. Early experience with customized, meal‐triggered gastric electrical stimulation in obese patients. Obes. Surg. 25: 174–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Horbach, T. et al 2015. abiliti closed‐loop gastric electrical stimulation system for treatment of obesity: clinical results with a 27‐month follow‐up. Obes. Surg. 25: 1779–1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Tang, M. et al 2006. Implantable gastric stimulation alters expression of oxytocin‐ and orexin‐containing neurons in the hypothalamus of rats. Obes. Surg. 16: 762–769. [DOI] [PubMed] [Google Scholar]
- 81. Zhang, J. et al 2008. Optimal locations and parameters of gastric electrical stimulation in altering ghrelin and oxytocin in the hypothalamus of rats. Neurosci. Res. 62: 262–269. [DOI] [PubMed] [Google Scholar]
- 82. Andrews, P.L.R. & Lawes I.N.C.. 1992. A protective role for vagal afferents: a hypothesis In Neuroanatomy and Physiology of Abdominal Vagal Afferents. Ritter S.R., Ritter R.C. & Barnes C.D., Eds.: 221–248. Boca Raton: CRC Press. [Google Scholar]
- 83. Williams, R.M. , Berthoud H.R. & Stead R.H.. 1997. Vagal afferent nerve fibres contact mast cells in rat small intestinal mucosa. Neuroimmunomodulation 4: 266–270. [DOI] [PubMed] [Google Scholar]
- 84. Adriaensen, D. et al 1998. Pulmonary intraepithelial vagal nodose afferent nerve terminals are confined to neuroepithelial bodies: an anterograde tracing and confocal microscopy study in adult rats. Cell Tissue Res. 293: 395–405. [DOI] [PubMed] [Google Scholar]
- 85. Abe, C. et al 2017. C1 neurons mediate a stress‐induced anti‐inflammatory reflex in mice. Nat. Neurosci. 20: 700–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Fetissov, S.O. & Dechelotte P.. 2011. The new link between gut–brain axis and neuropsychiatric disorders. Curr. Opin. Clin. Nutr. Metab. Care 14: 477–482. [DOI] [PubMed] [Google Scholar]
- 87. Borovikova, L.V. et al 2000. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 405: 458. [DOI] [PubMed] [Google Scholar]
- 88. Wang, H. et al 2003. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature 421: 384–388. [DOI] [PubMed] [Google Scholar]
- 89. Rosas‐Ballina, M. et al 2008. Splenic nerve is required for cholinergic antiinflammatory pathway control of TNF in endotoxemia. Proc. Natl. Acad. Sci. USA 105: 11008–11013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Huston, J.M. et al 2006. Splenectomy inactivates the cholinergic antiinflammatory pathway during lethal endotoxemia and polymicrobial sepsis. J. Exp. Med. 203: 1623–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Pavlov, V.A. & Tracey K.J.. 2012. The vagus nerve and the inflammatory reflex–linking immunity and metabolism. Nat. Rev. Endocrinol. 8: 743–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Berthoud, H.R. & Powley T.L.. 1993. Characterization of vagal innervation to the rat celiac, suprarenal and mesenteric ganglia. J. Auton. Nerv. Syst. 42: 153–169. [DOI] [PubMed] [Google Scholar]
- 93. Berthoud, H.R. & Powley T.L.. 1996. Interaction between parasympathetic and sympathetic nerves in prevertebral ganglia: morphological evidence for vagal efferent innervation of ganglion cells in the rat. Microsc. Res. Tech. 35: 80–86. [DOI] [PubMed] [Google Scholar]
- 94. Bratton, B.O. et al 2012. Neural regulation of inflammation: no neural connection from the vagus to splenic sympathetic neurons. Exp. Physiol. 97: 1180–1185. [DOI] [PubMed] [Google Scholar]
- 95. Martelli, D. , McKinley M.J. & McAllen R.M.. 2014. The cholinergic anti‐inflammatory pathway: a critical review. Auton. Neurosci. 182: 65–69. [DOI] [PubMed] [Google Scholar]
- 96. de Jonge, W.J. et al 2005. Stimulation of the vagus nerve attenuates macrophage activation by activating the Jak2‐STAT3 signaling pathway. Nat. Immunol. 6: 844–851. [DOI] [PubMed] [Google Scholar]
- 97. Matteoli, G. et al 2014. A distinct vagal anti‐inflammatory pathway modulates intestinal muscularis resident macrophages independent of the spleen. Gut 63: 938–948. [DOI] [PubMed] [Google Scholar]
- 98. McAllen, R.M. et al 2015. The interface between cholinergic pathways and the immune system and its relevance to arthritis. Arthritis Res. Ther. 17: 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Komegae, E.N. et al 2018. Vagal afferent activation suppresses systemic inflammation via the splanchnic anti‐inflammatory pathway. Brain Behav. Immun. 73: 441–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Koopman, F.A. et al 2016. Vagus nerve stimulation inhibits cytokine production and attenuates disease severity in rheumatoid arthritis. Proc. Natl. Acad. Sci. USA 113: 8284–8289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Janig, W. & Green P.G.. 2014. Acute inflammation in the joint: its control by the sympathetic nervous system and by neuroendocrine systems. Auton. Neurosci. 182: 42–54. [DOI] [PubMed] [Google Scholar]
- 102. Liebig, C. et al 2009. Perineural invasion in cancer: a review of the literature. Cancer 115: 3379–3391. [DOI] [PubMed] [Google Scholar]
- 103. Gil, Z. et al 2010. Paracrine regulation of pancreatic cancer cell invasion by peripheral nerves. J. Natl. Cancer Inst. 102: 107–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Zhao, C.M. et al 2014. Denervation suppresses gastric tumorigenesis. Sci. Transl. Med. 6: 250ra115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Hayakawa, Y. et al 2017. Nerve growth factor promotes gastric tumorigenesis through aberrant cholinergic signaling. Cancer Cell 31: 21–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Partecke, L.I. et al 2017. Subdiaphragmatic vagotomy promotes tumor growth and reduces survival via TNFα in a murine pancreatic cancer model. Oncotarget 8: 22501–22512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Renz, B.W. et al 2018. Cholinergic signaling via muscarinic receptors directly and indirectly suppresses pancreatic tumorigenesis and cancer stemness. Cancer Discov. 8: 1458–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Renz, B.W. et al 2018. β2 Adrenergic‐neurotrophin feedforward loop promotes pancreatic cancer. Cancer Cell 33: 75–90.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Saloman, J.L. et al 2016. Ablation of sensory neurons in a genetic model of pancreatic ductal adenocarcinoma slows initiation and progression of cancer. Proc. Natl. Acad. Sci. USA 113: 3078–3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Schemann, M. , Schaaf C. & Mader M.. 1995. Neurochemical coding of enteric neurons in the guinea pig stomach. J. Comp. Neurol. 353: 161–178. [DOI] [PubMed] [Google Scholar]
- 111. Worl, J. et al 1994. Neuronal and endothelial nitric oxide synthase immunoreactivity and NADPH‐diaphorase staining in rat and human pancreas: influence of fixation. Histochemistry 102: 353–364. [DOI] [PubMed] [Google Scholar]
- 112. Berthoud, H.R. 1996. Morphological analysis of vagal input to gastrin releasing peptide and vasoactive intestinal peptide containing neurons in the rat glandular stomach. J. Comp. Neurol. 370: 61–70. [DOI] [PubMed] [Google Scholar]
- 113. Berthoud, H.R. , Patterson L.M. & Zheng H.. 2001. Vagal‐enteric interface: vagal activation‐induced expression of c‐Fos and p‐CREB in neurons of the upper gastrointestinal tract and pancreas. Anat. Rec. 262: 29–40. [DOI] [PubMed] [Google Scholar]
- 114. Wan, S. et al 2008. Presynaptic melanocortin‐4 receptors on vagal afferent fibers modulate the excitability of rat nucleus tractus solitarius neurons. J. Neurosci. 28: 4957–4966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Gautron, L. et al 2012. Melanocortin‐4 receptor expression in different classes of spinal and vagal primary afferent neurons in the mouse. J. Comp. Neurol. 520: 3933–3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Rosario, W. et al 2016. The brain‐to‐pancreatic islet neuronal map reveals differential glucose regulation from distinct hypothalamic regions. Diabetes 65: 2711–2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Williams, E.K. et al 2016. Sensory neurons that detect stretch and nutrients in the digestive system. Cell 166: 209–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Stanley, S.A. et al 2015. Remote regulation of glucose homeostasis in mice using genetically encoded nanoparticles. Nat. Med. 21: 92–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Stanley, S.A. et al 2012. Radio‐wave heating of iron oxide nanoparticles can regulate plasma glucose in mice. Science 336: 604–608. [DOI] [PMC free article] [PubMed] [Google Scholar]


